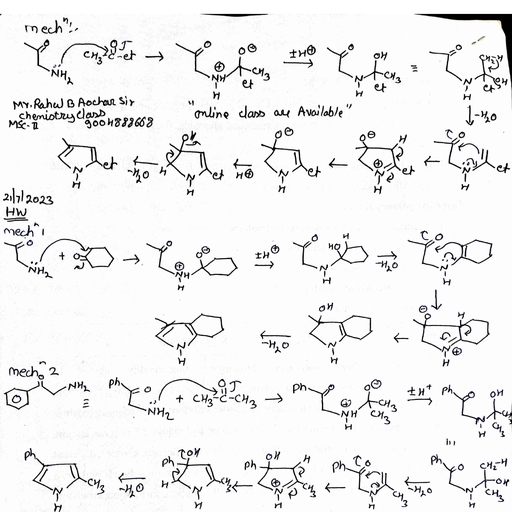
Page 1 :
The d-block Elements, , Syllabus 60 ER EEE RN NEN RLERN MER ERR REAKReManeRRSeN aD o, , Elements of first, second and third transition series, General characteristics of d-block elements, a) Metallic character b ) Molar volume and densities c) Atomic radii d) Ionic Radii_e) Melting and, boiling points f) lonization Energies g) Reactivity h) Oxidation states i) Standard electrode, potential j) Reducing Properties k) Colour |) Magnetic properties m) Catalytic Properties, n) Tendency to form comp lexes ., , (O8L 20/30M), , Introduction:, , All elements consists in periodic table are classified into four blocks-s, p, d and f. The element, in which outer most (last) electron enters into d-orbital of penultimate shell (last but one shell i.e., (n-1) d shell) are called as d-block elements. These elements have (n—1)d'"°, ns°? General, electronic configuration and are belonging to 3-12 groups of the periodic table., , The d-block elements are also called as transition elements because their properties have been, intermediate between those of s and p-block elements. The electronic configuration of transition, elements is (n-1)d', ns’ whereas the electronic configuration of d-block elements is (n-1)d?,, ns’?. Hence the elements Zn (zinc), Cd(cadmium), Hg (Marcary) and Cn (Copernicium) are, excluded from transition elements because their atoms as well as their ions have completely filled, d-subshell. Thus they are considered as non-transitional elements., , The d-block elements are divided into four series of 10 elements and are shown as below., , THB | IVB VB VIB | VIOIB vit IB | DB, GB) | @ (5) () | (7) _| (8) | (9) | GO) | GD | G2), 1 Series | 2Sc Ti Vv Cr Mn | Fe |] Co] Ni Cu | 3oZn, , 2™ Series | 3oY Zr Nb Mo Tc | Ru] Rh| Pd | Ag | 4gCd, 3" Series s7La | Hf Ta Ww Re | Os] Ir Pt Au | soHg, 4" Series goaAc | Rf | Ha/Db | Sg Bh | Hs| Mt} Ds | Rq } j2Cn, , , , , , Group>, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , The 1* transition series called 3d series and contains nine elements from Sc (21) to Cu (29), (i.e. Cu as in Cu* ion), The II" transition series called 4d series and contains another nine, elements i.e. from Y(39) to Ag (47) (i.e. Ag as in Ag” ion). The 3™ transition series called 5d., series and contains nine elements from La (57) to Au (79) (i.e. Au as in Au” ion). The 4" series, called 6d series and contains nine the elements from Ac(89) to Rg (111), , Position of d-block elements in periodic table:, In the long form of periodic table, the d-block elements are placed between highly, electropositive s-block on the left and highly electronegative p-block elements on right i.e. these
Page 2 :
elements (40) are located in the middle part of periodic table. All d-block elements are from 4",, 5", 6" and 7" row (period) of each 10 elements of the periodic table. As well as they constitute, TB (3), IVB(4), VB (5), VIB (6), VIIB(7), VII (8,9,10), IB(11) and IIB (12) groups of the, periodic table., , 3.1. Elements of first to third transition series :, (Electronic Configuration of Transition elements), T) Elements and Electronic Configuration of First (3d) Series :, The elements from Sc (21) to Zn (30) constitute the 1‘ transition series and their outer, electronic configuration is given in the following table., , , , , , , , , , , , Se Ti Vv Cr Mn Fe Co Ni Cu Zn, Subshell, 21 22 23 24 25 26 27 28 29 30, 3d 1 2 3 § 5 6 7 8 10 10, 4s 2 2 2 1 2 2 2 Z 1 2, , , , , , , , , , , , , , , , , , , , , , , , II) Element and Electronic Configuration of Second (4d) Series :, The elements from Y (39) to Cd (48) constitute the 2™ transition series and their outer, electronic configuration is given in following table., , , , , , , , , , Subshell Y Zr Nb Mo Te Ru Rh Pd Ag Cd, 39 40 41 42 43 44 45 46 47 48, 4d 1 2 4 5 5 7 8 10 10 10, , 5S Z 2 1 1 2 1 1 0 1 2, , , , , , , , , , , , , , , , , , , , , , , , , , , , III) Elements and Electronic Configuration of Third (5d) Series :, The elements from Hf (72) to Hg (80) constitute the 3™ transition series and their outer, electronic configuration is given in following table, , , , , , , , , , H, Ta WwW Re Os Ir Pt Au Hg, , Subehell m2 | 723 | 74 | 2 | 7 | 7 | 78 | 79 | 80, 5d 2 3 4 5 6 7 10 | 10 | 10, , 6s 2 2 2 2 2 2 0 1 2, , , , , , , , , , , , , , , , , , , , , , , , , , IV) Elements and Electronic Configuration of Fourth (6d) Series :, The element having atomic number R; (104) to Cn (112) (i.e. Ry to onward) constitute the 4h, transition series and their outer electronic configuration is given in table., , , , , , , , , , , , Rf Db Sg Bh Hs Mt Ds Rg Cn, Subshell "7 o;-| 105 | 106 | 107 | 108 | 109 | 110 | 111 | 112, 6d 2 3 4 5 6 7 9 10 | 10, , 7s 2 2 2 2 2 2 1 1 2, , , , , , , , , , , , , , , , , , , , , , , , The general outer electronic configuration of d-block element is (n-1)d'""° ns”? in which (n-1), shell is last but one shell (penultimate shell) while n is the outermost shell In above tables certain
