Page 1 :
Is Matter Around Us Pure, , Solutions, Solutions-An Overview, Large amount of salt is dissolved in seawater. This makes it unfit for drinking directly. Can, we say that the amount of salt in the sea is the same everywhere?, The air contains gases like oxygen, carbon dioxide and ozone along with various small, particles like pollen grains and dust. Are the gases and the particles present in equal, amounts in air?, Soil contains a lot of substances, e.g., clay, organic matter, minerals, pebbles, etc., , Why do the amounts of clay, organic matter, minerals, etc. in soil vary from place to place?, All of the above substances (soil, air and seawater) are examples of mixtures. Let us go, through the lesson to find out what mixtures are., Mixtures, A mixture may be defined as a material having two or more types of pure forms of matter., For example, milk is a mixture as it contains a combination of water molecules, fat, molecules and protein molecules. The constituents of a mixture can be separated by certain, physical processes such as evaporation and boiling. Constituents of certain mixtures can, also be separated manually. For example, a mixture of stones and sand can be separated, manually. On the other hand, salt cannot be manually separated from saltwater. One needs, to boil the mixture to separate the salt from water.
Page 2 :
Solutions, Now that we know what mixtures are, let us study about solutions. Whenever we talk, about solutions, we instantly think of liquids. But is it necessary for all solutions to be, liquids?, , No. A solution is simply a homogeneous mixture of two or, more substances. Solutions can be solid, liquid and gaseous. Alloy is an example of a solid, solution, while air is a gaseous solution., A mixture is called solution when it has homogeneity at the particle level. A solution is, formed when a solute is dissolved in a solvent., Examples of solutions, Solutions, 1. Saltwater, 2. Solution of iodine in, alcohol, 3. Vinegar, 4. Soda water, 5. Air, , Solvents, Water, Alcohol, , Solutes, Salt, Iodine, , Water, Water, , Acetic acid, Carbon dioxide
Page 3 :
Nitrogen (present in the, largest amount), , Other gases (present in, relatively smaller amounts), , Properties of Solutions, , , , , , , , They are homogeneous mixtures of solutes and solvents., The solute particles in a solution are extremely small in size. They are less than 1 nm, (10−9 m) in diameter., The solute particles are not visible to the naked eye., As a result of the small size of the solute particles, a solution does not scatter a beam of, light passing through it., Being small in size, the solute particles get dissolved in the solvent. Hence, the solute, cannot be separated from the solvent by filtration., The solute particles do not settle down when left undisturbed., Solved Examples, Hard, Example:, For each of the given substances, state whether or not it is a mixture. Also, if it is a, mixture, then identify it as homogeneous or non-homogeneous., Substance, , Mixture, , Homogeneous, , Non-homogeneous, , 1.Solder, 2.Soap water, 3.Silver, 4.Green tea, , Solution:, Substance, , Mixture, , Homogeneous, , 1.Solder, 2.Soap water, , Non-homogeneous, —, , —
Page 4 :
3.Silver, 4.Green tea, , —, , —, —, , Know More, Further Connect, Like a gas, a liquid exerts pressure of its own due to evaporation. This pressure is known as, the vapour pressure of the liquid., When a liquid solution is formed, it exerts its own vapour pressure. This results from the, individual pressures of the solute and the solvent., In 1882, a scientist named Francois-Marie Raoult established that for a solution containing, volatile liquids, the partial vapour pressure of each component of the solution is, proportional to its mole fraction present in solution., p1∝ x1, p1=p1o x1, where p1o is the vapour pressure of pure component at the same temperature., Did You Know?, 1. The addition of solutes to solvents can lower the freezing point, elevate the boiling point, and lower the vapour pressure of the solvents., 2. A solution is formed when two similar substances are mixed. For example, water and salt, form a solution, but water and oil do not. This is because:, , , , Water and salt are polar substances (wherein the centres of positive charge and negative, charge do not coincide), so they can mix with each other., Oil is a non-polar substance, so it does not mix with water to form a solution., 3. The solubility of a solute in a solvent is affected by temperature. Usually solubility, increases with increase in temperature., Concentration of a Solution, Concentration of a Solution
Page 5 :
We come across many solutions in daily life, for example, saltwater, lime juice, squashes,, coconut water, etc. How do we rate these solutions? We do so by classifying them as sweet,, salty, etc., on the basis of our senses., In chemistry, however, such classification of solutions is not very informative or beneficial., So, chemists usually use words such as ‘saturated’, ‘unsaturated’, ‘supersaturated’, ‘dilute’, and ‘concentrated’ to define the concentration of a given solution., Uses of Concentration in Real Life, , , , , , , Maintenance of the ionic balance in our body, Preparation of dyes, Preparation of juices, Labelling of alcoholic drinks, Addition of antifreeze to vehicles, Let us learn more about concentration in this lesson., Know More, Antifreeze, Antifreeze solutions are added to a liquid in a cooling system (such as the water in an, automobile engine) to lower its freezing point and prevent ice build-up in the system at, very low temperatures., Functions of antifreeze, , , , , , It contains chemicals that prevent corrosion and scale formation in the engine and radiator, of a vehicle., It provides protection against boiling during summers. At 1 atmosphere pressure, water, boils at 100°C; but a 50-50 blend of water−ethylene glycol boils at 106°C., A mixture of antifreeze and distilled water (in the ratio, one part antifreeze to one part, water) provides freeze protection down to −36.67°C and boil-over protection up to, 129.4°C., Precautions, , , , , Avoid using concentrated antifreeze in a cooling system. At least 40% of the mixture should, be water., Do not increase the concentration of antifreeze above 60% as it damages the freezing and, overheating protection of the engine.
