Page 1 :
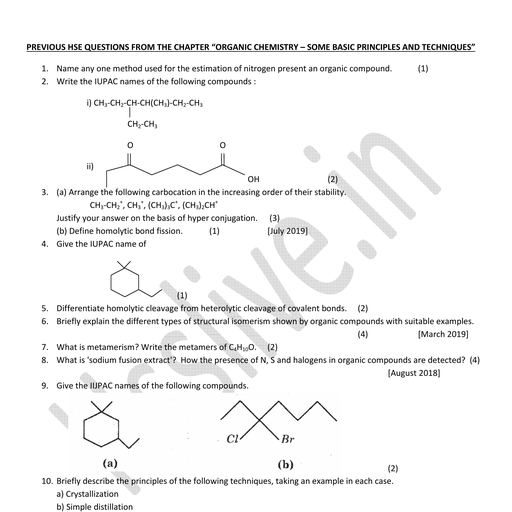
2. STRUCTURE OF ATOM, HAIZEL G.ROY, H.S.S.T. (HG) CHEMISTRY, GOVT. H.S.S. KALAMASSERY, ERNAKULAM
Page 2 :
INTRODUCTION, , , According to Dalton’s atomic theory, all, matter, , is, , composed, , of, , extremely, , small, , particles called atoms., , , The atom consists of smaller particles such, as electron, proton and neutron., , , , These particles are regarded as fundamental, particles.
Page 3 :
STRUCTURE OF ATOM
Page 4 :
DISCOVERY OF ELECTRON, The electrons were discovered by conducting, an experiment by using a simple apparatus, known as discharge tube or Crookes tube., , WILLIAM CROOKES
Page 5 :
DISCHARGE TUBE EXPERIMENT
Page 6 :
➔, , Discharge Tube consists of a sealed glass, tube of about 50 cm lengths with two metal, electrodes fused to the ends and a side tube, connected to a vacuum pump., , ➔, , The tube is filled with a gas under study and, the two electrodes are connected to a source, of high voltage.
Page 7 :
OBSERVATIONS, , , When the discharge tube containing the gas is at, 1 atm pressure and at high voltage, the gas, remains non conducting., , , , When the pressure of the gas is reduced to about, 10−2 atm, the gas becomes conducting and light is, emitted by the residual gas in the tube., , , , The colour of the light depends up on the nature, of the gas.
Page 8 :
, , When the pressure of the gas in the discharge, tube is further reduced, the glow becomes weak., , , , At about 10−4 atm pressure, the glow within the, tube stops but the gas continues to conduct, electricity., , , , The glow surrounding the cathode or the negative, electrode detaches itself leaving a space between, it and the electrode., , , , This is known as Crookes dark space.
Page 9 :
, , When the pressure is reduced to below 0.001 mm of, Hg, the dark space fills the entire tube., , , , The emission of coloured light stops., , , , The discharge tube begins to glow with a faint, greenish light., , , , This is due to the striking on the glass tube by some, invisible rays coming from the cathode., , , , The rays are called cathode rays., , , , These rays are found to consist of negatively charged, material particles called electrons.
Page 10 :
PROPERTIES OF CATHODE RAYS, , , Travel in straight lines., , , , Produce mechanical effects., , , , When an electric field is applied to a stream, of cathode rays, they get deflected towards, the positive plate which indicates that, cathode rays themselves are negatively, charged.
Page 11 :
, , When, , a, , magnetic, , field, , is, , applied, , perpendicular to the path of cathode rays,, they get deflected in the direction expected, for negative particles., , , The direction of deflection shows that, cathode rays are –vely charged., , , , When cathode rays are allowed to strike a, thin metal foil, it gets heated up.
