Page 1 :
I, , N D E X, , Topic, , Page No., ORGANIC CHEMISTRY, NOMENCLATURE, , 1., , Common Name, , 01, , 2., , Derived System, , 14, , 3., , Nomenclature of Saturated unbranched hydrocarbon, , 17, , 4, , Nomenclature of Saturated branched hydrocarbon, , 18, , 5., , Nomenclature of Unsaturated unbranched hydrocarbon, , 22, , 6., , Nomenclature of Unsaturated branched hydrocarbon, , 23, , 7., , Nomenclature of Functional group compounds, , 26, , 8., , Nomenclature of Polyfunctional group compounds, , 32, , 9., , Nomenclature ofAlicyclic/Cyclic compounds, , 39, , 10., , Nomenclature of Bicyclo compounds, , 41, , 11., , Nomenclature of Spiro compounds, , 42, , 12., , Exercise - 1, , 46, , Exercise - 2, , 53, , Exercise - 3, , 58, , Exercise - 4, , 61, , 13., , Answer Key, , 63, , 14., , Hints/Solution, , 64, , NO MENC L ATU R E
Page 2 :
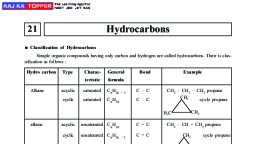
1, , NOMENCLATURE OF ORGANIC COMPOUNDS, Mainly three systems are adopted for naming an organic compound : –, (i), (ii), (iii), , Common Names or Trivial System, Derived System, IUPAC system or Geneva System, , COMMON OR TRIVIAL SYSTEM, On the basis of, , Source, , Property, , Discovery, , Structure, , (i) On the basis of source from which they were obtained., S.No. Organic, Compound, , Trivial Name, , 1., , CH3OH, , 2., , NH2CONH2, , Wood spirit or Methyl, spirit, Urea, , Obtained by destructive distillation, of wood., Obtained from urine, , 3., , CH4, , Marsh gas (fire damp), , It was produced in marsh places., , 4., , CH3COOH, , Vinegar, , Obtained from Acetum - i.e. Vinegar, , Oxalic acid, , Obtained from oxalis plant., , Formic acid, , Obtained from formicus [Red ant], , Lactic acid, , Obtained from lactous (milk), , 6., , COOH, |, COOH, HCOOH, , 7., , CH 3 – CH – COOH, , 5., , Source, , |, OH, , 8., , CH 2 – COOH, |, CH(OH)COOH, , Malic acid, , Obtain from Apple, , 9, , CH3CH2CH2COOH, , Butyric acid, , Obtained from butter., , 10., , CH3(CH2)4COOH, , Caproic acid, , Obtained from goats., , 11., , C2H5OH, , Grain alcohol, , Obtained from barley.
Page 4 :
GROUPS, Atom or a group of atoms which possess any ‘free valency’ are called as Groups., If their are two structure of same molecular formula then some prefix (n, iso, neo) are used two, differentiate them., Normal group : –, (a) It is represented by ‘n’., (b) Groups having no branch (Straight chain)., (c) Free bond will come either on Ist carbon atom or on last carbon atom., n – butyl, CH3 – CH2 – CH2 – CH2 –, n – propyl, CH3 – CH2 – CH2 –, Iso group : –, When one methyl group is attached to the second last carbon of the straight carbon chain is named, as iso group., CH 3 C H CH 2 , , H3 C CH , |, CH3, , e.g., , CH 3 C H CH 2 CH 2 , , |, , |, , CH 3, , Isopropyl, , CH 3, , Isobutyl, , Isopentyl, , Exception :, , CH 3, , CH 3, , |, , |, , CH 3 C C H CH 2 , , CH 3 C CH 2 C H CH 2 , |, , |, , |, , CH 3, , CH 3, , |, , CH 3 CH 3, , (i) Iso octyl, , (ii) Iso heptyl, , Neo group : –, (a), When two methyl groups on second last carbon of a straight carbon chain is attached to other four, carbon atom group is named as neo group., (b), It is represented by following structure -, , (c), , C, |, CCC, |, C, , for eg., , C, |, CCC–, |, C, , Neo pentyl, , There should be one 4° carbon and atleast three methyl group on 4° carbon., , NOTE : (Optically Active) = If all valency are attached to different atoms., Amyl group : –, H, |, CH3 CH2 C CH2 , |, CH3, , Active amyl, , CH3 CH2 CH –, |, CH2, |, CH3, , Secondary amyl, , CH3 CH2 CH2 CH , |, CH3, , Active secondary amyl, , CH3 CH CH , |, |, CH3 CH3, , Active iso secondary amyl