Page 6 :
, , Do not add too much water to the cooling system as it lowers the concentration of the, corrosion inhibitor and antifreeze. This results in decreased protection against corrosion, and freezing., Did You Know?, The coke or colas (aerated drinks) available in the market are actually super saturated, solution of CO2 in water., Experiment to Demonstrate Solubility, , Procedure: Take two beakers and label them A and B. Fill each with 50 mL of water., Dissolve sugar in beaker A and barium chloride in beaker B. Keep dissolving the solutes in, the respective beakers till the solutions become saturated and no more solute can be, further dissolved. After this, heat the solutions and dissolve one more spatula of the solutes, in the respective beakers. Slowly cool down the solutions after the solutes added further, get completely dissolved., Result:, , , , , To saturate 50 mL of water, different amounts of sugar and barium chloride is added., Upon heating, the saturated sugar solution is able to dissolve more amount of sugar., When cooled, the sugar (dissolved after the saturated solution is heated) precipitates out, from the solution., Explanation: Upon heating, a saturated solution can accommodate more amount of solute., This is called a super-saturated solution. This solution is quite unstable as the molecules, of the solute (dissolved after the saturated solution is heated) crystallise easily., Conclusion: A particular amount of solute (here sugar or BaCl2) can saturate a particular, type of solvent (here water) at a particular temperature only. If temperature is changed,
Page 7 :
then the solubility of the solvent also changes. Solubility of a solute in a solvent is the, maximum amount of solute that can be dissolved in 100 g of that solvent at a particular, temperature. It is expressed in terms of concentration. For example, a maximum of 36 g, sodium chloride can be dissolved in 100 g water at 20°C. Therefore, the solubility of, sodium chloride in water is 36 g at 20°C., Concentration and Its Classification, A solution is a homogeneous mixture of two or more substances. The substance that is, dissolved in a solution is called the solute, and the substance that dissolves the solute is, called the solvent. The amount of solute in a solution may vary., The amount of solute present in a given quantity of solution is called the concentration of, that solution. The concentration of a solution helps us to determine the amount of solute, present in the solution., , Depending on the amount of solute present, a solution can be classified as follows:, 1.Dilute solution, 2.Concentrated solution, 3.Saturated solution, 4.Unsaturated solution, 5.Supersaturated solution, Concentration in terms of Mass by Mass Percentage, Concentration in terms of Molarity, It is defined as the number of moles of solute present in 1000 mL of the solution. Molarity, is represented by M.
Page 8 :
A 10 M solution indicates that 10 moles of solute are present in 1 L or 1000 mL of the, solution. One mole of any amount is equal to the gram molecular mass to be taken., Consider the example of NaCl, the amount of 58.5g/mole(molecular weight) of NaCl is, called one mole of NaCl. If the substance is in the elemental form, then its atomic mass will, have to be considered for 1 mole amount., Brush Up, , Summary, The following chart shows the types of solutions that can be formed by varying the, concentration.
Page 9 :
Suspensions, Suspensions: An Overview, In our day to day activities, we encounter many examples of suspensions. Here are a few:, , , , , , , Paint, Dust in air, Muddy water, Suspended particulate matter in air, Silt in the water on a river bank, Non-homogeneous systems in which solids are dispersed in liquids are called, suspensions., In other words, a suspension is a heterogeneous mixture in which the solute particles are, spread throughout the bulk of the liquid (solvent), rather than being dissolved in it. The, solute particles in a suspension can be seen with the naked eye.
