Page 1 :
Copyrights and Attributions, The lesson plans created by India Literacy Project are not for, profit or for sale. The content here has been compiled as open, source and can be used, reproduced, derived of or modified by, anyone as long as it is used for educational and non-profit use, and released as open source., , All images and videos used in this presentation are sourced from, the Internet and are owned and copyrighted as appropriate by the, original owners., India Literacy Project neither owns nor claims any copyright over, them., , ಉಚಿತ ವಿತರಣೆ, , Free Distribution, India Literacy Project
Page 2 : For Teachers, • ILP, a voluntary organization, has prepared this digital resource for free delivery., • Its objectives are:, o To enable Smart Class in school., o To provide digital resource to school students to help understand the concept in addition to a text book., o Teachers and children gain multi-dimensional learning experience., , • Its features are:, o This digital resource reinforces the concepts given in the textbook., o Using this digital resource in conjunction with a representative teaching method (Blackboard,, Experiments, Activities, and Others) will help you transform your classroom into a smart class., o PPT is a textbook application. When using this PPT, children should have a textbook, notebook and pen, ready., o 2-5 minute videos are used to interpret concepts to complement learning., o This content can be changed to support learning., o Teachers can use this digital tool to their advantage., o The ILP Science Experiment Kit can be used if you have one., o Teachers/ students can feel free to give their suggestions to improve these lesson plans., • India Literacy Project - email:
[email protected]
Page 3 :
Think…, Have you noticed changes in the, weather from morning to evening?, When do you feel cold and when do, you feel warm?, Why does this happen?
Page 4 :
Answer:, What do these pictures tell us?
Page 5 :
Answer:, What is the difference between the figures A and B?, A, , B, , 1, , 2, , Temperature
Page 6 :
Answer:, 1. What kind of clothes do we wear in different seasons?, 2. How do we find out how cold or hot an object is?
Page 7 :
Grade: 7, , Chapter: 4, , Heat, , Subject: Science
Page 9 :
Hot and Cold, We see that some objects are cold and, some are hot. We also know that some, objects are hotter than the others and, some are colder., How do we decide which object is, hotter than the other?, We decide by either touching the object, by hand or tasting it.
Page 10 :
Activity:, Take three small tubs/ containers. Label them as A, B and C. Put cold, water in mug A and hot water in mug B. Mix some cold and hot, water in mug C., Now dip your left hand in mug A and the right hand in mug B. After, keeping the hands in the two mugs for 2–3 minutes, put both the, hands simultaneously in mug C., Do both the hands get the same feeling?, (A), , Cold Water, , (B), , Hot Water, , (C), , Mixed Water, , Feeling Water in Three Containers., , Make sure that water is not so hot that you burn your hand.
Page 11 :
Observation:, Boojho says, “My left hand, tells me that the water in mug, C is hot and the right hand, tells me that the same water, is cold. What should I, conclude?”, , Boojho’s confusion shows that we cannot always rely on our, sense of touch to decide whether an object is hot or cold., Sometimes it may deceive us. Then, how do we find out how, hot an object really is?, , A reliable measure of the hotness of an object is its, temperature.
Page 12 :
Measuring Devices - Thermometer, What do we use these instruments for?, What are the units of measurement in, these instruments?, , Weight - mg/ g/ kg, Length - cm/ m/ km, Temperature is measured by a device called, thermometer., There are mainly two types of thermometers, a. Clinical or Medical thermometers, b. Laboratory thermometers, , Units of Temperature, Celsius (°C) or Fahrenheit (°F), , a, , b
Page 13 :
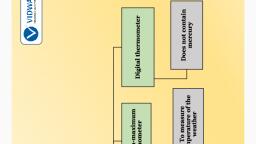
Measuring Temperature, Have you seen a thermometer? Recall that when you or someone, else in your family had fever, the temperature was measured by a, thermometer. The thermometer that measures our body, temperature is called a clinical thermometer., , Clinical Thermometer, Hold the thermometer in your hand and examine it carefully., If you do not have a thermometer, request a friend to share it with, you., A clinical thermometer looks like the one shown in the above, picture.
