Page 1 :
Aim, , To determine the density of solid (denser than, water) by using a spring balance and a, measuring cylinder., , Theory, , 1. Density: The density of a substance is, defined as the mass per unit volume,[D=, +1, , V, , Here, D = Density of the body, M = Mass of the body, , V = Volume of the body., , 2. S.. unit of density = Kgnv? or Kg/n?, c.g.s. unit of density = g/cm or g cm, , 3. Floating bodies: The density of water is 1, g/cm® (c.g.s. system) and 1000 kg/m (S11., system)., , 4. Case (a) If the density of a body is more, than 1 g/cm? or 1000 kg/m* then the body, will sink in water., , 5. Case (b) If the density of a body is less than, 1 g/cm? or 1000 kg/m® then the body will, float on water., , 6. Case (c) If the density of a body is same i.e., 1 g/cm? or 1000 kg/m* then the body will, half float and half submerge in water.
Page 2 :
6. Case (C) IT the density of a boay Is same 1.e., 1 g/cm® or 1000 kg/m then the body will, half float and half submerge in water., , , , Weight, , 1. The force due to the gravitational attraction, of the earth that acts on a body is called, weight., , 2. (Weight) Force = mass x acceleration., Force = mass x acceleration due to gravity, (9), , Force = mass X g, , ie Weight=mxg, 3. Weight of a body = Force on the body., 4. S.|. unit = Newton = 1 kg m/s?, , N =1 kgf= 1 kilogram force,, , ie g=9.8m/s?, 5. Weight is measured by spring balance.
Page 3 :
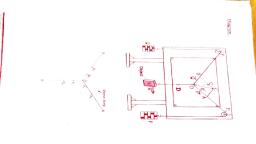
Materials Required, , A spring balance, a measuring cylinder, a beaker, with water, a metal bob (or any body that is, heavier than water and does not dissolve in, water), a cotton string, a stand (optional)., , Procedure, , 1. Tie a metal bob (or any solid) with the, string of cotton to the hook of the spring, balance. The spring balance should be, checked for any error. Let the zero error be, x, , 2. Hold the spring balance (or tie it to the, stand), suspended with the metal bob in air., Measure the weight of the bob. Let its, weight be ‘We', , 3. Pour the water in the measuring cylinder, and record the initial volume of water, let it, be ‘Vj1', , 4. Suspend the metal bob into the measuring, cylinder with water. The bob should not, touch the base, nor the sides of the, cylinder., , The water level rises, measure the, increased water level, let this volume be, Ve
Page 4 :
5. Record all your observations in the, observation table and do the calculation to, find the density of a given solid metal bob., , Spring, balance, , , , , , , , , , , , Observations, WEIGHT OF THE SOLID (METAL BOB) (M), Initial Reading of Final Reading of spring _ Weight of the Metal Bob, Spring balance, x balance with Metal Bob (W,) W-W,-x, 0 400 400
Page 5 :
1. Volume of water displaced by solid (metal, , bob) = 20 mL., 40.89, 2. Density of a solid (metal bob) = ml =, 2.04 g/cm?, , 1 mL of water = 1 cm?, , Result, , The density of given solid (Metal Bob) is 2.04, g/cm?, , Precautions, , 1. The spring balance should be sensitive., , 2. The zero error in the spring balance should, be recorded before it is used to find the, weight of solid., , 3. Record the readings carefully of both spring, balance and measuring cylinder by keeping, the level of eye and the mark of reading, same/parallel., , 4. The solid/metal bob should not touch the, bottom, or sides of the measuring cylinder.