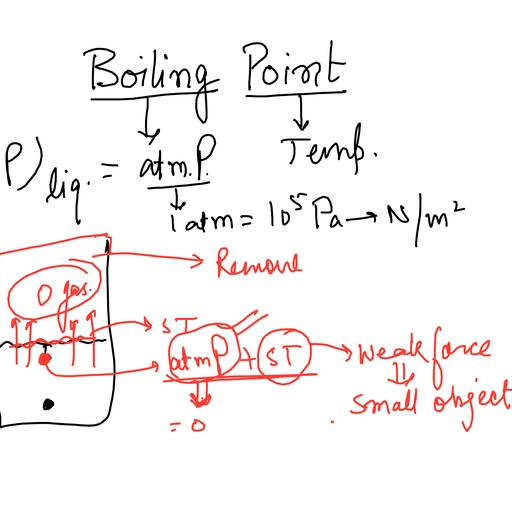
Page 3 :
------·-, , -, , 12/18, , hht•, ■13::1:.i-i, , NEW. COURSE CHEMl'S TRY· (XI), , ,•i•j•I] ·, , the multiple bonds) of the organic molecule. For chains from one to four carbon atoms, special word roots, , (based upon the common names of alkanes) are used but for chains of five or more carbon atoms Greek, number roots are used as given below:, · ,, ··, , Hept (a)=Oct (a)- _, Non (a)-.--, , MethEthProp (a)-_, But (a)- _, Pent (a):...., Hex (a)-, , . Dec (a)-, , ,-, , Undec (a)-_, Dodec (a)- .-, , Extra ' a' given in parenthesis is used only if the primary suffix (explained later) to be added to the, word root begins with a consonant. In general, the word root for any carbon chain is ~k., ., ., _2. S_yffix. There are two types of suffixes :, ., ·, - ·, · ·, ··, ~, rimary suffix. A primary suffix is always added to the word root to indica~e .whether the carbon, chain is -saturated or unsaturated. The three basic primary suffixes are given below :, , ~, , ., , (a) Saturated (containing single bonds only), , (b) Unsaturated with one double bond, (c) Unsaturated with. one, .., triple bond, , -ane, -ene, -yne, , Alkane, ... Alkene-, , -, , Alkyn~ -, , the parent carbon chain contains two, three, four or more double or triple bonds numerical prefixe$, such as di (for two), tri (for three), tetra (for four), etc. are a~ded to the primary suffix. For example,, ,-....Jr....n.1., , -: :: :~-.;--Type~o!_ca5~o~'itj~ii: -~.j;::· -~:.,i_ .., , tf\7L"~·, , (a) Unsaturated with ~ o double bonds, (b) Unsaturated with two triple bonds, , :~~ -~, -diene, -----., , --diyne, , --..e., , Alkadiene, , AJkadiyne, , The following examples illustrate the use of word roots and primary suffixes in naming organic, compounds :, ·, , Organic ~mpo~, 'r,_•, , •, , --.., , l, , .r_.-,, , CH 3CH2CH2CH3, CH 3CH =CH2, CH=CH, CH 2 =CH-CH= CH2, HC = C--C :CH, , But, Prop, Eth, Buta•., Buta•, , ane, ene, yne, diene, diyne, , Butane, Propene, , •, , Ethyne, , Butadiene, Butadiyne, , . Secondary sufflx. A _secondary s~, is then added t, rima SU, to indicate the nature of, the unctional group present ,n the or anzc com our:,,d. Secondary suffixes of some important funcuon, groups are given below :, rim~rv s, i.e·•diene or diyne be ., · -t,, • Ex:n·a .a , has been added. to the word root since the =--J, ~, gms wi.th aconsonan~, ., a, vowel, as, 1s, the, case, rn, e, last, two examples., •, ., ', instea, ____, 0, -.., ·, i.e.,, 11, , -, , ~-v
Page 19 :
12/34, , ., , 18, , r-.rew ·couR·se 'cHeivlasTiRv ·cxh '!1•110, , PRADEEP ' S, , L?west set of Jocants rule. When two or more substituents ar~ present, the lowest set of locants rule, applied. According to this rule, when two or more different sets of locants containing the same number, , of terms is possible, then that set of locants is the lowest which when compared term by term with other, sets, each in order of increasing magnitude, has the lowest term at the first point ofJiif/0 rence ., . For deciding the