Page 13 :
12, , MCQ, Q.1, , Which of the following are secondary radicals :, |, , Q.2, , |, , (a) CH 3 C H C 2 H 5 (b) CH 2 C CH 3 (c) CH2=CH–, , (d) (CH3)2CH–, , (A) a, b, c,, , (D) a, b, d, , (B) a, d, c, , (C) b, c, d, , Common name of the structure C H 2 OH, |, , CH 2 OH, (A) Ethylene Glycol, , Q.3, , (B) Propionamide, , (D) Acetic amide, , (A) CH 3 C H C 2 H 5, , (B) CH3–CH=CH–CH2–, , (C) CH 3 CH 2 C| CH 3, , (D) CH 2 CH 2 C CH 3, |, , Which one is structure of Maleic acid, O, ||, H C C OH, ||, (A) HO C C H, ||, O, , (C), , Q.6, , (C) Butyramide, , The structure of 2–butenyl radical is :, |, , Q.5, , (D) Ethylene alcohol, , O, ||, CH, , CH, , Common name of the compound, 3, 2 C NH2 is -, , (A) Acetamide, Q.4, , (B) Ethene dialcohol (C) Glycerol, , (B) HO CH COOH, |, CH 2 COOH, O, ||, H C C OH, ||, (D), H C C OH, ||, O, , HO CH COOH, |, HO CH COOH, , Common name of the structure CH 3 C O CH CH 2 is :, ||, , O, , (A) vinyl acetate, Q.7, , (B) acryle acetate, , (C) methyl acrylate, , (D) Vinyl ethanoate, , Which is the structural formula of isoprene, , (A) CH 3 C CH 2, |, CH 3, Cl, |, (C) CH 2 C CH CH 2, , CH 3, |, (B) CH 2 C CH CH 2, , (D) CH3–CH=CH–CH3
Page 16 :
EXERCISE-2 (Exercise for JEE Advanced), [REASONING TYPE], , (A), (B), (C), (D), , These questions consists of two statements each, printed as Statement-Iand Statement-II. While answering, these Questions you are required to choose any one of the following four responses., If both Statement-I & Statement-II are True & the Statement-II is a correct explanation of the StatementI., If both Statement-I & Statement-II are True but Statement-II is not a correct explanation of the, Statement-I., If Statement-I is True but the Statement-II is False., If Statement-I is False but the Statement-II is True., , Q.1, , Statement-I : Pentane and 2-methyl pentane are homolo-gues., Statement-II : Pentane is a straight-chain alkane, while 2-methyl pentane is a branched-chain alkane., [2030113623], , Q.2, , Statement-I : All the C atom o but-2-ene lie in one plane., Statement-II : Double-bond C atoms are sp2-hybridised., , Q.3, , [2030113674], , Statement-I : The IUPAC name of citric acid is 2-hydroxy-propane-1, 2, 3-tricarboxylic acid., COOH, HOOC, , COOH, OH, Citric acid, , Statement-II : When an unbranched C atom is directly linked to more than two like-functional groups,, then it is named as a derivative of the parent alkane which does not include the C atoms, of the functional groups., [2030113725], Q.4, , Statement-I : Rochelle’s salt is used as complexing agent in Tollens reagent., Statement-II : Sodium potassium salt of tartaric acid is known as Rochelle’s salt. The IUPAC name of, , Rochelle’s salt, , NaOOC, , OH, COOK, , is