Page 14 :
Clinical Thermometer, When is clinical thermometer used? Who uses it?, , A clinical thermometer consists of a long, narrow, uniform glass, tube. It has a bulb at one end. This bulb contains mercury., Outside the bulb, a small shining thread of mercury can be, seen., If you do not see the mercury thread, rotate the thermometer a, bit till you see it. You will also find a scale on the thermometer., A clinical thermometer reads temperature from 35°C to 42°C; do, you know why?
Page 15 :
Reading a Clinical Thermometer, Wash the thermometer, preferably with an, antiseptic solution., Hold it firmly and give it a few jerks. The, jerks will bring the level of mercury down., Ensure that it falls below 35°C., Now place the bulb of the thermometer, under your tongue., After one minute, take the thermometer, out and note the reading. This is your body Correct Method of, Reading a Clinical, temperature., Thermometer, The temperature should always be stated, with its unit, °C.
Page 16 :
Temperature of Human Body, The normal temperature of human body is 37°C or 98.6°F. If it, goes above 37°C or 98.6°F, it indicates fever., , 37.0, , 0C, , Temperature of human body in Celsius, Scale (0C), , Temperature of human body in, Fahrenheit Scale (0F)
Page 17 :
Activity:, Measure the body temperature of some of your friends (at least, 10) with a clinical thermometer. Record your observations in a, table., Name, , Temperature (°C), , Is the body temperature of every person 37°C?, The temperature of every person may not be 37°C.
Page 18 :
Temperature in Different Objects, 100 0C - Temperature of Boiling point of Water, 0 0C - Temperature of Melting point of Ice
Page 19 :
Think…, Boojho got a naughty idea., He wanted to measure the, temperature of hot milk, using a clinical thermometer., Paheli stopped him from, doing so. Do you know why?, , What happens if we try to weigh 10 kg material, in a 1 kg weighting scale?
Page 20 :
Precautions, Precautions to be observed while using a clinical thermometer:, Thermometer should be washed before and after use,, preferably with an antiseptic solution., Ensure that before use the mercury level is below 35°C., Read the thermometer keeping the level of mercury along the, line of sight., Handle the thermometer with care. If it hits against some hard, object, it can break., Don’t hold the thermometer by the bulb while reading it., Caution: Do not use a clinical thermometer for, measuring the temperature of any object other, than the human body. Also avoid keeping the, thermometer in the sun or near a flame. It may, break.
Page 21 :
Laboratory Thermometer, How do we measure the temperature of other, objects?, For this purpose, there are other thermometers., One such thermometer is known as the laboratory, thermometer., , The range of a laboratory thermometer is, generally from –10°C to 110°C., , Caution: The laboratory thermometer should be kept upright not, tilted. The bulb should be surrounded from all sides by the, substance of which the temperature is to be measured. The bulb, should not touch the surface of the container.
Page 22 :
Activity:, Take some tap water in a beaker or a mug. Dip, the thermometer in water so that the bulb is, immersed in water but does not touch the, bottom or the sides of the container. Hold the, thermometer vertically., Observe the movement of mercury in the, thermometer. Wait till the mercury thread, becomes steady., , Note the reading. This is the temperature of water at that time., Compare the temperature of water recorded by each student, in the class., Repeat the activity with hot water., Are there any variations in the readings?
Page 23 :
Think…, Why does the mercury not fall or rise in a clinical thermometer, when taken out of the mouth?, Why does mercury levels decrease when a laboratory, thermometer is taken out of the fluid or the substance which is, being tested?, , Let us find out the difference between a clinical and a, laboratory thermometer.
Page 24 :
Difference Between a Clinical and a Laboratory Thermometer, Clinical Thermometer, , Laboratory Thermometer, , Used to measure the temperature, of the human body only., , Used to measure room, temperature and other substances., , A kink near the bulb prevents, mercury level from falling on its, own., , The level of mercury begins to fall, as soon as the thermometer is, taken out of the fluid or the, substance being tested., , The temperature is read after the, thermometer is taken out of the, mouth., , The temperature must be read, while the thermometer is in the, fluid or the substance being tested.