Page 10 :
Take a beaker and fill it with 50 mL water. Add one spatula of chalk powder to it. Stir the, contents of the beaker using a glass rod and use a torch to direct a beam of light through, the beaker. You will observe that the light gets scattered due to the presence of the chalk, particles in water., Then, leave the mixture undisturbed for five to ten minutes. The chalk particles will settle, down when left undisturbed. This indicates the unstable nature of a suspension. Further,, owing to the large size of the solute particles, you can filter them using a filter paper., Characteristics of Suspensions, A suspension is a heterogeneous mixture.The solute particles in a suspension are quite, large. The diameter of these particles is more than 100 nm.The solute particles can be, easily seen with the naked eye. A beam of light passing through a suspension gets scattered., This happens due to the presence of the large-sized solute particles., The characteristics of suspensions are:
Page 11 :
Suspended Particulate Matter, Suspended particulate matter (SPM) refers to the small particles (having liquid or solid, origin) present in Earth’s atmosphere., , Sources and Effects of SPM, Sources of SPM, Man-made, i) Burning of fossil fuels, ii) Industrial processes, iii) Emissions from factories and power, plants, iv)Vehicular emissions, v) Household emissions, vi) Burning of crackers, , Natural, i) Volcanoes, ii) Dust storms, iii) Forest fires, iv) Sea sprays, v) Vegetation (pollens, fungi, moulds,, yeasts), vi) Bacteria and viruses, , Effects of SPM, , , Changes the amount of incoming solar radiation
Page 12 :
, , , , , , , , Increases the absorption power of Earth’s crust, thereby increasing the surface, temperature, Causes respiratory problems such as lung cancer and asthma, Causes birth defects, Causes cardiovascular issues, Clogs stomatal openings of plants, Corrodes metals and soil structures, Impairs visibility, Colloidal Solutions, , , , Colloidal Solutions-An Introduction, , , , You know that the solute particles of a true solution are much smaller than those of, a suspension|chem. Now, there is a third kind of mixture which has solute particles, intermediate in size to those of a true solution and a suspension. This mixture is, known as a colloid or a colloidal solution. Things such as milk, butter, cream and fog, fall in this category., , , , The particles in a colloidal solution are small enough to make it seem like a, homogeneous mixture. However, this is not the case. A colloidal solution is a, heterogeneous mixture in which the solute particles are smaller than the solute, particles of a suspension, but bigger than those of a true solution.The seeming, homogeneity of a colloidal solution is due to the uniform distribution of its particles, throughout the solution. Nevertheless, it is a heterogeneous mixture., , , , Some Colloidal Solutions from Daily Life, , Gelatin, Mayonnaise, , Foam, , Classification of Colloidal Solutions, Colloids are classified according to the states of the dispersion medium and, the dispersed phase., , Sponge
Page 13 :
Dispersed Phase, Dispersion, Medium, , Solid, , Liquid, , Gas, , Solid sol, Coloured gemstone, ruby, glass, , Gel, Cheese, jelly, , Foam, Sponge, foam,, rubber, , Liquid Sol, Mud, milk of magnesia, , Emulsion, Milk, cream, , Gas, , Aerosol, Fog, cloud, , Foam, Shaving, cream, None, All gases are, soluble, , Solid, , Aerosol, Smoke, automobile exhaust, , Classification of Colloidal Solutions, Based on the nature of interaction between the dispersed phase and the dispersion, medium, colloids can be classified as:, , , , Hydrophilic colloids: These are water-loving colloids.The colloid particles are attracted, toward water. They are also called reversible sols., Hydrophobic colloids: These are opposite in nature to hydrophilic colloids. The colloid, particles are repelled by water. They are also called irreversible sols., Properties of Colloidal Solutions, 1. A colloid is a heterogeneous mixture., 2. The solute particles in a colloid are bigger than those in a true solution and smaller than, those in a suspension. They are between 1 nm and 100 nm in diameter.
Page 14 :
3. The solute particles present in a colloidal solution cannot be seen even with a, microscope., 4. The solute particles in a colloidal solution are large enough to scatter a beam of light, passing through the solution., 5. The solute particles of a colloid cannot be filtered using a filter paper., 6. Colloids are quite stable. Their particles do not settle down when left undisturbed., Summary, , Tyndall Effect, , It is named after the British physicist John Tyndall. It is the scattering of light by small, colloidal particles or particles in suspension.Due to the presence of scattered particles in a, colloid or a suspension, light is unable to travel in straight line.The incident light collides, with the particles, thereby making the path of light visible.For Tyndall effect to occur, the, size of the colloidal particles should be larger than the wavelength of the incident light.The
Page 15 :
longer-wavelength light is more transmitted.The shorter-wavelength light is more, scattered.Tyndall effect increases linearly with concentration.This phenomenon is similar, to Rayleigh scattering., Some real life examples of Tyndall effect:, , , , , , , Blue colour of the smoke emitted from vehicles, Blue colour of the iris, Scattering of sunlight in a forest, Bright-coloured sunrise and sunset, Milky glass appears blue but the transmitted light is yellowish in colour., Know More, Rayleigh scattering, , It is named after the British physicist Lord Rayleigh. It is the elastic scattering of light or, electromagnetic radiation by particles much smaller than the wavelength of the, radiation. It occurs when light travels through transparent solids and liquids. It is most, prominent in gases., , Know Your Scientist
Page 16 :
