Page 2 :
C nae 1, , As we look at our surroundings, we see a large, variety of things with different shapes, sizes, and textures. Everything in this universe is, made up of material which scientists have, named “matter”. The air we breathe, the food, we eat, stones, clouds, stars, plants and, animals, even a small drop of water or a, particle of sand — every thing is matter. We, can also see as we look around that all the, things mentioned above occupy space and, have mass. In other words. they have both, mass* and volume**., , Since early times, human beings have, been trying to understand their surroundings., Early Indian philosophers classified matter in, the form of five basic elements - the, “Panch Tatva"— air, earth, fire, sky and water., According to them everything, living or nonliving, was made up of these five basic, elements, Ancient Greek philosophers had, arrived at a similar classification of matter., , Modern day scientists have evolved two, types of classification of matter based on their, physical properties and chemical nature., , In this chapter we shall learn about, matter based on its physical properties., Chemical aspects of matter will be taken up, in subsequent chapters., , 1.1 Physical Nature of Matter, , 1.1.1 MATTER IS MADE UP OF PARTICLES, , For a long time, two schools of thought prevailed, regarding the nature of matter. One school, believed matter to be continuous like a block, of wood, whereas, the other thought that matter, was made up of particles like sand. Let us, perform an activity to decide about the nature, of matter — is it continuous or particulate?, , , , meee!, , MATTER IN Our SURROUNDINGS, , Activity, , * Take a 100 mL beaker., , * Fill half the beaker with water and, mark the level of water., , * Dissolve some salt/ sugar with the help, of a glass rod., , "Observe any change in water level., , * What do you think has happened to, the salt?, , * Where does it disappear?, , = Does the level of water change?, , In order to answer these questions we, need to use the idea that matter is made up, of particles. What was there in the spoon, salt, or sugar, has now spread throughout water., This is illustrated in Fig. 1.1., , 1.1, , , , , ‘Water ‘Water ‘Water, =., Add salt ‘Sur ‘Salt, , Particles of water ‘Salt, magnified millions, of times, , Fig. 1.1: When we dissolve salt in water, the particles, of salt get into the spaces between particles, of water., , 1.1.2 How SMALL ARE THESE PARTICLES, OF MATTER?, , Activity 1.2, , * Take 2-3 crystals of potassium, permanganate and dissolve them in, 100 mL of water., , , , * ‘The SI unit of mass {s kilogram (kg)., , "* The SI unit of volume is cubic metre (m*), The common unit of measuring volume Is, litre (L) such that IL = 1 dm*, 1L = 1000 mL, | mL = 1 cm’.
Page 3 :
* Take out approximately 10 mL of this, solution and put it into 90 mL of clear, water,, , * Take out 10 mL of this solution and, put it into another 90 mL of clear water., , * Keep diluting the solution like this 5 to, 8 times., , * Is the water still coloured ?, , 10 mb, , ef Fs i, , Fig. 1.2: Estimating how small are the particles of, matter. With every dilution. though the colour, becomes light. it ts still visible., , , , This experiment shows that just a few, crystals of potassium permanganate can, colour a large volume of water (about, 1000 L). So we conclude that there must be, millions of tiny particles in just one crystal, of potassium permanganate, which keep on, dividing themselves into smaller and smaller, particles., , The same activity can be done using, 2 mL of Dettol instead of potassium, permanganate. The smell can be detected, even on repeated dilution., , , , 1.2 Characteristics of Particles of, Matter, , 1.2.1 PARTICLES OF MATTER HAVE SPACE, BETWEEN THEM, , In activities 1.1 and 1.2 we saw that particles, of sugar, salt. Dettol, or potassium, permanganate got evenly distributed in water., Similarly, when we make tea, coffee or, lemonade (nimbu paani), particles of one type, of matter get into the spaces between particles, of the other. This shows that there is enough, space between particles of matter., , 1.2.2 PARTICLES OF MATTER ARE, CONTINUOUSLY MOVING, , Activity 1.3, , © Put an unlit incense stick in a corner, of your class. How close do you have to, go near it so as to get its smell?, , * Now light the incense stick. What, happens? Do you get the smell sitting, , at a distance?, , * Record your observations., , Activity 1.4, , Take two glasses/beakers filled with, water., , * Put a drop of blue or red ink slowly, and carefully along the sides of the first, beaker and honey in the same way in, the second beaker., , * Leave them undisturbed in your house, or in a corner of the class., , * Record your observations., , * What do you observe immediately after, adding the ink drop?, , * What do you observe immediately after, adding a drop of honey?, , * How many hours or days does it take, for the colour of ink to spread evenly, , throughout the water?, , Activity 1.5, , ™ Drop a crystal of copper sulphate or, potassium permanganate into a glass, of hot water and another containing, cold water. Do not stir the solution., Allow the crystals to settle at the, bottom., , * What do you observe just above the, solid crystal in the glass?, , * What happens as time passes?, , * What does this suggest about the, particles of solid and liquid?, , * Does the rate of mixing change with, temperature? Why and how?, , From the above three activities (1.3, 1.4 and, 1.5), we can conclude the following:, , Scmvce
Page 4 :