lowest set of locants, the carbon atoms of tlie parent chain are numbered from all, possible directions and a locant assigned to each substituent from each direction. The set of locants from, ~ach direction is then compared term by term till the first point of difference is reached. That set of locants, is preferred which h~s a lower number at the first point of difference. That is why this rule is also sometimes, called as first point of difference rule., ·, - · Consider, for example, the following alkanes. Each alkane can be numbered in two different ways as, shown in structures I and II., 2, , 1, , CH GH, , _, , 2, , I, , 6, , 7, , TH2CH3, , 3, , ~ ~GH-€H-t- CH 2ciq, 4I, C, ), , ___:_ j, , CH 3-CH· CH-CH 2CH, 3, ., , 5, , 4.,, , (:'.H 2C~ 2CH 3, 5, , 6, , CH 2CH 2CH 3, , 1, , 3, , I (correct), , 2, , 1, , II (wrong), Set of locants = 4, 5, , Set of locants = 3, 4, , Out of two sets of locants _i1, 4) and ~ S), the first set is lower and hence preferred because the first, term, i.e., 3 in the first set (3, 4) is lower than the first term, i.e., 4 in the second set (4, 52,, .....--. ., ..,. · · ·· · - · ·, . CH, ·cH-- . ... .. ., CH 3, CH 3, . I 3, I 3, I, 3, I, s, s, 4, 3, 2, · 1, r;;/ cH ~ C-CH ~CH-CH, CH 3-:---'C-CH 2-<;H-CH 3, 3, J '' 3 r, 2, I, CH, CH3, , -, , ., , r, , 3, , Set of locants = 2, 2., 4 (correct), 1, , 2, , Set of locants = 2, 4, 4 (wrong), , 3, , 4, , 5, , 6, , 8 ~ Correct numbering, , 7, , GH3· yW-CH2-CH 2;:-TH-TH-C H2-CH3, CH 3, , CH 3 CH 3, Set of locants = 2, 5, 6, , 8, , 7, , 6, , 5, , 4, , ,3, , 1 ~ Wrong numbering, , 2, , CH 3-yH· . CH 2-CH 2-TH-yH-CH 2-CH, 3, CH 3, , CH 3 CH 3, Set of locants = 3, 4, 7, , Here out of two sets of locants (2, S, 6) and (3, 4, 7), the first set is preferred because the first term,, ., first set (2, S, 6) is lower than the first term, i.e., 3 in the second set (3, 4, 7). Thus, the correct, 2 in, i.e:, ., t i the alkane is 2, 5, 6-trimethyloctane., , the, , name o, .. Name of the brr cbed cbaj~ e•~ane. _Prefix _t~e name of the substituent (i.e., the alkyl groups) to, - if the parent alkane and indicate its position (on the parent chain) by writing before it the, the name.: h arbon atom carrying the substituent. The name of the substituent is separated from its, . al, ·, nu mbe r o, t he chen (-). The ft na l name oif the alkane is, ways wntten, as one word. Some examples are, locant by a YP, · ·, ·, , given below for illustration., , CH3, , I, , -, , 3, , :, 4, , CH3 -2cH-CH2 CH3, z-Methylbutane, , I, , 2, , CH 2CH, , 31, , 3, 4, , .., , s, , 6, , c~ CH2-CH -cH2CH2CH3, 3-Ethylhexane
Page 20 :