John Tyndall (1820−1893) was a physicist of Anglo-Irish origin. He made a number of, discoveries with respect to the processes occurring in the atmosphere, one of them being, Tyndall effect. In the late 1850s, he started studying the effect of radiant energy on the, components of air. Here are a few of his significant contributions to the world of science., , 1. He was the first to establish that the heat in Earth’s atmosphere is due to the absorption, of the radiation emitted from Earth’s surface, by the molecules of gases present in the, atmosphere., 2. In the process of his study on the effect of radiant energy on the components of air, he, discovered that the dust particles present in air cause scattering of light. This phenomenon, is now known as Tyndall effect., 3. He was the first to discover thermophoresis in aerosol mixtures. Thermophoresis is the, phenomenon wherein moving particles show variable responses to temperature gradient., , Lord Rayleigh (1842−1919), born John William Strutt, was an English physicist. He, discovered argon along with William Ramsay, and for this he was awarded the Nobel Prize, in Physics in 1904. He is credited with the discovery of the phenomenon now known as, Rayleigh scattering. This phenomenon explains why the sky appears to be blue. His initial, researches were mainly mathematical, concerning optics and vibrating systems. Later, he
Page 17 :
worked over almost the entire field of physics, covering sound, wave theory, colour vision,, electrodynamics, electromagnetism, light scattering, flow of liquids, hydrodynamics, gas, density, viscosity, capillarity, elasticity and photography., Separating Mixtures by Centrifugation, Distillation and Sublimation, Why Do We Need to Separate Substances?, Most substances occurring in nature are mixtures. To obtain pure substances, it is, necessary to separate the components of the mixtures. For example, seawater is the, mixture of water and salt. If we want to use water and salt separately, then we have to, separate the salt from the water., Pure substances have great importance in chemical industries. These are used in, laboratories to study their chemical properties., Components of heterogeneous mixtures can sometimes be separated very easily as the, components are physically distinct from each other. For example, methods like, handpicking, filtering, sieving etc., can be used to separate the components., However, to separate the components of a homogeneous mixture, we need to use some, special separating techniques., The commonly used techniques for separating the components of mixtures are as follows:, , , , , , , , , , , , Evaporation, Centrifugation, Separation of immiscible liquids by a separating funnel, Separation of miscible liquids by:, Simple distillation, Fractional distillation, Sublimation, Crystallization, Chromatography, Separation of solids using solvent and filtration, Evaporation, Evaporation is the process in which a liquid is changed into its vapour state by heating. It is, used to separate solid substances dissolved in a liquid., Separation of the coloured component (dye) from blue or black ink, Blue or black ink is prepared by dissolving blue or black dye in water. The dye can be, separated from ink by evaporation. Let us perform an activity to do this.
Page 18 :
Procedure:, , , , , , Half fill a beaker with water., Put a watch glass on top of the beaker., Add a few drops of blue or black ink on the watch glass., Now, heat the beaker till a solid mass is obtained in the watch glass., Observation:, You will observe that a solid residue of the dye is obtained in the watch glass. Ink is a, colloidal solution. It is a heterogeneous mixture of dye and water. Heating leads to the, evaporation of water. This leaves behind the dye in the watch glass., Centrifugation, , Milk is a heterogeneous mixture of proteins, fats and other nutrients. Cream is separated, from milk as a fat-rich substance by the process of centrifugation. The given figure shows, this process.
Page 19 :
It will be observed that cream collects in the upper layer of milk after centrifugation. Fat, (cream) is the lightest component of milk, so it forms a layer on top., The technique of centrifugation is used in the:, , , , , , Drying of wet clothes in the spin tub of a washing machine, Extraction of DNA for forensic and experimental purposes, Treatment of wastewater, Processing of sugar and milk, , Separation of Two Immiscible Liquids, Examples of immiscible liquid mixtures: Oil and water, Petrol and water, Separation of Two Miscible Liquids, They can be separated by Simple distillation (for liquids having at least 20−25 K, difference in boiling points) and Fractional distillation (for liquids having very similar, boiling points). Examples of miscible liquid mixtures are alcohol and water, acetone and, water, Fractional Distillation, This method is used to separate miscible liquids in a mixture. The apparatus used is similar, to that used in simple distillation, with the exception of a fractionating column fitted, between the condenser and the distillation flask. The fractionating column is packed with, glass beads. These provide a large surface area for the hot vapours to cool and condense, repeatedly.
Page 20 :
Did You Know?, Air is a homogeneous mixture of different gases such as oxygen, nitrogen and carbon, dioxide. The oxygen cylinders in hospitals and cylinders of carbon dioxide in cars and, buildings are prepared by separating individual gases from air., Project Ideas, Aim: To separate a mixture of alcohol, water, salt and sand., Apparatus required: Beaker, burner, funnel, filter paper, tripod stand, wire gauze and, fractionating column, Theory: The given mixture contains alcohol, salt, water and sand. The separation of the, mixture depends upon the characteristic properties of its constituents., Alcohol and salt are soluble in water, but sand is insoluble in water. So, sand can be, removed easily by filtration., The remaining mixture now contains alcohol, salt and water. The boiling points of these are, as follows:, , , , , Alcohol = 78°C, Salt = 108°C, Water = 100°C, It is evident from the above information that alcohol can be separated from the mixture by, simple distillation. During this process, alcohol will boil first and can then be collected in a, beaker., The remaining solution now contains salt dissolved in water which can be separated by, evaporation. The water vapours can be condensed back to water, while the salt is left, behind in the flask., Procedure:, , , , , , , , , , , Pour the mixture in a beaker., Take an empty beaker and place the funnel on it., Cover the inner side of the funnel with filter paper., Pour the mixture into the funnel, allowing sand to separate from the liquid mixture., Dry the sand collected on the filter paper., Pour the remaining solution in a distillation flask and heat to boil., Vapours of alcohol condense in the condenser and liquid alcohol is collected in the beaker, attached to the condenser., Heat the remaining mixture till water starts evaporating.