Page 12 :
Produce, x-rays when they strike, hard metals like tungsten, copper, etc., , , , Produce, , , , fluorescence, , on, , glass, , walls, ZnS etc., Penetrate through thin metallic foils., , , , Affect photographic plates., ,
Page 13 :
DETERMINATION OF, CHARGE TO MASS RATIO (e/m) OF ELECTRONS, , In 1897 J.J. Thomson determined the e/m of, the electron by measuring the deflection of, cathode, , rays, , under, , the, , simultaneous, , influence of electric and magnetic, fields., , J.J. THOMSON
Page 15 :
, , A narrow beam of cathode rays is generated by, electric discharge in a gas at low pressure and, it produces fluorescence on the screen at the, other end of the tube., , , , If an electric field is applied at right angles to, the beam, the beam having negative charge is, attracted to the positive plate of the field., , , , The beam thus traverses a parabolic path and, strikes at another point on the screen.
Page 16 :
, , A magnetic field is now applied to the beam in a, direction at right angles to that of the electric field., , , , The beam undergoes deflection in the opposite, direction., , , , The strength of the two fields are so adjusted that, the beam strikes the screen at the original position., , , , From the strength of the two fields, the ratio e/m can, be calculated., , , , The e/m value is found to be 1.76 x 108 Coulombs/g.
Page 17 :
DETERMINATION OF CHARGE OF AN ELECTRON, , The charge of an electron was determined by, Robert Millikan in 1909 by oil drop experiment., , ROBERT MILLIKAN
Page 18 :
MILLIKAN'S OIL DROP EXPERIMENT
Page 19 :
, , A spray of oil droplets is produced by an atomizer., , , , The oil droplets enter the apparatus through a small, hole., , , , It is allowed to fall in between two charged plates., , , , The motion of the droplets is observed with a, telescope., , , , The space between the charged plates is irradiated, with x-rays., , , , The x-rays ionize the molecules of the air.
Page 20 :
, , One or more electrons produced may be absorbed by, an oil droplet., , , , The oil droplet as a result becomes negatively charged., , , , By measuring the velocity of a given oil droplet as it, falls freely under the influence of gravity and then in an, electric field, it is possible to calculate the charge on, the droplet which was considered to be electronic, charge., , , , The charge on the electron is found to be 1.602 x 10−19, coulombs.
Page 21 :
MILLIKAN'S OIL DROP EXPERIMENT, From the values of e and e/m, the mass (m) of the electron, is calculated by dividing e by e/m.
Page 22 :
ANODE RAYS OR CANAL RAYS, Goldstein in 1886 discovered the existence of a, new type of rays in the discharge tube., , GOLDSTEIN
Page 24 :
, , Goldstein repeated the discharge tube experiment by, using a perforated cathode., , , , He evacuated the discharge tube and a high voltage, is applied across the electrodes., , , , He observed a new type of rays streaming behind the, cathode., , , , These rays were named as anode rays or canal rays., , , , These rays travel in opposite directions to the, cathode rays.
Page 25 :
PROPERTIES OF ANODE RAYS, , , Travel in straight lines., , , , Consist of material particles., , , , Anode rays are positively charged., , , , When a magnetic field is applied, they get deflected in, the direction expected for positive particles., , , , Produce heating effects., , , , The e/m values of anode rays are much smaller than, that of the cathode rays.
Page 26 :
DISCOVERY OF PROTON, , , Protons were discovered by E. Goldstein., , , , A proton is a subatomic particle having a unit, positive charge and a mass nearly equal to that of, hydrogen., , , , Charge of proton = 1.602 x 10−19 C, , , , Mass of proton = 1.672 x 10−24 g
Page 27 :
ORIGIN OF, CATHODE RAYS AND ANODE RAYS, , , Under the influence of high electric field, the, gas in the discharge tube is ionized., , , , This results in the formation of particles with, positive and negative charge., , , , The, , negatively, , charged, , particles, , towards the anode at very high speeds., , move
Page 28 :
, , On their way they collide with the atoms, of the gas producing more electrons and, positively charged particles., , , , The electrons moves towards the anode, in the form of cathode rays while the, positive ions move towards cathode in, the form of anode rays.