2iis1J, : ; . '.,·: ·/?\,,,'.']:J1~, •, . •, ., ., phabetical order :f·the sid, , ~ c~ams. When two,or more alkyl groups (side chains) are present on, ·ent chain, each alkyl, the, prefixed by its positional number is arranged in alphabetical order, (irrespective of its positio nal, er) before the name of the pa,:ent alkane. For example,, S., , !::u:, , I, , 2, , 4, , 3, , 2, , 1, , CH 2 -CH3, , 6, , 5, , ., , 4, , 31, , CH3- CH-C HCH CH CH, 3, 2, 2, I, I, CH 3 CH 2ctt 3, , CH3 -CH -CH -CH 2CH3, I, CH 2-CH2 -CH 3, 6, , 5, , 7, , 4-Ethyl-3-methylheptane, , 3-Ethyl- 2-methy lhexane, , alkyl groups, prefixes, It may be note? here that while deciding the alphabetical order of the various, ., while the prefixes sec and, iso and neo are cons1dered to be part of the fundamental name of the alkyl group, ,, tert are not. For examp le,, CH(CH 3 ) 2, 3, , 2, , I, , 6, , 5, , 1, , 4, , CH3 ~Hz CHz- CH -, , 8, , 7, , 10, , 9, , ?H-C H2 CH2 CH2 CHzCH3, ., , CH3 -CH -CH2 CH3, , ., , 5-sec-Butyl-4-isopropyldecane, , alkyl groups are, umbering of different alkyl groups at equivalent positions. If two different, that the alkly, way, a, such, present at equiva lent positions, -the-numbering of -the-parent chain is done in, gets the lower-number. For ., group which comes-firs( in the·alphabeticah,rder (written first in the name), •, - - -- --example,, 3, , 2, , I, , 7 f- Correct numbering, , 6, , 5, , 4, , CH -CH 2 -CH. - CH 2-CH- CH 2 -CH, 3, 1 f- Incorrect numbering, 3, 2, 3I, 4, 5I, 6, 7, CH 3, CH 2 CH 3, , i;, I, I, , 3-Ethyl-5-methylheptane, , more than, ng same alkyl groups at different positi.o~s. When the same alkyl group oc~urs, is separated by, th parent chain at different positions, the posmonal number of each alkyl group, 0, (for four), etc. are attached to the, nd itable pre;;ves such as di (for two), tri (for three), tetra, com, h l ha, 'd, ·, ·, ...,..,.,, su group. Howev er, the prefixes di, tn, etc. are not consz ered w h'l, le deci·d·mg t ea p betical, a alkyl, of the, namemas, order of the _alkyl s roup:J_-_, , CH3 CH 3, 31, 21, I, I, 2, 3 ·, 1, 5, 6, 4, C-CH CH, -CH, I, 21, 3, 4, 5, 3, CH -CH - C' - CH 2-CH -C~ CH3, 2, I, I, I, H3, 2, 3, CH -CH -CH 2 -C-C, 4, I, I, 3, CH 3 -CH- CH 2 -CH 3, . CH3, CH 3-CH 2, 6, 5, CH3, CH3, CH3, , CH 3, , 3-Ethyl-2, 3, 4-trimethylhexane, , 4-Ethyl-2, 4-dimethylhexane, ·, , ., 2 2 4-Trime thylpen tane, , atom, its positional number is also, lkyl roup occurs twice on the same carbon, ' ', ., g, •, a, In case the same, repeate d twice. For example,, CH, CH, . •., CH 3, 3, 2I, I, H3, -C-C, CH, I, 3, CH 3, 2, 2-Dime thylprop ane, , CH 2 CH3, , I, , 2, , 31, , 4, , 3, , 3, , 5, , CH CH -C-C H 2 CH3, I, 2, 3, CH 2 CH 3, 3, 3-Diethylpentane, , 6, sl, 4, 3, 21, -CH, -CH, H, CH3-C-C H-C 2, 3, I ., I, 1, , H 3C, , ~H 2CH3, 3-Ethyl-2, 2, 5-trimet hylhexa ne, , '.
Page 22 :
·, , ·, , ~, l•li::( : it•t•, •;mm t· I <\r--CH3, , ,, CH3-GH CH3, 1, 2 I., 3I ., 4, ~H _ 5, 3 CH 2 -CH 2 - C, - CH-- CH 3, ~, 1I, CH3, CH3- C.H II,, , .ci,,, , ,, , 2-~ ethyl-3, 3-.bis( l ~1J1et9ylethyl)hexane, ---7, , r - - - - -·, CH3, 1I, 2, I3, , j, , H3, -C-C, CH 3 -CH, 1, ,, 2, -, , L, IO, , 9, , 8, , 7_ _, , 6_5 '_4- ~3, , I, , 2, , ___,e- CH 2 -CH 2 -CH- CH 3, CH3- CH 2 -CH 2 -CH 2 -CH., . 