Page 21 :
, , Crystals of salt will be left in the flask., Result: Separation of the mixture is done as follows:, , , , , , Sand from the mixture: By filtration, Alcohol from the remaining mixture: By distillation, Salt from water: By evaporation, Real life extension: List some of the industrial uses of the separation techniques used in, this experiment., , Separation of Gases by Fractional Distillation, , Stages involved in separation of the components of air:, , , , , , Air is first filtered to remove dust particles. Then, it is compressed under high pressure., This compressed air is then passed through a water condenser to decrease its temperature., The cold compressed air is now passed through a separator. Here, carbon dioxide separates, as dry ice. Due to repeated compression, air becomes cold and turns into liquid., The liquid air coming out of the separator is passed through an expansion jet into the, distillation column. Here, it is warmed slowly. Liquid nitrogen, having the boiling point of, −196°C, boils first to form liquid nitrogen gas. This gas is collected from the upper part of, the column. Argon, having a boiling point of −186°C, is collected next. Oxygen, having a, boiling point of −183°C, is collected last., Sublimation
Page 22 :
Crystals of ammonium chloride and common salt look similar. It is difficult to separate, their mixture by ordinary methods. Sublimation is the best way to separate ammonium, chloride from common salt. Let us understand this process with the help of an activity., , Procedure:, , , , , , , Take a mixture of common salt and ammonium chloride in a china dish., Place the dish on a tripod stand and cover it by inverting a funnel over it (as shown in the, figure)., The funnel should be plugged on the top with cotton to prevent the vapours of ammonium, chloride from escaping into the atmosphere., Heat the china dish using a burner., On heating, ammonium chloride sublimes. As a result, ammonium chloride gets separated, from common salt and solidifies on the cold walls of the funnel., Note: For any substance undergoing sublimation, the energy required to convert its solid, form into liquid is greater than the energy required to convert the solid form into gas., Did You Know?, Sublimation Printing, , , , , , , , , , , , It involves using dye sublimation printer to print on a substrate., The printer uses heat to transfer dye onto the substrate., Due to the heat, the dye transitions from solid to gas without passing through the, intermediate liquid phase., The dye sublimation printer produces a continuous tone., The obtained prints are dry and can be used immediately., The process is clean since no liquid dye is formed., The coloured ribbons and the heating head must match the size of the substrate., Only specially coated papers accept sublimated dye., The sublimated ink is prone to diffusion, so the obtained prints are not sharp.
Page 23 :
, , , , , Once used, the coloured ribbons cannot be used again; so, a lot of dye is wasted., A negative of the print appears on the coloured ribbons., Dye sublimation papers and ribbons are very sensitive and can be destroyed by skin oils., For effective prints, the ribbons need to be free from dust., Crystallization, We can obtain pure copper sulphate from an impure sample through the process, of crystallization. Let us understand this process with the help of an activity., Procedure:, , , , , , , , , Take a small amount (5 g) of impure copper sulphate in a china dish., Dissolve the sample in 10 mL of water., Filter it to remove impurities., Evaporate the filtered solution on a water bath till small crystals are formed, indicating that, a concentrated solution has been obtained., Cover the solution with a filter paper and leave it undisturbed at room temperature for the, rest of the day., It will be observed that crystals of pure copper sulphate are obtained and the impurities, are left in the solution., , Sedimentation, Decantation and Filtration, Mixtures of mud in water and chalk in water can be separated by using methods such, as sedimentation, decantation, and filtration. The definitions of these three methods are, given in the following table.