Page 29 :
EARLIER ATOM, MODELS
Page 30 :
THOMSON'S MODEL OF ATOM, , The first atom model was proposed by, J.J. Thomson.
Page 31 :
, , An atom may be considered as a, sphere of positive charge., , , , The electrons are uniformly distributed, in it to make the atom as a whole, electrically neutral., , , , Therefore this atom model is known as, plum pudding model.
Page 34 :
RUTHERFORD'S α-PARTICLE, SCATTERING EXPERIMENT, In order to find out the arrangement of electrons, and protons, Rutherford in 1911 conducted a, scattering experiment., , ERNEST, RUTHERFORD
Page 35 :
EXPERIMENT
Page 36 :
, , Rutherford bombarded a thin gold foil with a, stream of fast moving +vely charged α−particles, emitting from a radioactive element., , , , A movable circular screen coated with zinc, sulphide was placed at the back of the gold foil, to detect whether the α −particles undergo any, deviation in their path on passing through the, foil.
Page 37 :
OBSERVATIONS, , , Most of the α-particles passed through, the gold foil without any deviation., , , , Some of the α -particles were deflected, by small angles., , , , A very few α -particles bounced back.
Page 38 :
CONCLUSIONS, , , Since most of the α -particles pass through, the gold foil without any deviation, it indicates, that most of the space in an atom is empty., , , , α-particles, , being, , positively, , charged, , and, , having considerable mass, could be deflected, only by some heavy positively charged centre.
Page 39 :
, , The small angle of deflection of α-particles, indicated the presence of heavy positive, centre in the atom., , , , Rutherford named this +ve centre as, nucleus., , , , A few of the α-particles are bounced back, due to the direct collision with the nucleus.
Page 41 :
RUTHERFORD'S NUCLEAR MODEL OF THE ATOM, , , In an atom, the entire mass and the positive, charge is concentrated in a very small, region at the centre known as nucleus., , , , The magnitude of the positive charge on, the nucleus (number of protons) is different, for different atoms.
Page 42 :
, , The nucleus is surrounded by negatively, charged, , electrons, , which, , balance, , the, , positive charge on the nucleus., , , The electrons are not stationary but are, revolving around the nucleus at very high, speeds., , , , Most of the space in an atom is empty.
Page 43 :
RUTHERFORD'S ATOM MODEL
Page 44 :
PLANETARY MODEL OF ATOM, , , Nuclear model of an atom can be compared with the solar, system., , , , In an atom, the electrons revolve around the nucleus like, planets revolve around the sun., , , , The nucleus represents the sun and the electron, represents the planets., , , , Therefore this model is also referred to as planetary, model of atom and the electrons are called planetary, electrons.
Page 45 :
ATOMIC NUMBER, , , The number of unit positive charges carried by, the nucleus of an atom is termed as the atomic, number., , , , The atomic number is numerically equal to the, number of protons present in the nucleus of the, atom., , , , The number of protons in an atom is equal to the, number of electrons.
Page 46 :
DISCOVERY OF NEUTRON, , Neutrons were discovered by Chadwick, in 1932.
Page 47 :
, , Beryllium is bombarded with α-paricles., , , , The emitted radiation consists of a new, particle carrying no charge., , , , Chadwick called the particle, neutron.
Page 48 :
NEUTRON, , , A neutron is a subatomic particle having a, mass equal to that of hydrogen atom and, carrying no electrical charge., , , , The mass of neutron is found to be 1.675 x, 10−24 g.
Page 49 :
MASS NUMBER (A), , , The mass of an atom is mainly due to protons and, neutrons., , , , Protons and neutrons are collectively known as nucleons., , , , The total number of protons and neutrons in the nucleus, is called mass number of the atom. It is generally, represented by ‘A’., , , , Mass number = No. of protons + Number of Neutron, , , , No. of Neutrons = Mass Number − Atomic Number, , , , i.e., (A―Z)
Page 50 :
ISOTOPES, , , Atoms of an element with the same atomic, number but different mass numbers are called, isotopes., , , , For example, hydrogen has three isotopes., , , , They are Protium (1H),, , Deuterium (2H) and, , Tritium (3H)., , , Carbon has three isotopes namely, 12 C, 13 C, 14 C.