2, ·, , r 3 - T, , -1-f - - 7, , ~ -H, , .,---, , I CH3- CH2- C-CH 3 I, I, IL _ _ _ _ _~H, , 2__~, , 3, , 5, 5-Bis (1 , l-dimethy lpropyl)- 2-methyl decane, , •, , Sampl e P r ob!em, , ~ Structures and IUPAC names of some hydrocarbons are given below., , Explain why the names given in the parenthesis, , are, , incorrect., , Nt::ER T Solved ; Exan,, ple, , (i) CH 3-CH- CH 2 -CH 2 -CH- CH-C H 2 -CH 3, I, I, I, CH, CH, CH 3, 3, 3, 2, S, 6-'Iiimethyloctane, (and not 3, 4, 7-Trimethyloctane), I, , (ji), , I, , CH 3-CH 2 -CH- CH 2 -CH- CH 2 -CH 3, I, I, CH3, CH 3CH2, 3-Ethyl-S-methylheptane, (and not S-Ethyl-3-methylheptaile), , Solution. (i) The set of locants (2, 5, 6) is lower than the set of locants (3, 5, 7)., 4>9sJti.ons, Jow_estJocant i~. giv~n to that substituent which comes first, (ii) When substitu ents are at equiv.al.e1;1_, 5 is assigned to methyl, in the alphabetical order. Therefore, lower locant 3 is assigned to ethyl and higher locant, group., , TIC E, PR AC, Uk PfftD, fll" ■■ LEMS FII R, :44, f?Ac#@&, $&&, .+, 2, , •111., 1. Give the fiJPAC names of the following alkanes :, H3 C, , C2H5, , I, I, (ii) CH 3 CH 2 -C -CH- C 2 H 5, I, CH 2 CH 2 CH 3, , @, , H, I, (iii), , CH 3 -C -, , C4 H 9, I, , C-CH 3, , I, I, C 2 H 5 CH 3, , (v) C1½CH 2 CH? CHCH CH CH, 3, 2, 2, - I, , C(CH 3 ) 3
Page 24 :
[•1;tc:M,~I t•-i :I =Ml f.i i;ffl.1•P, I=I =t·J..4 "• :J;) m-il~ M=f.-tUI •Ii 3ij: WM•1:t:-J, , 4, , '-- 2 · !f bor!, double and triple bonds are present, the numbering of the parent chain should always be, done fro~n that e,i d which is nearer to the double or the triple bond, i.e., the lowest set of locants rule/or, rhe mulrrple bonds must be follmved . For example,, 7, , 6, , 5, , 4, , 1, , 2, , I, , 8, , CH 3 -CH =C H-CH 2 -C = C-CH 2 -CH 3, Set nf locants, , 5, , 6, , 7, , 8, , = 2, 5, , r--, , (correct), I, , 2, , 3, , 4, , CH 3 - CH =C H- CH 2 -C = C-CH 2 -CH 3, Set of locants = 3, 6 (wrong), , 0_/Jf, howeve,: there is a choice in numbering, the double bond is always given preference over the, triple bond. For example,, 5, , 4, , 3, , 2, , I, , ~ Correct nwnbering, , I, , 2, , 3, , 4, , 5, , ~, , CH= C-CH 2 -CH = CH 2, , Wrong numbering, , sit", 4. If the organic compound 0•1tains only one double or the triple bond, its locant or the, number is always placed before its suffix in accor ance with 1993 recommen anons for IUPAC nomenclature, ', organic compounds. For example,, , of, , 4, , 3, , 2, , I, , CH 3 -CH = CH-CH 3, But+ 2-ene = But-2-ene, , e, heir locants are written b, If, however, both double and tri le bonds are rese, suffixes, the tenninal ' e' from e suffix 'ene' is dropped while writing the complete name of the or.&anic, compound. lt may be emphasized here that the organic compound is named as derivative of alkyne rather, •, alkene':For example,, , nu;,,, , 4, , 5, , 3, , 1, , 2, , CH 3 -CH = CH-C = CH, Pen t + 3-en (/) + 1-yne = Pent-3-en-l-yne, , These rules are further iIIustrated by the following additional examples:, CH3, CH 3, , 5, 4, 2 31, 1, HC = C-CH-C = CH, , 4, 3, 2I, I, CH 2 = C-CH = CH 2, , 3-Methylpenta-l, 4-diyne, , 2-Methylbuta- l , 3-diene, , In some cases all the double and triple bonds present in the molecule cannot be included in the IonK;st, chain. In such cases, the following pi:efixes ~re used for double and triple bonded groups., HC a CC!f2 = CHCH3 CH =, CH =, 2, ~ thynyl, ymyl or ethen:>.:l, Ethylidene, Methyle~, , •, , CH 2, 1, 2, 311, For example, CH 2 = CH-C-CH =CH2, 5, , 4, , enta- l , 4-diene, , ~, 2, , l, , 3, , 5, , 4, , .CH =CH-CH ::::CH-yH-~, , -H, , 2, , /', , 5-Eth,; n~ I, doubl, , CH= CH 2, 6, , 7, , .