sodium potassium -2, 3-dihydroxy butane-1, 4-dioate., , OH, , [2030113776], Q.5, , Statement-I : The IUPAC name of isoprene is 2-methyl buta-1, 3-diene., Statement-II : Isoprene unit is a monomer of natural rubber., , [2030113827], , [MULTIPLE CORRECT CHOICE TYPE], Q.6, , Which of the following statements is/are wrong ?, (A) CnH2n is the general formula of alkanes, (B) In homologous series, all members have the same physical properties, (C) IUPAC means International Union of Physics and Chemistry, (D) Butane contains two 1º C atoms and 2ºC atom, [2030113825]
Page 17 :
EXERCISE-3 (Miscellaneous Exercise), Q.1, , [2030113777], , Q.2, , [2030113828], , Q.3, , [2030113523], , Q.4, , [2030113574], , Q.5, , [2030113625], , Q.6, , O2N, , OH, , [2030113676], , O, , Q.7, , [2030113727], OH, , Q.8, , [2030113778], , Q.9, , [2030113829], , Q.10, , [2030113524]
Page 18 :
EXERCISE-4, SECTION-A, (IIT JEE Previous Year's Questions), Q.1, , The IUPAC name of the compound having the formula is:, CH 3, |, H 3C C CH CH 2, |, CH 3, , (A) 3,3,3-trimethyl-1-propene, (C) 3,3-dimethyl-1-butene, , (B) 1,1,1-trimethyl-2-propene, (D) 2,2-dimethyl-3-butene, , [JEE 1984], , Q.2, , Write the IUPAC name of CH3CH2CH = CHCOOH, , [JEE 1986], , Q.3, , The IUPAC name of the compound CH2=CH–CH(CH3)2 is:, (A) 1,1-dimethyl-2-propene, (B) 3-methyl-1-butene, (C) 2-vinyl propane, (D) None of the above, , [JEE 1987], , Q.4, , The number of sigma and pi-bonds in 1-butene 3-yne are:, (A) 5 sigma and 5 pi (B) 7 sigma and 3 pi (C) 8 sigma and 2 pi, , Q.5, , Write I.U.P.A.C name of following:, (a), , [JEE 1989], (D) 6 sigma and 4 pi, , Me = methyl group, , [JEE 1990], , CH 3, |, H 3C N — CH CH 2CH 3, |, |, CH 3 C 2 H 5, , [JEE 1991], , Q.6, , Write IUPAC name of succinic acid., , [JEE 1994], , Q.7, , The IUPAC name of C6H5COCl is, (A) Benzoyl chloride, (C) Benzene carbonyl chloride, , (b), , (B) Benzene chloro ketone, (D) Chloro phenyl ketone, , [JEE 2006]
Page 20 :
EXERCISE-1, Q.1, Q.5, Q.9, Q.13, Q.17, Q.21, Q.25, Q.29, Q.33, Q.37, Q.41, Q.45, Q.49, , (A), (D), (C), (A), (B), (D), (B), (B), (C), (C), (B), (D), (D), , Q.2, Q.6, Q.10, Q.14, Q.18, Q.22, Q.26, Q.30, Q.34, Q.38, Q.42, Q.46, Q.50, , (C), (B), (A), (B), (D), (C), (B), (D), (A), (B), (C), (A), (B), , Q.3, Q.7, Q.11, Q.15, Q.19, Q.23, Q.27, Q.31, Q.35, Q.39, Q.43, Q.47, , (C), (D), (B), (A), (C), (C), (D), (C), (D), (D), (C), (B), , Q.4, Q.8, Q.12, Q.16, Q.20, Q.24, Q.28, Q.32, Q.36, Q.40, Q.44, Q.48, , (C), (B), (D), (B), (B), (B), (A), (C), (D), (B), (B), (A), , (A), (A), (B), (C), , Q.4, Q.8, Q.11, , (B), (A), (B), (C), (A), (B), (C), (D), , EXERCISE-2, Q.1, Q.5, Q.9, Q.12, , (B), (B), (C), (D), (A), (B), (C), (D), , Q.2, Q.6, Q.10, , (A), Q.3, (A), (B), (C) Q.7, (A), (B), (C), (D), Q.13, , Q.14, , (A), (B), (C), (D), , Q.15, , (A), (B), (C), (D), , Q.16, Q.18, Q.20, , [(A) Q; (B) R; (C) S; (D) P], [(A) R, Q; (B) P; (C) S ], [(A) R; (B) S; (C) P; (D) Q; (E) U; (F) T], , Q.17, Q.19, , [(A) R; (B) P; (C) S; (D) Q], [(A) Q, R; (B) R, S; (C) P ], , (A), (B), (D), , EXERCISE-4, SECTION-A, Q.1, Q.8, , (C), (B), , Q.3, Q.9, , (B), (C), , Q.4, , (B), , SECTION-B, Q.10, , (D), , Q.7, , (C)