Page 1 :
UNIT-1, SOME BASIC CONCEPTS OF, CHEMISTRY, HAIZEL G. ROY, H.S.S.T. (HG) CHEMISTRY, GOVT. H.S.S. KALAMASSERY, ERNAKULAM
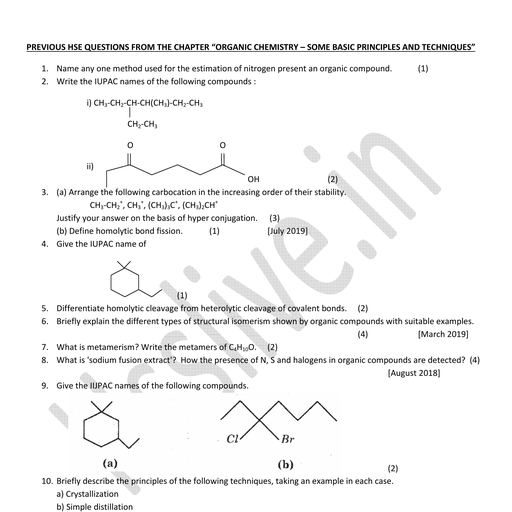
Page 2 :
CHEMISTRY, The branch of science which deals with, the, , composition,, , structure, , and, , properties of matter and the changes, which matter undergoes.
Page 3 :
BRANCHES OF CHEMISTRY, ✔, , Inorganic Chemistry, , ✔, , Medicinal Chemistry, , ✔, , Organic Chemistry, , ✔, , Industrial Chemistry, , ✔, , Physical Chemistry, , ✔, , Hydrochemistry, , ✔, , Analytical Chemistry, , ✔, , Electrochemistry, , ✔, , Polymer Chemistry, , ✔, , Green Chemistry, , ✔, , Biochemistry, , ✔, , Pharmaceutical Chemistry
Page 4 :
MATTER, Anything which occupies space and has, mass and can be perceived by our senses., Eg: Air, Water, Stone etc.
Page 5 :
STATES OF MATTER, , , Solid state, , , , Liquid state, , , , Gaseous state, , , , Plasma state, , , , Bose-Einstein condensate, , , , Fermionic condensate
Page 6 :
CLASSIFICATION OF MATTER, 1. PHYSICAL CLASSIFICATION, , 2. CHEMICAL CLASSIFICATION
Page 7 :
PHYSICAL CLASSIFICATION, , SOLIDS, , GASES, LIQUIDS
Page 8 :
SOLIDS, , , Have definite mass, volume and shape., , , , The constituent particles are tightly packed, , , , Intermolecular distances are short., , , , Intermolecular forces are strong., , , , Constituent particles have fixed positions., , , , They can only oscillate about their mean positions., , , , Incompressible and rigid.
Page 9 :
LIQUIDS, , , , , , , , , , , Liquids are not rigid, but have definite volume., They do not have a definite shape., They take the shape of the container in which they, are placed., The constituent particles are loosely packed., The intermolecular distances will be large as, compared to that of solids., Intermolecular forces are weak compared to solids., The rate of diffusion will be greater than solids.
Page 10 :
GASES, , , , , , , , , , Gases have neither definite shape nor definite volume., The particles are far apart from one another., The intermolecular distances are very large., The intermolecular forces of attraction are very weak., Gases are highly compressible., Gases exert pressure equally in all directions., Gases have much lower density than solids and liquids., Gases mix evenly and completely in all proportions, without any mechanical aid.
Page 12 :
CHEMICAL CLASSIFICATION, , ELEMENTS, , MIXTURES, , COMPOUNDS
Page 13 :
ELEMENTS, , , An element is a pure substance., , , , It cannot be broken down into two or more, simpler substances by physical and chemical, means., , , , It is made up of only one kind of atom., , , , Eg: Copper, Silver, Gold, Iron etc.
Page 15 :
COMPOUNDS, , , A compound is a pure substance., , , , It is made up of two or more elements, combined, , chemically, , proportion., , , Eg: H2O, NH3 , CH4 etc., , in, , fixed
Page 17 :
MIXTURES, A mixture is made up of two or more, elements or compounds or both., Eg: Brass is a mixture of Cu and Zn., Bronze is a mixture of Cu and Sn.
Page 19 :
CLASSIFICATION OF MIXTURES
Page 20 :
HOMOGENEOUS MIXTURE, , , In a homogeneous mixture, the components, completely mix with each other., , , , Its composition is uniform throughout the, mixture., , , , Eg: Ethanol – Water mixture, Salt solution, sugar solution etc.