-1. _ ·e e (_ca rn:cl), ,-, , rence over the, , ~, , Jfx1Fn ts ( 1. 3, 6), uble bonds), , 7, , 6, , 5, , 4, , 3, , 1, , 2, , CH 3 -C = C-CH-CH 2 -CH=CH, 2, I, CH=GH 2, 4-Ethenylhept- l -en~5-yne or 4-Vinylhept-l-en-5-)'<1le, 3, 4, 5, 6., 7, , -C, CH 2 = CH-CH =_GH-C_H_, , I, , = CH, , CH= CH 2, 2, , •, , 3-Ethynylhepta-l, 4, 6-triene (wrong), , (~ouble bonds are 1jven prefellnu over the, tnpl~ bond but a higher set of locants ( J, 4, 6 ), 15 assigned to the three double bonds)
Page 27 :
12/42, , ·., , PRADEEP ' S NEW COURSE CHEMISTRY (XI), , i!l•j•u, , '"f t~mbering the chain terminating functional groups. When a chain terminating functional gmup, such as -CHO, -COOH, -COOR, -CONH , -COCl, - C = N.i etc. is present, ~ is alw~ys given ~u?1ber .!_., 2, and number 1 is usually omitted from the final name of the compound when. there 1s 00 amhigmty. For, example,, 4, , 3, , 2, , ', , CH 3 -CH 2 -CH-CH 2 -CH 3, I, COOH, 1, 2-Ethylbutan-l-oic acid or simply 2-Ethylbutanoic acid, , However, in the following examples, the numerical locant 1 is always included when another numerical ·, · locant appears in the same name., , 5, , 4, , 3, , 2 ,, , I, , 4, , CH 3, , 4, , 2I, , 3, , CH 3 -CH-C-CH -CH, 3, I, II, I, CH 30, 0 OC 2H 5, , CH 3 -CH 2 -C -CH 2CH 3, I, . •, 1CHO, , 1,-Ethoxy-4-methoxypentan-3-one, , 2-Ethyl-2-methylbutan-l-a!, , Br, 3, , ~ ·, , 4, , ~, , C, , 5, , 4, , ', , 3, , I, , 3-Chloro-2-methylbutan-1-oic acid, , 3, , 1, , But-2-en-I-ol, , . '-7, , 2, , 2, , CH 3 -CH = CH-CH 2 0H, , Ethyl 2-bromobutan- 1-qate, , ., , 2, , 0, , I2, , 111, CH 3 -<;H 2 -~H-~G-OC 2 H 5, 4, , 3, , CH3 -CH-CH-CH, 3, I, I, Cl iCOOH, , 1, , 1, , 2, , 3, , 4, , 5, , 6, , 7, , CH 3 -C = CH-C-CH = C-CH 3, I, II, I, CH 3, 0, CH 3, , CH3 -C:C-CH 2 -CH=0, Pent-3-yn-1-al, , 2, 6-Dimethylhepta-2, 5-dien-4-one, , If a compound contains two or more like functional groups, the numerical prefixes di , tri, tetra, etc., ~e used and the terminal 'e' froJJl the primary suffi x is retained (not dropfieq) while writing the IUPAC, , !, \,, , I, , ~, , ·, , ,, , l, , -, , 2, , 1, , CH 2 -CH 2, I, I, OH, OH, , , 3, , 1, , 2, , CH 2 -CH-CH, I, I, I 2, OH, OH OH, , Propane-I, 3-diol, , Propane-I, 2, 3-triol, , 2, , 1, , 2, , 3, , 4, , HOOC-COOH, , N_C-CH = CH-CN, , Ethane- I, 2-dioic acid, , But-2-en:,-I , 4-dinitrile, , 1, , 2, , 3, , 3, , CH 2 -CH 2 -CH 2, I, I, OH, OH, , Ethane-1, 2-diol, , 1, , 2, , 1, , 0, 3, , 4 11, , 5, , CH 3-C-CH 2 -C-CH 3, Pent~, , 2, , 3, , 4, , HOOC-CH = CH-COOH, But-2-ene- I, 4-dioic acid, , 4, , -, , 0, 211, , 1, , OHC-CH = CH- CHO, , ~, , >, , Diethyl bJ'!we l , 4-dtoate