Particles of matter are continuously, moving, that is, they possess what we call, the kinetic energy. As the temperature rises,, particles move faster, So, we can say that with, increase in temperature the kinetic energy of, the particles also increases., , In the above three activities we observe, that particles of matter intermix on their own, with each other. They do so by getting into, the spaces between the particles. This, intermixing of particles of two different types, of matter on their own is called diffusion. We, also observe that on heating, diffusion, becomes faster. Why does this happen?, , 1.2.3 PARTICLES OF MATTER ATTRACT, EACH OTHER, , Activity 1.6, , * Play this game in the field— make four, groups and form human chains as, , suggested:, , * ‘The first group should hold each, other from the back and lock arms, like Idu-Mishmi dancers (Fig. 1.3)., , , , Fig. 1.3, , ® The second group should hold hands, to form a human chain., , * The third group should form a chain, by touching each other with only their, finger tips., , * Now, the fourth group of students, should run around and try to break the, three human chains one by one into, as many small groups as possible., , * Which group was the easiest to break?, Why?, , Marrer in OuR SuRROUNDINGS, , * If we consider each student as a, particle of matter, then in which group, the particles held each other with the, maximum force?, , Activity Lz, , * Take an iron nail, a piece of chalk and, a rubber band., , * Try breaking them by hammering,, cutting or stretching., , * In which of the above three, substances do you think the particles, are held together with greater force?, , Activity 1.8, , * Take some water in a container, try, cutting the surface of water with your, fingers., , * Were you able to cut the surface of, water?, , * What could be the reason behind the, surface of water remaining together?, , The above three activities (1.6, 1.7 and 1.8), suggest that particles of matter have force, acting between them. This force keeps the, particles together. The strength of this force of, attraction varies from one kind of matter to, another., , uestions, 1. Which of the following are, matter?, , Chatr, air, love, smell, hate,, almonds, thought, cold, lemon, , water, smell of perfume., 2. Give reasons for the following, , observation:, The smell of hot sizzling food, reaches you several metres, away, but to get the smell from, cold food you have to go close,, 3. A diver is able to cut through, water in a swimming pool. Which, property of matter does this, observation show?, 4. What are the characteristics of, the particles of matter?, I
Page 5 :
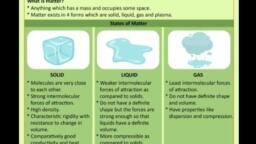
1.3 States of Matter, , Observe different types of matter around you., What are its different states? We can see that, matter around us exists in three different, states— solid, liquid and gas. These states of, matter arise due to the variation in the, characteristics of the particles of matter,, , Now, let us study about the properties of, these three states of matter in detail., , 13.1 THE SOLID STATE, , Activity 1.9, , * Collect the following articles— a pen,, a book, a needle and a piece of wooden, stick,, , * — Sketch the shape of the above articles, , in your notebook by moving a pencil, around them., , * Do all these have a definite shape,, distinct boundaries and a fixed volume?, , * What happens if they are hammered,, pulled or dropped?, , * Are these capable of diffusing into each, other?, , * Try compressing them by applying, force. Are you able to compress them?, , All the above are examples of solids. We, can observe that all these have a definite, shape, distinct boundaries and fixed volumes,, that is, have negligible compressibility. Solids, have a tendency to maintain their shape when, subjected to outside force, Solids may break, under force but it is difficult to change their, shape, so they are rigid., , Consider the following:, , (a) What about a rubber band, can it, change its shape on stretching? Is it, a solid?, , (b) What about sugar and salt? When, kept in different jars these take the, shape of the jar. Are they solid?, , (c) What about a sponge? It is a solid, yet we are able to compress it. Why?, , All the above are solids as:, * A rubber band changes shape under, force and regains the same shape when, , a, , the force is removed. If excessive force is, applied, it breaks., , * The shape of each individual sugar or, salt crystal remains fixed, whether we, take it in our hand, put it ina plate or in, a jar., , * A sponge has minute holes, in which, air is trapped, when we press it, the air, is expelled out and we are able to, compress it., , 1.3.2 THE LIQUID STATE, , * Collect the following:, {a) water, cooking oil, milk, juice, a, , cold drink,, , (b) containers of different shapes. Put, a50 mL mark on these containers, using a measuring cylinder from, the laboratory., , * What will happen if these liquids are, , spilt on the floor?, , * Measure 50 mL of any one liquid and, transfer it into different containers one, by one. Does the volume remain the, same?, , * Does the shape of the liquid remain the, same ?, , * When you pour the liquid from one, container into another, does it flow, , easily?, , We observe that liquids have no fixed, shape but have a fixed volume. They take up, the shape of the container in which they are, kept. Liquids flow and change shape, so they, are not rigid but can be called fluid., , Refer to activities 1,4 and 1.5 where we, saw that solids and liquids can diffuse into, liquids. The gases from the atmosphere, diffuse and dissolve in water. These gases,, especially oxygen and carbon dioxide, are, essential for the survival of aquatic animals, and plants., , All living creatures need to breathe for, survival. The aquatic animals can breathe, under water due to the presence of dissolved, oxygen in water. Thus, we may conclude that, solids, liquids and gases can diffuse into, liquids. The rate of diffusion of liquids is, , 1.10, , Sarnce