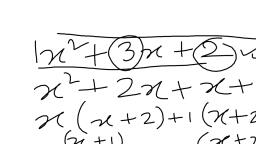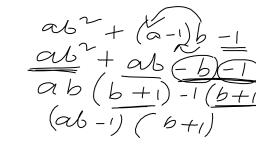Page 1 :
Question 3., , Define the term ‘allotropy’. Give q, reason why carbon exhibits allotropy., Answer 3:, , Allotropy : -Allotropy is occurrence of, an element in two or more forms Which, have the same chemical Properties but, differ in physical properties., , Diamond graphite,, buckminsterfullerene, , Carbon has a difference in atomic, , arrangement in the crystal struct, which results in allotropy. E, , Question 4., , Name two crystalline and &, , amorphous allotropes of carbon. a
Page 2 :
~usstion 4., , Name _ two crystalline and four, , amorphous allotropes of carbon., Answer 4:, , two crystalline allotropes of carbon, , diamond, graphite., , four amorphous allotropes of carbon, , coal,, , coke, lampblack, is, , a wood charcoal. Ee, , Question 5., , Compare the structure of the crystal of, diamond & graphite with special, reference to the reason for diamond, , Oe er OCCU eK ek Ce
Page 3 :
Question 5., , Compare the structure of the crystal of, diamond & graphite with special, reference to the reason for diamond, being the hardest natural substance, while graphite one of the softest., Compare the electrical & thermal, conductivity of the two crystalline, , allotropes of carbon., Answer 5:, , Structure of crystal of diamond :, +4, fe It is the hardest natural, , substance due to the stren, and uniformity of carbon., exhibits carbon covalent bo, i.e. tetrahedron structure. @, SS, It is a bad conductor, , electricity and heat bo, because of no availability free
Page 4 :
electricity and heat both,, because of no availability free, electrons to move., , Structure of graphite :, , 4 It is soft and greasy, because, , of the parallel layers of atoms —, of carbon held together by /, weak forces and forming a, hexagonal structure. 7, , a, It is a good conductor of, , electricity and heat, because, of the availability of free, electrons., , Question 6., , With reference to the structure of, two crystalline allotropes of car, , state why diamond is_ inert, unreactive while — graphite ei, comparably more reactive., , Answer 6:, , The structure of diamond is compact
Page 5 :
With reference to the structure of tne, two crystalline allotropes of carbon,, state why diamond is_ inert or, unreactive while graphite _—is, comparably more reactive., , Answer 6:, , The structure of diamond is compact, and hence it is unreactive., , Graphite has an open structure which, makes it more prone to chemical, attack., , Question 7., State the reasons for, , (a) Use of diamond - as an. item of, jewellery,, , (b) Use of graphite - f, , (i) as a lubricant for heated mac, parts, @, (i) as a lining for crucibles used, manufacture of high grade steel,, , (iii) as an electrode in electroplating., , Answer 7:, la) Use of diamond — Diqmond ics used