Page 26 :
Think…, Boojho wonders why the, level of mercury should, change at all when the bulb, of the thermometer is, brought in contact with, another object?
Page 27 :
Caution, , There is a lot of concern over the use of mercury in thermometers., Mercury is a toxic substance and is very difficult to dispose of if a, thermometer breaks. These days, digital thermometers and, thermal scanners are available which do not use mercury.
Page 28 :
Answer:, Why does a clinical thermometer measure from 35 °C to 42 °C?, The element present in clinical thermometers is ___________., The sense organ that senses heat is _______., _______ ________ is required to measure room temperature., Name the substances which are in solid, liquid and gaseous, state in room temperature., 6. The substance that exists in all three states of matter – solid,, liquid and gas is ___________., 7. The average normal temperature of a human body is _______., 8. Human body temperature increases with the increase in, atmospheric temperature. True/ False., 1., 2., 3., 4., 5.
Page 29 :
Think…, How do we feel when we touch, ice?, , How do we feel when we touch a, cup of hot coffee?
Page 30 :
Flow of Heat, You might have observed that a frying pan becomes hot when, kept on a flame., It is because the heat passes from the flame to the utensil., When the pan is removed from the fire, it slowly cools down., Why does it cool down?, The heat is transferred from the pan to the surroundings., So you can understand that in both cases, the heat flows from, a hotter object to a colder object.
Page 31 :
Think…, Why does a spoon if kept inside a, vessel of boiling Rasam gets heated?, , It is always cooler on top of a hill., Why?, , How does the heat of the sun reach us?
Page 32 :
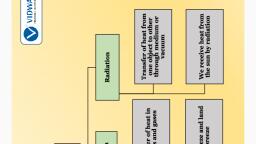
Transfer of Heat, Modes of Heat Transfer, , Conduction, Convection, Radiation
Page 33 :
Conduction, The process by which heat is transferred from the hotter end to, the colder end of an object is known as conduction. In solids,, generally, the heat is transferred by the process of conduction., , Conduction of heat occurs when there is an increase in the, temperature of molecules; they vibrate, and this vibration and, movement passes the heat energy to the surrounding molecules.
Page 35 :
Answer:, Answer the following questions after doing the experiment “Heat, Transfer in Solids”., What happens to the shoe tack nails?, Do these nails begin to fall? Which nail falls the first?, Do you think that heat is transferred from the end nearest to, the flame to the other end?
Page 36 :
Convection, When water is heated, the water near the, flame gets hot. Hot water rises up., The cold water from the sides moves down, towards the source of heat., This water also gets hot and rises and water, from the sides moves down., This process continues till the whole water, gets heated. This mode of heat transfer is, known as convection., , Convection of Heat, in Water, , Molecules can move in liquids and gases. Heated molecules, expand and rise. The colder molecules descend.
Page 38 :
Atmospheric Temperature, Are room temperature and atmospheric temperature one and the, same? Temperature depends on the following three factors., The earth receives heat through, radiation from the sun’s rays. The, landmass of the earth gets heated. This, is radiation., Hot landmass when comes in contact, with air, transfers heat to the air. This is, conduction., Hot air rises up and cool air on top, descends down to the ground. This is, convection., , Radiation, Convection, Cool Air, , Hot Air, Conduction, , Sun does not heat up the air in the earth’s atmosphere., Temperature decreases with the decrease in altitude.