Page 51 :
ISOBARS, Atoms having the same mass number but, different, Isobars., Examples, , atomic, , numbers, , are, , called
Page 52 :
ISOTONES, Atoms having the same number of neutrons, but different mass numbers are called, isotones., Example
Page 53 :
NATURE OF ELECTROMAGNETIC RADIATIONS, , CORPUSCULAR THEORY, , NEWTON
Page 54 :
NEWTON’S CORPUSCULAR THEORY, , , Light is propagated as a stream of minute, particles called corpuscles of light., , , , This theory of light could explain the phenomena, of reflection and refraction., , , , It failed to explain the phenomena of diffraction, and interference., , , , Therefore the corpuscular theory was rejected.
Page 55 :
ELECTROMAGNETIC RADIATIONS, , , James Clark Maxwell in 1856 suggested that light, propagates through space in the form of waves., , , , These waves are associated with electric and, magnetic fields., , , , Therefore,, , these, , electromagnetic, radiations., , waves, , waves, , or, , are, , called, , electromagnetic
Page 56 :
CHARACTERISTICS OF ELECTROMAGNETIC RADIATIONS, , Electromagnetic radiations propagate through space in, the form of waves., All Electromagnetic radiations travel with a velocity of, light., It require no medium for transmission., The energy of electromagnetic radiation is directly, proportional to its frequency or inversely proportional to, its wavelength.
Page 57 :
CHARACTERISTICS OF A WAVE
Page 58 :
WAVE LENGTH (λ), , The distance between two adjacent, crests of troughs is called wavelength., It is denoted by the Greek letter, lambda (λ) and is generally expressed, in terms of Angstrom units., 1Å = 10−10 m or 10−8 cm
Page 59 :
FREQUENCY (υ), The number of waves which pass through, a given point in one second is known as the, frequency., It is denoted by the Greek letter nu (υ)., The units of frequency are cycles per, second or Hertz (Hz)., 1 Hz = 1 cycles / sec
Page 60 :
VELOCITY (c), The distance travelled by a wave in, one second is called the velocity of a, wave., It is generally denoted by the letter (c).
Page 61 :
AMPLITUDE (a), , ➔It is the height of the crest or depth, of the trough of a wave., ➔It is generally denoted by the letter, (a).
Page 62 :
WAVE NUMBER, It is defined as the number of wavelengths per unit, length., It is the inverse of wavelength., It is denoted by nu bar., It is expressed in cm −1 or m −1
Page 63 :
RELATIONSHIP BETWEEN, WAVELENGTH, FREQUENCY AND VELOCITY, Velocity = Wavelength x Frequency, c=λxυ, The velocity of electromagnetic radiations is, 3 x 108 m/s.
Page 64 :
ELECTROMAGNETIC SPECTRUM, The arrangement of different types of, electromagnetic radiations according to, the increasing wavelength or decreasing, frequency is known as electromagnetic, spectrum.
Page 66 :
The different regions of the electromagnetic, spectrum are arranged as follows., Cosmic rays, γ-rays, x-rays, UV region, Visible, region, Infrared region, Microwaves, Radiowaves, etc., The wavelength of different colours constituting, the visible light are as follows.
Page 67 :
BLACK BODY RADIATION, ➔The, , ideal, , body, , which, , emits, , and, , absorbs all frequencies is known as black, body., ➔The radiation emitted by the black body, is known as black body radiation.