Page 24 :
SEDIMENTATION, It is the process of, settling down of the, heavier components, present in a mixture., , DECANTATION, It is the process of, transferring a liquid from, one container to another, without disturbing the, sediments that are, present at its bottom., , FILTRATION, It is the process of separating, the undissolved components, from a mixture. It is done by, passing the mixture through a, material containing fine holes, that will allow only one, component of the mixture to, pass through., , Filtration is a better method than decantation to separate mixtures as some of the solid, particles also pass along with the liquid during the process of decantation while filtration, allows one to obtain a cleaner liquid. However, decantation is used in cases where, recovering the solid substance from the filter paper is difficult., Sedimentation, Decantation and Filtration, Chromatography, Chromatography can be used to separate the coloured components of a mixture on the, basis of the difference in the speeds of the components on chromatograph paper, when, dissolved in the same solvent. The adsorbent paper acts as the stationary phase; it carries, the components of the mixture on the paper. The mixture acts as the mobile phase and the, components get separated. The component which moves slowly (i.e., the less-soluble, component) appears as a spot on the lower side of the paper. The component which moves, faster (i.e., the more-soluble component) appears as a dot on the higher side of the paper., Separating coloured components of ink by chromatography
Page 25 :
Procedure:, , , , , , , , On a thin strip of filter paper, make a boundary at least 3 cm above the lower portion. At, the centre of the boundary, pour a drop of ink and let it dry., Repeat the above action two to three times, letting the drop dry each time, in order to, concentrate the mixture. Pour some amount of water in a cylindrical jar., Place the paper in the jar in a manner that the lower end of the paper dips in water., Be careful not to dip the ink in water., Fix the upper end of the paper to the lid of the jar or on the top edge of the jar., Leave the apparatus undisturbed for some time., Observation: As the water rises on the filter paper, it carries the drop of ink with itself;, after some distance, the ink separates into its constituent colours., Project Ideas, Aim: To separate the green pigment from plant leaves with the help of chromatography., , Theory: Plants survive by making their own food with the help of a green pigment known, as chlorophyll. They absorb water from the ground with the help of their roots and carbon, dioxide gas from the atmosphere. They use sunlight to convert the absorbed water and, carbon dioxide into glucose. Glucose is a sugar which is used by plants as food to supply, energy and as the building block for growth. This entire process is named photosynthesis., Chlorophyll helps in this process of manufacturing food and provides green coloration to, plants. In winters, there is often shortage of light and water for photosynthesis to be, carried out. During such times, the plants rest and live off the food they stored during, summers. When plants stop manufacturing food, chlorophyll starts to disappear and, shades of yellow or orange become visible. This is the reason leaves change colour in, autumn., Materials required: Leaves, small beakers, covers lids for beakers, rubbing alcohol, paper, coffee filters, shallow pan, hot water, tape, pen, plastic spoon, clock
Page 26 :
Procedure:, , , , , , , , , , , , , , , , , Collect two to three big leaves from different trees., Cut each leaf into tiny pieces and place them in a beaker labelled with the name or location, of the tree that the leaf came from., Add rubbing alcohol to the beakers in order to cover the cut pieces of the leaves., Using a plastic spoon, carefully grind the leaves in the alcohol., Cover the beakers loosely with lids., Place the beakers in a shallow tray containing up to 1 inch of hot water., Keep the beakers in water for at least thirty minutes, until the alcohol is coloured (the, darker the better)., Swirl each beaker gently every five minutes. Replace the hot water if it cools off., Cut out a long thin strip of coffee filter paper for each beaker and label it accordingly., Remove the beakers from water and uncover them., Place a strip of filter paper into each beaker such that one end is in the alcohol. Tape the, other end to the beaker after bending it around the corner., The alcohol will travel up the paper, dragging the colours with it., After thirty to ninety minutes (or longer), the colours will travel to different distances on, the paper as the alcohol evaporates., Different shades of green, and possibly some yellow, orange or red (depending on the type, of leaf) will start showing up on the paper., Remove the strips of filter paper, dry them and then tape them to a piece of plain paper., Result: List the different colours into which the green pigment got separated., Real life extension: Find out the applications and uses of chromatography in daily life., Separation of Solids using a Solvent, Separating a mixture of sodium chloride and sand using water, Procedure:, , , , , , , , , Place the mixture of salt and sand in a beaker., Add water to the mixture and stir well., Allow the mixture to stand for a while., Filter the mixture to obtain salt water and sand., Allow the sand to dry., Evaporate the salt water in china dish., Observation: Salt being soluble in water, will get dissolved in it. The remaining sand is, filtered off. Salt can be recovered from the solution by evaporation. The crystals of sodium, chloride are obtained on the walls of the china dish., Separating a mixture of carbon and sulphur using carbon tetrachloride, Procedure:
Page 27 :
, , , , , , , Place the mixture of carbon and sulphur in a beaker., Add carbon tetrachloride to the mixture and stir well., Allow the mixture to stand for a while., Filter the mixture to obtain carbon and solution of sulphur., Allow the carbon to dry., Evaporate the solution of sulphur in china dish., Observation: Sulphur is soluble in carbon tetrachloride solution. It gets dissolved in it, leaving carbon behind. The sulphur is obtained by evaporating the solution., Classifying Substances as Mixtures and Compounds, Physical and Chemical Changes: An Overview, So many changes take place around us daily. The given images show examples of such, changes., , Scientists classify the various changes occurring in nature under two categories:, , , , Physical changes, Chemical changes, Wondering what these categories imply? Read on to find out., Physical and Chemical Changes, Physical changes are those changes in which only the forms of substances get modified;, the chemical natures and compositions of the substances involved are not altered.