Page 39 :
How does air move?, Air expands when heated and exerts less pressure., Cool air contracts and exerts more pressure., When the air pressure changes, the air flows from a high, pressure area to a low pressure area., The greater the difference in air pressure in the atmosphere,, the faster the air flows., In hurricanes, the pressure difference in the air from one area, to the other is much greater., , Higher Air, Pressure, , Hot Air – Low Pressure, , Lower Air, Pressure, , Cool Air – High Pressure
Page 40 :
Convection in Coastal Areas, People on the coast of our country do, not use wool., They do not experience cold. Why?, The land and the sea water get heated, after receiving the solar radiation., Land surfaces absorb much more solar, radiation than water., The people living in the coastal areas, experience an interesting, phenomenon., , Sea breeze during the day Land breeze during the night
Page 41 :
Convection in Coastal Areas - Day, During the day, the land gets heated faster than the water. The air, over the land becomes hotter and rises up., The cooler air from the sea rushes in towards the land to take its, place., The warm air from the land moves towards the sea to complete the, cycle. The air from the sea is called the sea breeze., , Hot, Air, High Pressure, , Cool, , Low Pressure, , Sea Breeze During the Day
Page 42 :
Conduction in Coastal Areas - Night, At night it is exactly the reverse. The water cools down more slowly, than the land. So, the cool air from the land moves towards the sea., This is called the land breeze., To receive the cooler sea breeze, the windows of the houses in, coastal areas are made to face the sea., , Air, , Hot, , Cool, ತಂಪು, , Low Pressure, High Pressure, , Land Breeze During the Night
Page 43 :
How does the sun’s heat reach the earth?, When we come out in the sun, we feel warm. How does the, heat from the sun reach us?, It cannot reach us by conduction or convection as there is no, medium such as air in most part of the space between the earth, and the sun.
Page 44 :
Radiation, From the sun the heat comes to us by another process known, as radiation., The transfer of heat by radiation does not require any medium., It can take place whether a medium is present or not.
Page 46 :
Conductors and Insulators, The materials which allow heat to pass through, them easily are conductors of heat., For example: aluminium, iron and copper., The materials which do not allow heat to pass, through them easily are poor conductors of, heat such as plastic and wood., For example: plastic, wood, water and air., Poor conductors are known as insulators. The, water and air are poor conductors of heat., Then, how does the heat transfer take place in, these substances?
Page 47 :
Kinds of Clothes We Wear in Summer and in Winter, What kind of clothes do we wear in summer and in winter?, , Wool is a poor conductor of heat. Air trapped between wool fibres, prevents the flow of heat from our body to the cold surroundings.
Page 48 :
Answer:, 1. Boiled milk cooling down is an example for ___________., 2. We feel cold when we touch ice because, the heat from our, body being higher in amount moves to the ice. True / False, 3. Write the correct order of the following ways by, which heat is transferred through the atmosphere:, , 1. Conduction, 2. Radiation, 3. Convection, 4. People in Mangaluru do not wear woollen clothes. Why?
Page 49 :
Dispersion of Light, 1. Have you seen a rainbow?, 2. How many colours are there in a rainbow?, 3. When do we see a rainbow?
Page 51 :
Effect of Heat on Colours, You know that in summer we prefer light-coloured clothes and in, winter we usually wear dark-coloured clothes. Why is it so? Let us, find out., Dark surfaces absorb more heat and, therefore, we feel, comfortable with dark coloured clothes in the winter., , Light coloured clothes reflect most of the heat that falls on, them and, therefore, we feel more comfortable wearing them, in the summer., What happens if you wear a black dress during the summer?, Also, what happens if you wear white clothes in winter?
Page 53 :
Trapped Layers, We often use electricity and fuels like coal and wood to keep, our houses cool or warm. Is it possible to construct buildings,, that are not affected much by heat and cold outside?, This can be done by constructing outer walls of buildings so that, they have trapped layers of air., One way of doing this is to use hollow bricks, which are, available these days.
Page 55 :
Keywords, Celsius scale, Conduction, Conductor, Convection, Insulator, Land breeze, Radiation, Sea breeze, Temperature, Thermometer
Page 56 :
Summary, Our sense of touch is not always a reliable guide to the degree, of hotness of an object., Temperature is a measure of the degree of hotness of an object., Thermometer is a device used for measuring temperature., Clinical thermometer is used to measure our body temperature., The range of this thermometer is from 35°C to 42°C. For other, purposes, we use the laboratory thermometers. The range of, these thermometers is usually from –10°C to 110°C., The normal temperature of the human body is 37°C., The heat flows from a body at a higher temperature to a body, at a lower temperature. There are three ways in which heat can, flow from one object to another. These are conduction,, convection and radiation.