Page 68 :
PHOTOELECTRIC EFFECT, ➔When a light of suitable frequency strikes a metal,, electrons are ejected., ➔The phenomenon is known as photoelectric effect., ➔The emitted electrons are called photoelectrons., , HEINRICH HERTZ
Page 69 :
IMPORTANT ASPECTS, The ejection of electrons from the surface of a metal, will take place only if the incident radiation has a, certain minimum frequency., The minimum frequency of light, required to cause, the emission of electrons from a metal surface is, called threshold frequency (υ0).
Page 70 :
The kinetic energy of the ejected electrons is, proportional to the frequency of the incident, radiation and is independent of its intensity., The number of electrons ejected from the metal, surface is proportional to the intensity of the, radiation.
Page 71 :
PLANCK’S QUANTUM THEORY, In order to explain the phenomena of black body, radiation and photoelectric effect, Max Planck in 1900, put forward a new theory called quantum theory of, radiation., , MAX PLANCK
Page 72 :
QUANTUM THEORY, Radiant energy is emitted or absorbed not, continuously or discontinuously in the form of, small packets of energy called quanta., Each packet of wave is associated with a, definite amount of energy. In the case of light,, the quantum of energy is often called a photon.
Page 73 :
The amount of energy associated with a, quantum of radiation is proportional to the, frequency of radiation.
Page 74 :
DUAL NATURE OF, ELECTROMAGNETIC RADIATIONS, The particle nature of light could explain certain, phenomena like photoelectric effect and black body, radiation., But the phenomena such as diffraction and interference, can be explained only on the basis of wave nature., The experimental facts suggest that light has dual, character., i.e. particle character as well as wave character.
Page 75 :
SOLAR SPECTRUM, When white light is passed through a prism, it, splits into a series of colour bands known as, VIBGYOR., Sunlight, , is, , electromagnetic, , composed, waves, , of, , a, , collection, , of, , having, , different, , light, , different, , wavelengths., The, , prism, , bends, , the, , wavelengths to different extents., , of
Page 76 :
The phenomenon of splitting if light into, seven colours is known as dispersion and the, series of colour bands is called a spectrum., In this spectrum, one colour merges into the, other, , without, , any, , gap, , or, , discontinuity., , Therefore the spectrum is known as continuous, spectrum.
Page 77 :
ATOMIC SPECTRA, The spectra of atoms are not continuous., The atomic spectra consist of sharp well, defined lines or bands corresponding to, definite wave lengths., Atomic spectra are of two types., They are Emission Spectra and Absorption, Spectra.
Page 78 :
EMISSION SPECTRA, If the light emitted from an excited substance, is dispersed by using an instrument called, spectroscope, the spectrum obtained is not, continuous but consists of a series of sharp well, defined lines., Each line in the spectrum corresponds to a, definite wavelength.
Page 80 :
A spectrum containing lines of definite, wavelengths, , is, , called, , discontinuous, , spectrum or line spectrum., The line spectrum is also known as, atomic spectrum.
Page 81 :
ABSORPTION SPECTRA, When a continuous electromagnetic radiation is, allowed to pass through a gas or a solution of, some salt and the transmitted light is analyzed in a, spectroscope,, contains, , some, , we, , obtain, , dark, , continuous spectrum., , a, , lines, , spectrum, in, , an, , which, , otherwise
Page 82 :
A spectrum containing a few dark lines due to, absorption, spectrum., , of, , light, , is, , known, , as, , absorption
Page 83 :
SPECTRUM OF HYDROGEN ATOM, Obtained by passing an electric discharge through, hydrogen, , gas taken in a discharge tube under low, , pressure., The, , light, , emitted, , is, , analyzed, , by, , using, , a, , spectroscope., The spectrum consists of a large number of lines, appearing in different region of, spectrum., , electromagnetic
Page 84 :
Some of the lines are found in the visible region, while others in ultraviolet and Infra red region., The different series are Lymann Series, Balmer, Series, Paschen Series, Brackett Series and Pfund, Series., Lymann series appear in the ultraviolet region., Balmer series appear in the visible region., Paschen, Brackett and Pfund series appear in the, infra-red region.