Page 28 :
Examples: Melting of butter, boiling of water, glowing of a bulb and cutting of trees, Characteristics:, , , , , No new substance is formed during a physical change., Most physical changes can be reversed easily., The chemical composition of a substance undergoing physical change remains the same., Chemical changes are changes that involve reaction of substances with one another. Such, reactions result in alterations in the chemical compositions of the substances involved., These changes lead to the formation of new substances., Examples: Rusting of iron, burning of paper and cooking of food, Characteristics:, , , , , , , One or more new substances are formed during a chemical change., Most chemical changes cannot be reversed easily., The chemical composition of a substance undergoing chemical change does not remain the, same., A chemical change is always accompanied by a change in energy., Classification of Substances as Mixtures and Compounds, On the basis of physical and chemical changes, substances are classified as mixtures and, compounds., , , , , Compounds are formed when two or more elements combine together in fixed proportion., Example: water, common salt and sugar, Mixtures are formed by the physical combinations of different elements and, compounds. Example: air, Difference between mixtures and compounds, , Mixtures, , Compounds, , 1. They are obtained by the physical, combinations of either elements or, compounds or both., , 1. They are obtained by the chemical, combinations of elements., , 2. The compositions of the constituents, of mixtures are not fixed., , 2. The compositions of the elements, present in compounds are fixed.
Page 29 :
3. A mixture shows the properties of all, its constituents. For example, a mixture, of sulphur and iron displays the, properties of both sulphur and iron., , 3. A compound may or may not show the, properties of its constituent elements. For, example, the compound obtained on, heating sulphur with iron does not display, the properties of iron., , 4. The constituents of a mixture can be, separated by physical methods. For, example, in case of an iron−sulphur, mixture, iron can be easily separated, with the help of a magnet., , 4. The constituents of a compound can be, separated only by chemical and, electrochemical methods., , Classification of Pure Substances, Matter−Pure or Impure, You must have seen cartons of milk, ghee etc., with markings on them stating that the, products inside are pure. Do you think the products are pure?, You might say that they are pure. This is because most people use the term “pure” to, describe a product that is not adulterated or a product that is free from harmful substances, such as bacteria, fungi, etc. However, for a scientist, a substance is “pure” if it is composed, of only one type of particles or molecules., Let us first define the term ‘substance’., Substance: A substance may be defined as a form of matter that has a definite chemical, makeup. Some substances can be separated into other types of matter by physical, processes, while some cannot., Pure substance: It is a substance composed of only the same type of particles. It has, definite properties throughout., Milk is not made up of the same type of molecules. It consists of molecules of water,, proteins, fats, etc. Therefore, chemically, milk is not pure., Salt-water solution is not pure as salt can be separated from its solution by evaporation (a, physical process). However, salt (sodium chloride) is a pure substance and cannot be, separated into its constituents by any physical process., Most of the substances that we see around us are actually impure form of matter., Examples: gold in ornaments, steel or aluminium utensils, etc., Classification of Matter
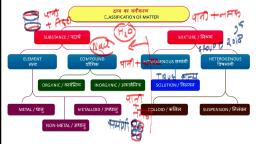
Page 30 :
Pure Substances and Mixtures, Mixture: It may be defined as a material having two or more types of pure forms of matter., For example, milk is a mixture as it contains a combination of water molecules, fat, molecules, and protein molecules., Constituents of a mixture can be separated by physical processes such as evaporation,, boiling, etc. Constituents of certain mixtures can also be separated manually. For example, a, mixture of stones and sand can be separated manually. On the other hand, salt cannot be, manually separated from a salt-water solution. One needs to boil the mixture to separate, salt from water. Some examples of mixtures are air, soil, milk, petrol, brass, blood, salt, solution, etc., Pure substances: Unlike mixtures which contain two or more types of particles, pure, substances are composed of the same type of particles. Pure substance can be divided into, two parts, that is, elements or compounds., , Pure Substances, , Mixtures
Page 31 :
1. It is a pure form of matter and has a, definite composition., , 1. It is a substance containing two or, more types of pure forms of matter., , 2. It cannot be separated into its chemical, constituents by any physical process., , 2. Constituents of a mixture can be, separated from each other by physical, processes such as evaporation, boiling,, etc., , Example: Sodium chloride contains only, one kind of pure particle and cannot be, separated into its chemical constituents by, any physical process., , Example: Salt-water solution is a, mixture that is made up of more than, one pure form of matter., , Classification of Elements, The term ‘element’ was first used by Robert Boyle. Later, a French chemist, Lavoisier, defined elements as: ‘the basic form of matter which cannot be broken down into simpler, substances by chemical reactions.’ Examples of elements are iron, carbon, mercury and, oxygen. Elements can be further classified as metals, metalloids and non-metals., Properties of metals:, , , , , , , , , , They are generally hard and strong., They have lustre (shine) and can be polished., They are good conductors of heat and electricity., They are ductile. It means that they can be drawn into thin wires., They are malleable. It means that they can be hammered into thin sheets., They are sonorous. It means that they make ringing sound when struck., They generally have high boiling and melting points., Iron, gold, silver, copper, sodium, potassium, etc., are the examples of metals., Classification of Elements, Properties of non-metals:, , , , , , , , , , They are generally soft and are not strong like metals., They are dull in appearance and are bad conductors of heat and electricity., They are not ductile. They break on stretching., They are not malleable. They break into pieces when hammered., They are not sonorous., They have lower melting and boiling points., Hydrogen, oxygen, carbon, iodine, etc., are the examples of non-metals.