Page 3 :
elements shows different electronic configuration from the general electronic configuration eg. in, 1" series Cr and Cu shows 3d°4s' and 3d'°4s!' configuration instead of 3d*4s’ and 3d°4s”, respectively. This is because of a very small energy difference between (n-1) d and ns shell, as, well as half and completely filled sub-shell shows relatively more stability than other. Hence such, configurations are favoured whenever possible., , 3.2. Characteristics of d-block elements :, , 1) Metallic Character :, , The metallic and non metallic character of the elements depends upon its electronic, configuration. In d-block elements, as the number of electrons in the outer most shell is very less, (ie 1 or 2) and are readily lost, all these elements are metal. These elements are hard, malleable, and ductile, forms alloy with several metals. These are also good conductors of heat and electricity, and have metallic lustre due to delocalisation of their outer s-electrons over the entire crystal, lattice., , The covalent as well as metallic bonding exists among the atoms of the transition elements., The covalent bonding is favoured by the presence of unfilled d-orbitals while metallic bonding is, due to valance electrons. Hard and brittle nature of these elements is associated with the covalent, bonding between the metal atoms., , 2) Molar Volume and Densities :, , The molar volume of the transition elements are low as compared to the neighbouring s and, p-block elements. This is because as the atomic number the increases in a transition series, the, extra electrons are added to inner d-orbitals that increases nuclear charge. This increasing nuclear, charge attracts all electrons more strongly, which decreases atomic volume. So the transition, elements has higher densities. Generally densities of transition elements is greater than 5g cm?, except Sc (3g cm>), Y (44.47g cm®) and Ti (4.51 g cm”). The two elements with highest densities, are Os (22.57 g cm“) and Ir (22.61g cm). The densities of 3“ row elements are nearly double, than 2™ row elements due to lanthanide contraction., , In transition series when we move from left to right, the density first increases and then attains, maximum value on reaching group VIII. Then it start decreasing when we go further to group IB, and IIB due to their small radii and close packed structure., , 3) Atomic and Ionic Radii :, , 3.1 Atomic Radii :, , The atomic radii of the transition elements decreases from left to right in the series with, increase in atomic number. This is due to increase in the nuclear charge which tends to pulls the, electron charge cloud inword. However, there are some exceptions. e.g. the radii of the elements, from Cr to Cu are very close to one another because of successive addition of d electrons protects
Page 4 :
the outer 4s electrons from the inward pull of the nucleous. As a result, the size of the atom does, not change much in going from Cr to Cu as shown in following table., , In series of transition elements atomic radii goes on increasing from 1“ transtion series to 4", transition series due to development of new valance shell. But due to the lanthanide contraction,, the atomic radius of 2™ series and 3™ series (i.e. post Lanthanide elements) is practically same, eg. Zr and Hf, Nb and Ta, Tc and Re have same size, chemical and physical properties and they, are difficult to seperate from one another. Such pairs of elements are called chemical twins., , 3.2 Ionic Radii:, , The ionic radii of the transition elements follows the same trend as the atomic radii. For ions, having the same charge, the ionic radius decreases slowly with increase in atomic number. The, radii of double charged ions, are close with the radii of Ca™ ion (0.99A°). Hence their oxides are, less basic and less soluble in water., , Atomic and Ionic radii (A°) of 1“ transition series elements, , , , Element Sc Ti Vv Cr Mn Fe Co Ni Cu Zn, , , , Atomic Radii ae 1.62 | 1.47 | 1.34 | 1.27 | 1.26 | 1.26 | 1.25 | 1.24 | 1.28 | 