Page 85 :
RYDBERG EQUATION, Rydberg, , gave, , a, , general, , expression, , which, , is, , applicable to all series of lines in the hydrogen, spectrum., , where R is Rydberg constant. The value of R is 109677, cm─1 ., The expression is known as Rydberg Equation.
Page 88 :
FAILURE OF RUTHERFORD’S MODEL, Rutherford’s model failed to explain the, stability of atoms, Rutherford’s model also failed to explain the, existence of certain definite lines in the, Hydrogen spectrum.
Page 89 :
Rutherford’s, , atom, , model, , failed, , in, , view, , of, , electromagnetic theory proposed by Maxwell., According to Maxwell’s theory, a charged particle, when accelerated emits energy in the form of, electromagnetic radiations., According to Rutherford’s model, electrons are, revolving around the nucleus., This means that electrons would be in a state of, acceleration all the time.
Page 90 :
Since electrons are charged particles, the electrons, revolving in an orbit should continuously emit radiations., As a result, it would slow down and would no longer be, able to withstand the attractive force of the nucleus., Hence it would move closer and closer to the nucleus, and would finally fall into the nucleus by following a spiral, path., But we know that an atom is stable., Thus Rutherford’s model failed to explain the stability of, atoms.
Page 91 :
BOHR MODEL OF THE ATOM, In 1913, Niels Bohr proposed a model of the atom, which was based upon the Planck’s Quantum theory, of radiation., , NIEL'S BOHR
Page 92 :
The electrons in an atom revolve around the nucleus in, certain selected circular paths called orbits., As long as an electron revolves in a particular orbit, it, does not lose or gain energy., Therefore, these orbits are also called stationary orbits., Only those orbits are permitted in which the angular, momentum (mvr) of the electron is an integral multiple, of, When an electron jumps from one orbit to another, it will, absorb or emit radiation of a definite frequency.
Page 93 :
when an electron jumps from a lower level to a, higher level, it absorbs energy equal to E 2 − E 1 ., When the electron jumps back to the lower level, it, emits the same amount of energy., , This amount of energy absorbed or emitted is, given by the difference in energies of the two, levels concerned.
Page 94 :
MERITS OF BOHR MODEL, Bohr model could explain the stability of an atom., Bohr’s theory helped in calculating the energy of, the electron in a particular orbit of hydrogen atom., Bohr’s theory helped in calculating the radius of, each circular orbit by using the expression, rn = 0.529 x n2 where n = 1, 2, 3, .......
Page 95 :
Bohr model could explain the line, spectrum of hydrogen., , , Bohr, , model, , simultaneous, number, spectrum., , of, , could, , explain, , appearance, lines, , in, , of, , the, , a, , the, large, , hydrogen
Page 96 :
DEMERITS OF BOHR MODEL, It could not explain the line spectrum of multi electron, atoms., It could not explain the splitting of spectral lines under, the influence of magnetic field (Zeeman effect) and electric, field (Stark effect)., It could not explain the shape of molecules formed by the, combination of atoms., Bohr model could not explain the fine structure of the, spectral lines produced by hydrogen.
Page 97 :
DE-BROGLIE EQUATION, According to de-Broglie, the wave length (λ), associated with a particle of mass ‘m’ moving with, velocity (v) is given by the relation, , Where ‘λ’ is the wavelength, ‘h’ is Planck’s, constant, ‘P’ is the momentum.
Page 100 :
HEISENBERG'S UNCERTAINITY PRINCIPLE, , Heisenberg in 1927, pointed out that we cannot, measure accurately both the position and, velocity of a particle as small as electron., , WERNER HEISENBERG
Page 101 :
➔“It is impossible to measure simultaneously both, the position and velocity of a microscopic particle in, motion with absolute accuracy”., ➔Mathematically it can be expressed as, , ➔Where ∆x is the uncertainity in position and ∆p is, the uncertainity in momentum.