Page 32 :
Metalloids are the elements which exhibit certain properties of metals and non-metals., Boron, silicon, germanium, etc., are some examples of metalloids., Know More, The metalloids are also called semiconductors. They are used in the manufacture of, microchips., , Noble gases are the elements which do not react with other elements or compounds., These are also called as inert gases. Helium, neon, argon, krypton, xenon and radon are the, only six inert gases of the periodic table., , Distinguishing Metals, Non-Metals and Metalloids, , Metals, , Metalloids, , 1. They are generally hard and 1. These are elements, strong., having properties, intermediate to those of, metals and non-metals., 2. They are shiny (lustrous) 2. Boron, silicon and, and can be polished., germanium are examples of, metalloids., 3. They are good conductors, of heat and electricity., 4. They are ductile, so they, can be drawn into thin wires., 5. They are malleable, so they, can be hammered into thin, sheets., 6. They are sonorous, so they, make a ringing sound on being, struck., 7. They generally have high, boiling and melting points., 8. Iron, gold, silver, copper,, sodium and potassium are, examples of metals., , Non-metals, 1. They are not strong like, metals. They are generally, soft., 2. They are dull in, appearance., 3. They are bad conductors of, heat and electricity., 4. They are not ductile, so, they break when stretched., 5. They are not malleable, so, they break into pieces when, hammered., 6. They are not sonorous, so, they do not make a ringing, sound on being struck., 7. They have low melting and, boiling points., 8. Hydrogen, oxygen, carbon, and iodine are examples of, non-metals.
Page 33 :
9. Mercury is the only metal,, which is liquid at room, temperature., , 9. Bromine is a non-metal,, which is liquid at room, temperature., , Compounds, Compound is defined as the substance composed of more than one element. These, elements which make up a compound are combined in a definite proportion., Water, copper sulphate, ammonium chloride, sodium chloride (common salt), etc., are, some examples of compounds., • Compounds are formed when two or more elements combine chemically in fixed, proportions., • For example, water is a compound. It is formed when two atoms of hydrogen combine, chemically with one atom of oxygen. This composition of water is constant throughout the, universe, no matter where or how it is formed., • The physical and chemical properties of a compound may or may not be similar to its, constituents. For example, hydrogen is a combustible substance and oxygen supports, combustion. However, water (a compound of hydrogen and oxygen) neither burns nor, supports combustion. In fact, it is used as a fire extinguisher., Know Your Scientist, , Robert Boyle (1627−1691) was a chemist and physicist of Anglo-Irish origin. He is one of, the first modern chemists and a pioneer of the modern experimental scientific method. He, is best known for Boyle’s law which depicts that at a constant temperature, the pressure of, a gas is inversely proportional to its volume in a closed system. He is considered to be one, of the ‘fathers of modern chemistry’.
Page 34 :
Antoine-Laurent de Lavoisier (1743−1794) was a prominent chemist and biologist of, French origin. He is considered to be one of the ‘fathers of modern chemistry’. He is, credited with the identification and naming of oxygen and hydrogen gases. He also, predicted silicon to be an element. He was among the first to list down the known elements., He discovered that mass always remains the same irrespective of the changes in matter., , Compounds, Characteristics of compounds:, 1. A compound is formed by mixing two or more elements in a fixed ratio by mass. For, example, water is formed by mixing hydrogen and oxygen in the fixed ratio of 1:8 by mass., 2. The properties of a compound are entirely different from the properties of its, constituents., For example, oxygen supports combustion and hydrogen is an inflammable gas, while, water is neither combustible nor does it support combustion., 3. Whenever a compound is formed, it releases or absorbs heat. For example, when, nitrogen and hydrogen combines to form ammonia, it releases a lot of heat., 4. Since a compound is a pure substance, it will have fixed melting and boiling points., For example, ice melts at 0ºC, while water boils at 100ºC., 5. The constituents of a compound cannot be separated using simple physical methods., For example, water cannot be reduced to hydrogen and oxygen just by heating or filtering., Electrolysis of acidified water is the only way to separate the constituents., 6. Any compound is homogenous in nature., Let us perform an activity to show that sugar is a compound.
Page 35 :
Activity Time, Take some sugar in a test tube and heat it over a flame. What do you observe? You will see that, the sugar will melt and change its colour from white to brown. If you heat it further, you will, observe a black mass., , What do you call it?, This is known as charring of sugar., At the bottom of the test tube, you will observe water droplets. These are formed by the, decomposition of sugar and not by the condensation of water vapours. This shows that sugar is, formed by carbon and water. It also proves that sugar is a compound., , Classification of compounds:, Compounds can be classified into two categories. They are as follows:, (a) Organic compounds: Compounds obtained from living things, such as plants and, animals, are called organic compounds. For example: carbohydrates, proteins, fats, etc., obtained from plants and animals., (b) Inorganic compounds: Compounds obtained from non-living things, such as rocks,, minerals, etc., are known as inorganic compounds. For example: chalk, washing powder,, sodium hydroxide, etc.