Page 22 :
HETEROGENEOUS MIXTURE, The constituent particles are not uniformly, mixed., , The properties and composition vary, throughout the mixture., , Eg: Gun powder is a mixture of charcoal,, sulphur and nitre., , Muddy water, Chalk powder in water etc.,
Page 23 :
GUN POWDER, , CHALK POWDER IN WATER, , SAND IN WATER
Page 24 :
PROPERTIES OF MATTER
Page 25 :
PHYSICAL PROPERTIES, Properties which can be measured or observed, without, , changing, , the, , identity, , or, , the, , composition of the substance., Eg: Colour, odour, melting point, boiling point,, density etc.
Page 26 :
CHEMICAL PROPERTIES, ●, , Properties in which a chemical change, occur., , ●, , Eg: Chemical reactions of different, substances.
Page 27 :
MASS, ●, , Mass of a substance is the amount of, matter present in it., , ●, , The mass of a substance is constant., , ●, , The SI unit of mass is kg.
Page 28 :
WEIGHT, ●, , Weight is the force exerted by gravity, on an object., , ●, , Weight may vary from one place to, another due to change in gravity.
Page 29 :
DENSITY, ●, , Density of a substance is the amount of, mass per unit volume., ●, , ●, , The SI unit of density is kg/m, , 3
Page 30 :
TEMPERATURE, ●, , Temperature is the degree of hotness of a, body., , ●, , Three common scales are used to measure, temperature., , ●, , Degree Celsius (0C), , ●, , Degree Fahrenheit (0F), , ●, , Kelvin (K)
Page 31 :
●, , The Celsius scale is represented between zero, degree to hundred degree Celsius., , ●, , Fahrenheit scale is represented between 32 0, to 2120 F., , ●, , The Fahrenheit scale is related to Celsius scale, as, , ●, , The Kelvin scale is related to Celsius scale as, follows K = 0 C + 273.15
Page 32 :
ACCURACY & PRECISION, ACCURACY is the agreement of a particular value to, the true value of the result., ●, PRECISION, is, the closeness of, various, measurements for the same quantity., ●, Eg: If the true value for a result is 2.00 g and a, student ‘A’ takes two measurements and reports, the result as 1.95 g and 1.93 g., ●, These values are precise but are not accurate., ●
Page 33 :
SIGNIFICANT FIGURES, Significant figures are meaningful digits, which are known with certainty.
Page 34 :
RULES FOR DETERMINING SIGNIFICANT FIGURES, 1. All non zero digits are significant., Eg: 235 has 3 significant figures., 2. Zero’s to the left of the first non zero digit are not, significant., Eg: 0.002 has only one significant figure., 3. Zero’s between non zero digits are significant., Eg: 3.02 has 3 significant figures., 4. Zero’s to the right of the decimal point are significant., Eg: 2.00 has 3 significant figures.
Page 35 :
LAWS OF CHEMICAL, COMBINATION
Page 36 :
LAW OF CONSERVATION OF MASS, Matter can neither be created nor, destroyed., , Eg: 12 g carbon combines with, 32 g oxygen to form 44 g CO2., Here the total mass of the, reactants is equal to the total, mass of the products., , Antoine Lavoisier
Page 37 :
LAW OF DEFINITE PROPORTION, The same compound always contain the same, elements combined in the same fixed proportion by, mass., , Eg: NaCl may be obtained from sea water. It is, also prepared by chemical reactions between, NaOH and HCl. These samples on analysis are, found to contain Na and Cl in the ratio 23:35.5, , Joseph Proust, , by mass.
Page 38 :
LAW OF MULTIPLE PROPORTION, when two elements combines to form more than one, compound, the different masses of one of the elements, which combines with a fixed mass of the other element, are in the ratio of simple whole numbers., Eg: Carbon combines with oxygen to form two different, oxides CO and CO2 under different conditions. In CO2, 12, g of carbon combines with 32 g of oxygen and in carbon, monoxide 12 g carbon combines with 16g of oxygen. In, these two cases, the mass of oxygen combining with the, , John Dalton, , fixed mass of carbon are in the ratio 16:32 or 1:2.