Page 102 :
QUANTUM MECHANICS, Quantum mechanics was developed independently, in, , 1926, , by, , Werner, , Heisenberg, , and, , Erwin, , Schrodinger, , WERNER HEISENBER, , & ERWIN SCHRODINGER
Page 103 :
The branch of science that takes into account, the, , ➔, , dual behaviour of matter is called Quantum, mechanics., Quantum mechanics is a theoretical science that, , ➔, , deals with the study of the motions of the, microscopic objects that have the observable, wave like and particle like properties.
Page 104 :
SCHRODINGER WAVE EQUATION, On the basis of de-Broglie concept of matter waves, and Heisenberg’s uncertainity principle, a new, model of atom was developed, known as quantum, mechanical model., In this model, the behaviour of the electron in an, atom is described by an equation known as, Schrodinger Wave Equation.
Page 105 :
Schrodinger equation is represented as, , Where x, y and z are three Cartesian coordinates, E is the, total energy, V is the potential energy, m is the mass of the, electron, h is Planck’s constant and Ψ is the amplitude of, the electron wave.
Page 106 :
For a system such as an atom or a molecule, whose energy does not change with time, the, Schrodinger equation is written as H Ψ = E Ψ, Where H is a mathematical operator called, Hamiltonian Operator.
Page 107 :
SIGNIFICANCE OF Ψ, Ψ is the amplitude of the electron wave., Ψ has no physical significance but its square, i.e., Ψ2 has a physical significance., Ψ2 gives the intensity of the electron at any point., In other words, Ψ2 gives the probability of finding an, electron in a given region around the nucleus., Thus Ψ2 is termed as probability density and Ψ is called, probability amplitude.
Page 108 :
ORBITAL, An orbital may be defined as the region of space, around the nucleus where the probability of finding, the electron is maximum., Each orbital has definite amount of energy.
Page 109 :
DIFFERENCES BETWEEN, AN ORBIT AND AN ORBITAL
Page 110 :
ORBITAL, In an atom, the orbitals are designated by a set of, numbers known as quantum numbers., Inorder to specify energy, size, shape and orientation of, the electron orbital, three quantum numbers are required., In order to designate the electron, an additional quantum, number, called spin quantum number is required., Thus each orbital in an atom is designated by a set of, three quantum numbers and each electron is designated by, a set of four quantum numbers.
Page 111 :
PRINCIPAL QUANTUM NUMBER, It represents the main shell., It determines the energy of an electron., Principal quantum number is denoted by the letter ‘n’., This quantum number tells us in which principal energy level,, the electron is present., It can have any whole number value such as 1,2,3,4 ......, corresponding to K, L, M, N..., As the value of ‘n’ increases, the electron gets farther away, from the nucleus., Principal quantum number also determines the size of the orbital.
Page 112 :
Angular Momentum Quantum Number, or, Azimuthal Quantum Number( l ), This quantum number determines the angular momentum of the, electron. This is denoted by ‘l ’., The value of ‘ l ’ gives the sub shell in which the electron is, located. It also determines the shape of the orbital in which the, electron islocated., ‘ l ’ may have all possible whole number values from 0 to (n─1) for, each principal energy level., The various sub shells are designated as s, p, d, f depending, upon the value of ‘ l ’.
Page 113 :
For n = 1, ‘ l ’ can have only one value. i.e., 0., It means that electron present in the first energy level can, be present only in the s sub shell. i.e., 1s., For n = 2, ‘ l ’ can have only two values. i.e., 0 and 1. It, means that electron present in the second principal energy, level may be located either in the s sub shell or p sub shell., i.e., 2s and 2p., For n = 3, ‘ l ’ can have only three values. i.e., 0, 1 and 2., It means that electron present in the third principal energy, level may be located either in the s sub shell, p sub shell or, d sub shell. i.e., 3s, 3p and 3d.