1.33, , , , , , Tonic Mion < 0.90 | 0.88 | 0.84 | 0.80 | 0.76 | 0.74 | 0.72 | 0.72 | 0.74, radii M* ion [ 0.81 | 0.76 | 0.74 | 0.69 | 0.66 | 0.64 | 0.63 - - , , , , , , , , , , , , , , , , , , , , , , , , , , , , , 4) Ionisation Energy (1.E.) :, , Ionisation energy is the amount of energy required to remove the loosely bound outer most, electron from a free neutral gaseous atom., , The ionisation energies of these elements are high due to their small size. In most cases their, ionisation energy values lie in between those of s and p-block elements. This indicates that, transition elements are less electropositive than s-block elements and more electropositive than pblock element and may forms ionic or covalent compounds. The elements of 1" series have many, more ionic compound while that of 2“ and 3™ series elements have more covalent compound., , The ionisation energy of transition metals increases very slightly from left to right as their size, decreases very slightly from left to right and the extra electron added to inner (n-1)d shell which, shield the nuclear charge., , The higher values of the II" ionisation energy for Cr and Cu than those of their neighbours is, due to the extra stability of half-filled (a> ) and completely filled (d'°) d sub-shell of Cr and Cu, respectively. The ionisation energy (I-E.) of the elements of 1“ transition series are given below., , Element Se Ti Vv Cr | Mn | Fe Co Ni Cu Zn, 1" LE. (KIJ/mole) | 632 | 659 | 650 | 652 | 716 | 762 | 758 | 736 | 744 | 906, 2™ LE. (KI/mole) | 1245 | 1320 | 1376 | 1635 | 1513 | 1564 | 1647 | 1756 | 1961 | 1736, 3 LE (KJ/mole) | 2450 | 2721 | 2873 | 2994 | 3258 | 2963 | 3237 | 3400 | 3560 | 3838, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , 5) Melting Point and Boiling Point :, The d-block elements have very high melting point and boiling point. (i.e. near about 900°C, except Cd and Hg). This is due to their closely packed crystal structures as well as strong metallic
Page 5 :
bonding because of the overlapping of (n—1)d orbital and covalent bonding by the use of unpaired, d-orbital electrons. However Zinc, Cadmium and Mercury have low melting point i.e below, 900°C. This is due their completely filled d-orbitals and no covalent bonding amongst themselves., , The melting and boiling point of transition elements increases down the group due to increase, in the atomic mass. In general, greater the number of unpaired d-electrons, greater the possibility, of the covalent bond formation and hence higher the melting and boiling point., , 6) Reactivity :, , The transition elements show a tendency to behave as noble or unreactive ones. As the atoms, of these elements are rather small, their ionisation energy, melting point and heat of sublimations, are very high, the transition elements have tendency to remains noble or unreactive one e.g., platinum and gold. Thus d-block elements have a low tendency to react and the reactivity of these, elements goes on decreasing across the series., , Many of these elements are sufficiently reactive and they react with mineral acids ie. HCI,, H,SO, ete and liberate hydrogen gas. But few of these element possess noble character like Pt and, Au., , 7) Colour :, , Generally most of the compounds of transition elements are coloured while that of s and, p-block elements are colourless. The colour is associated due to the existance of incompletely, filled d-orbitals and the ability to promote an electrons from one d-energy level (orbital) to another, d-energy level by the absorption of light energy of the visible wavelength. This is known as, d-d-transition of electron. This absorption of energy in such transtion is small and hence causes, absorption in the visible region there by making the ion coloured. Hence transition metals ions are, coloured., , eg. Cu’* (d°) ion appears blue. This is due to d-d-transition of electron by absorption of red, light while its transmitted light has blue colour. But Cu” (d'°) ion is colourless, due to its, completely filled 3d orbital i.e. the absorption of light is take in U.V. region and not in the visible, region., , The transtion metal ions which have completely filled d-orbitals or completely empty, d-orbitals are colourless eg.Zn™*, Cd, Hg”, and Sc**, Ti* respectively. This is because of that,, there is no vacant d-orbital or no unpaired electrons in d-orbitals available for the d-d transition., , The ions of s and p-block elements are colourless because d-d transtion is not possible in these, elements.