Page 57 :
Summary, In solids, generally, the heat is transferred by conduction. In, liquids and gases the heat is transferred by convection. No, medium is required for transfer of heat by radiation., The materials which allow heat to pass through them easily are, conductors of heat., The materials which do not allow heat to pass through them, easily are called insulators., Dark-coloured objects absorb more heat than the lightcoloured objects. That is the reason we feel more comfortable, in light-coloured clothes in the summer., Woollen clothes keep us warm during winter. It is so because, wool is a poor conductor of heat and it has air trapped in, between the fibres.
Page 58 :
Exercise:, Paheli told us how to use a thermometer. The order, has been modified while writing. Rearrange the steps, in the correct order., 1. Now place the bulb of the thermometer under your tongue., , 2. Hold it firmly and give it a few jerks., 3. After one minute, take the thermometer out and note the reading., , 4. The jerks will bring the level of mercury down. Ensure that it falls, below 35°C., 5. Wash the thermometer, preferably with an antiseptic solution., 6. This is your body temperature. The temperature should always be, stated with its unit, °C.
Page 59 :
Exercise:, 1. State similarities and differences between the laboratory, thermometer and the clinical thermometer., 2. Give two examples each of conductors and insulators of heat., 3. Fill in the blanks :, a. The hotness of an object is determined by its __________., , b. Temperature of boiling water cannot be measured by a, c. _____________ thermometer., d. Temperature is measured in degree ______________., e. No medium is required for transfer of heat by the process, of __________.
Page 60 :
Exercise:, e. A cold steel spoon is dipped in a cup of hot milk. Heat is, transferred to its other end by the process of ___________., , f., , Clothes of ______________ colours absorb more heat, better than clothes of light colours., , 4. Match the following :, (i) Land breeze blows during, , (a) summer, , (ii) Sea breeze blows during, , (b) winter, , (iii) Dark coloured clothes are, preferred during, , (c) day, , (iv) Light coloured clothes are, preferred during, , (d) night
Page 61 :
Exercise:, 5. Discuss why wearing more layers of clothing during winter, keeps us warmer than wearing just one thick piece of clothing ., 6. Look at the figure beside, and mark where the heat, is being transferred by, conduction, by convection, and by radiation.
Page 62 :
Exercise:, 7. In places of hot climate it is advised that the outer walls of, houses be painted white. Explain., 8. One litre of water at 30°C is mixed with one litre of water at, 50°C. The temperature of the mixture will be;, (a) 80°C, , (b) more than 50°C but less than 80°C, , (c) 20°C, , (d) between 30°C and 50°C
Page 63 :
Exercise:, 9. An iron ball at 40°C is dropped in a mug containing water at, 40°C., a. The heat will, b. flow from iron ball to water., c. not flow from iron ball to water or from water to iron ball., d. flow from water to iron ball., e. increase the temperature of both., 10. A wooden spoon is dipped in a cup of ice cream. Its other end, a. becomes cold by the process of conduction., b. becomes cold by the process of convection., c. becomes cold by the process of radiation., d. does not become cold.
Page 64 :
Exercise:, 11. Stainless steel pans are usually provided with copper bottoms., The reason for this could be that, a. copper bottom makes the pan more durable., b. such pans appear colourful., c. copper is a better conductor of heat than the stainless, steel., d. copper is easier to clean than the stainless steel.
Page 65 :
Extended Learning — Activities and Projects, 1. Go to a veterinary doctor (a doctor who treats animals)., Discuss and find out the normal temperature of domestic, animals and birds., 2. Wrap a thin paper strip tightly around an iron rod. Try to burn, the paper with candle while rotating the iron rod continuously., Does it burn? Explain your observation..
Page 66 :
Do you know this?, , The Celsius scale was devised by a Swedish astronomer, Anders, Celsius in 1742. Strangely, he fixed the temperature of the, boiling water as 0°C and of freezing water as 100°C. However,, this order was reversed very soon.