Page 114 :
MAGNETIC QUANTUM NUMBER, This quantum number is denoted by ‘m’., It represents the different orientations of electron, cloud in a particular sub shell., These different orientations are called orbitals., Magnetic quantum number represents the number of, orbitals present in the sub shell., The possible values of ‘m’ ranges from + l to -l, including zero or ‘m’ can have (2 l+ 1) values.
Page 115 :
For l =0, m can have one value, m = 0, It implies that, s sub shell has only one orbital., For l =1, m can have three values, m = −1, 0, +1. It, implies that p sub shell has three orbitals., For l =2, m can have five values, m = −2, −1, 0, +1, +2., It implies that d sub shell has five orbitals., For l =3, m can have seven value, m = −3, −2, −1, 0,, +1, +2, +3., It implies that f sub shell has seven orbitals.
Page 116 :
SPIN QUANTUM NUMBER, It tells us about the direction of spin of the, electron., i.e., clockwise or anticlockwise., The spin quantum number can have only two, values, +1⁄2 and −1⁄2., The value +1⁄2 indicates clockwise spin and the, value −1⁄2 indicates anticlockwise spin.
Page 117 :
SHAPES OF ORBITALS
Page 118 :
s - ORBITALS, For s-orbitals, l = 0 and hence m can have only one value, i.e., m = 0., This means that the probability of finding the electron in, s-orbitals is same in all directions., In other words, s-orbitals are spherically symmetrical., In the case of 2s orbitals, there is a spherical shell where, the electron density is zero., This is called a node.
Page 120 :
p - ORBITALS, For p-orbitals, l = 1 and hence m can have 3 values i.e.,, m = +1, 0, −1., This means that there are three p orbitals in each p, sub shell., These are designated as P x , P y and P z ., P orbitals are dumb bell shaped.
Page 122 :
d - ORBITALS, ➔For p-orbitals, l = 2 and hence m can have 5 values, i.e., m = +2, +1, 0, −1, −2., This means that there are five d orbitals in each d, sub shell., These are designated as dxy , dxz , dyz , d x 2−y2 and, dz2 ., P orbitals are dumb bell shaped.
Page 124 :
Rules for filling of, orbitals in an atom
Page 125 :
PAULI'S EXCLUSION PRINCIPLE, “No two electrons in an atom can have the same, values for all the four quantum numbers”., Thus in any atom, the two electrons may have a, maximum of three quantum numbers the same, but the, fourth must be different., Eg:- Consider K shell where n = 1., n=1, , l=0, , m=0, , s = +1⁄2, , n=1, , l= 0, , m=0, , s = −1⁄2
Page 126 :
NOTE, On the basis of Pauli’s Exclusion Principle, it is, concluded that an orbital can have a maximum of two, electrons and they must have opposite spins., s-subshell can have a maximum of 2 electrons, p-subshell can have a maximum of 6 electrons, d-subshell can have a maximum of 10 electrons, f-subshell can have a maximum of 14 electrons
Page 129 :
(n + l ) RULE, ➔ Orbitals are filled in the order of increasing value of (n + l )., ➔ Eg:- 4s (n + l = 4 + 0 = 4) is filled before 3d (n + l = 3 + 2= 5), ➔ If two orbitals have the same (n + l ) value, the one with lower, ‘n’ value will be filled first., ➔Eg:- 2p (n + l = 2 + 1 = 3) is filled before 3s (n + l = 3 + 0 = 3), because 2p has lower value of n.
Page 130 :
HUND'S RULE, OF MAXIMUM MULTIPLICITY, Hund’s Rule states that electron pairing will not, take place in orbitals of same energy until each, orbital is first singly filled with parallel spin.
Page 131 :
ELECTRONIC CONFIGURATION, It is the distribution of electrons in the various, orbitals of the atom., The number of electrons in an atom will be equal, to the atomic number., These electrons are placed in the various, orbitals in the increasing order of energy.
Page 132 :
EXAMPLES
Page 133 :
THANK YOU