Page 39 :
GAY LUSSAC’S LAW, when gases combine to form gaseous products, a simple, ratio exists between the volumes of the reactants and, the products at constant temperature and pressure., , Eg: When H and Cl combine to form, HCl, a simple ratio by volume exists, between the gases H2, Cl2 and HCl, at constant, pressure., , temperature, , and, John Louis Gay Lussac
Page 40 :
AVOGADRO’S LAW, Equal volume of all gases under the same conditions of, temperature and pressure contains the same number, of molecules., , Avogadro
Page 41 :
DALTON’S ATOMIC THEORY, ●, , Matter is made up of small indivisible particles, called atoms., , ●, , Atoms of the same element are identical in mass, and other properties., , ●, , Atoms of different elements are different in mass, and other properties., , ●, , Atoms can neither be created nor be destroyed., , ●, , Since atoms are indivisible, they combine in small, whole numbers to form compound atoms called, molecules.
Page 42 :
ATOMS AND MOLECULES, ●, , An atom is the smallest possible unit of matter, that exhibits all the properties of matter that, may or may not have independent existence., , ●, , A molecule is the smallest unit of matter, which exhibits all the properties of that kind, of matter and is capable of independent, existence.
Page 45 :
ATOMIC MASS, Atomic mass of an element is defined as a, number which expresses how many times, the mass of one atom of the element is, greater than, , mass of a carbon-12 atom.
Page 46 :
ATOMIC MASS UNIT, One atomic mass unit is defined as mass exactly, equal to, , the mass of one carbon-12 atom., , amu is also known as unified mass (u)., 1 amu = 1.66056 x 10-24
Page 47 :
MOLECULAR MASS, , , Molecular mass is the sum of atomic, masses of the elements present in a, molecule., , , , It is obtained by multiplying the atomic, mass of each element by the number of its, atoms and adding them together.
Page 48 :
GRAM ATOMIC MASS OR GRAM ATOM, Atomic mass expressed in grams is called, gram atomic mass or simply gram atom.
Page 49 :
GRAM MOLECULAR MASS OR GRAM MOLE, The molecular mass expressed in grams is, called gram molecular mass or simply, gram mole.
Page 50 :
FORMULA MASS, ●, , The formula mass of a molecule is the sum of, the atomic weights of the atoms in the, empirical formula of the compound., , ●, , Eg: The formula mass of NaCl =, Atomic mass of sodium + Atomic mass of Cl, = 23 + 35.5 = 58.5
Page 51 :
MOLE CONCEPT, ●, , Mole is the unit used for counting very, large number of particles like atoms,, molecules or ions., , ●, , This is equal to 6.023 x 1023., , ●, , Mole is represented by the symbol ‘mol’.
Page 52 :
MOLAR VOLUME, ●, , The volume occupied by one mole of a, gas is called molar volume or gram, molar volume., , ●, , One mole of all gases occupies 22.4 L at, STP.
Page 53 :
PERCENTAGE COMPOSITION
Page 54 :
EMPIRICAL FORMULA, ●, , An empirical formula represents the, simplest whole number ratio of various, atoms present in a compound., , ●, , Eg: Empirical formula of Benzene is CH.
Page 55 :
MOLECULAR FORMULA, Molecular, , formula, , gives, , the, , exact, , number of different types of atoms, present in a molecule of a compound., Eg: Molecular formula of Benzene is C6H6.
Page 56 :
RELATION BETWEEN EMPIRICAL FORMULA, AND MOLECULAR FORMULA, Molecular formula = n x Empirical Formula
Page 57 :
STOICHIOMETRY, ●, , The word Stoichiometry is defined from two, Greek words., , ●, , ‘stoichion’ meaning element and ‘metron’, meaning measure., , ●, , Stoichiometry deals with the calculation of, masses of the reactants and the products, involved in a chemical reaction.
Page 59 :
REACTIONS IN, SOLUTIONS
Page 60 :
MASS PERCENTAGE, Mass percentage of a component in a, given solution is the mass of the, compound per 100g of the solution.
Page 61 :
MOLE FRACTION, It is the ratio of number of moles of one component to the total, number of moles of the solution., , If a substance ‘A’ dissolves in a substance ‘B’ and their number, of moles are nA and nB respectively. Then the mole fractions of A, and B are given as
Page 62 :
MOLARITY, ●, , It is defined as the number of moles of the, solute in one litre of the solution., , ●, , It is denoted by 'M'
Page 63 :
MOLALITY, ●, , The number of moles of solute present in 1, kg of solvent., , ●, , It is denoted by ‘m’.
Page 64 :
NORMALITY, ●, , It is defined as the number of gram, equivalents of the solute dissolved in one, litre of the solution., , ●, , It is denoted by 'N'
Page 65 :
LIMITING REACTANT, OR LIMITING REAGENT, The, , reagent, , which, , is, , completely, , consumed in a chemical reaction is called, limiting reactant or limiting reagent.