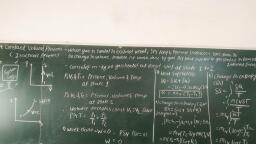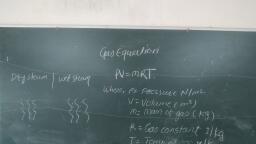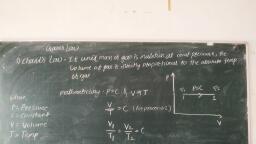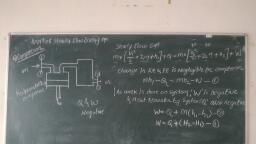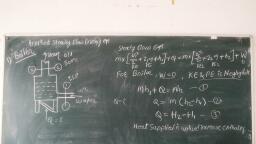Page 1 :
Fundamentals of Thermodynamics, , , , , , , , , , , , , , Syllabus :, , processes and cycles., , enthalpy and physical concept of entropy., , Engine, Heat Pump and Refrigerator., , , , 1.1 Basic concepts - Concepts of pure substance, types of systems, properties of systems, Extensive and, Intensive properties, flow and non flow process, specific volume, temperature, density, pressure,, , 1.2 Energy - Work, Heat Transfer and Energy Thermodynamic definition of work and heat, Difference, between heat and work, Potential Energy, Kinetic Energy, Internal Energy, Flow Work, concepts of, , 1.3. Laws of Thermodynamics - Zeroth Law, First law of Thermodynamics, Second Law of Thermodynamics Kelvin Planks, Clausius statements and their equivalence, Reversible and irreversible processes, Factors, making the process irreversible, reversible carnot cycle for heat engine and refrigerator.., , 1.4. Application of Laws of Thermodynamic Steady Flow Energy equation and its application to boilers,, engine, nozzle, turbine, compressor & condenser. Application of Second. law of Thermodynamics to Heat, , , , , , 1.1 Basic concept, , , , Thermodynamics :, , The science which deals with heat interaction or, transformation of energy between the material. i.e. heat, and work concern with, , 1. Concept of energy, 2. The conversion of one form of energy into another, , 3. Properties of working substance or media used to, obtained the energy conversion, , Unit : An orbitary constant amount of quantity to be, measure, which is assigned a value of unity., , Length : It is a dimension and meter is of its unit., , Time : It is in second and define as. 1/86400 part of a mean, solar days. 1N-m = 1 Joules, , (J or N-m Mechanical equivalent of heat = 427 kcal/kg°K), , 1.2 Concept of Pure Substance, , (MSBTE - S-09, S-13), , EEA, EH), , , , Q, What is pure substance 7, Q. Define working substance with example., , , , , , , , , , Pure Substance, , , , Definition : A substance that has a fixed chemical, composition throughout its mass is called Pure Substance, , , , , , — For example, water, nitrogen, helium, carbon-dioxide, are all pure substance., , — Pure substance does not have to be a single chemical, element or compound. A mixture of various chemical, elements or compound also qualifies as a pure, substance as long as a mixture is homogeneous., , — Eg. Air is a mixture of several gases but it is often, consider as pure substance because it has a uniform, chemical composition. A tpixture of coil with water is, not a pure substance,, , Working substance, , , , Definition : A substance which is capable of absorbing and, rejecting heat during the process is called Working, substance,, , , , , , , , — The working substance is most work producing and, absorbing devices in gas and vapour, or vapour and, , liquid., — Freon, Ammonia is the examples of working, substance., , Scanned with CamScanner
Page 2 :
wv Vrerial Enyineoting (MSATE) 1 Fundamentals of Thonn jinn,, {QA Thermodynamic System Q, _Diltorentiata between open system, 610860 systorn |, , (NSEYE © 8.07, 8-00, W009, 8-11, Weld, eth, We 16) and an isolated syatem with one axvample Of oach,, s Fs OT, HOD, WOO, HT 1, Weld, Seth, We, , Qo Detine the wan thormodyoamio ayaton, ETAT, , Q, Lint differant typos of systems., , ae, , {Q_ Datina ayatory ASA Q, Explain difforant typos of thormodynamic systerns,, , ‘Q Reyesent aysten, sutounding and boundary with Tay, aihtadle example, los |, , Q wt ma Q. Givo clanaitication of system with examples,, , a Soting thermodynamic system, — CET, , Sefore discussing types the following terms are known | Q. Explain Closed system and Open system. Give ty,, , (a) System oxamplos of each,, , Bg, , Q, What is isolated system 7, Q. Givo classification of system and explain each with, suitable examplo., , | Gefinition : The term system is defined as a specified, j mM Where traasfers of energy or mass are to be, , , , , j, , , , There are three types of system :, , OR, , , , , , | Dafinition : it is nothing but a model of equipment to be! Classification of System, | anatsed. In the system volume need not constant., , , , , , , , , , , , (a) Closed system, , Boundary, . (b) Open system, , Surrounding, Fig, 1.2.1 : System and surrounding (c) Isolated system, , , , , , , , , , , , , , , , b) Bound., (b) Boundary Fig C1.1 : Classification of System, , ‘a. Represent boundary with suitable example. =) (a) Closed system, , , , , , , , , , , , Definition : The envelope enclosing the system, which may|| — In this system only transfer of energy but no transfer, be rec! or hypothetical (imaginary) is known as boundary of mass across the boundary., | of system., f sys Eg. (1) Piston and cylinder without valve., , , , It is rigid or flexible., is rigid or (2) Refrigeration system., , dint air, (c) Surrounding (3) Boiling of water in a closed vessel, , , , , , Q. Represent surrounding with suitable example. Eg, , , , , , Definition : The region (space or matter) outside the, system., , , , ‘, , , , (d) Universe, , , , , , Definition : When system and surrounding put together is, known as universe., , , , , , , , , , , , , , , , 1.2.2 Types (Classification) of System, , (MSBTE - S-07, S-08, S-09, W-09, W-10, S-11,, , Fig. 1.2.2 : Closed or non-fl, W-11, S-14 W-14, S-15, S-17, W-17) muowsysiom, , , , — Fig. 1.2.2 shows a system is gas filled in piston, cylinder. If heat is added to the system, the g25 wit, , , , , , Q. Give classification of system. Ea, , , , , , weet, , Scanned with CamScanner
Page 3 :
Thermal Engine (MSBTE), , , , , , expand and work will be done by the gas on the, piston, Thus heat and work cross the boundary of the, system hence this system is known as closed system., (b) Open system, = Inthis system, both transfer of mass as well as energy, may cross the boundary., E.g. (i) Steam turbine, (ii) Steam Nozzle, (iii) Diffuser, , (c) Isolated system, , — In this system cannot transfer of either mass or energy, with the surrounding. It is a purely theoretical system., , - E.g. Tea present in a thermos flask. In this the heat and, the mass of the tea cannot cross the boundary of the, thermos flask. Hence the thermos flask is an isolated, , system., Mass in/High pressure, , |, 1, , , , , ee, System boundary, , Ww, , , , Heat one., , Mass out / Low pressure, , Fig. 1.2.3 : Open or flow system, 1.2.3 Property, , (MSBTE - S-09), , i, Q. Define property of system. Eg, , , , , , , , Q Emizn he ‘flewnp suas eet =, thermodynamics: : ES, , {) Intenseve property §=Ci) Extersive orccery, , , , 'Q. Explain Intersive an, , q, 4, , , , , , , , Fig. C1.2 : Classification of procertes, , {a) Intensive Property, , , , Definition : Intensive Property & &, | which do not cepend up, , , , , , , eg. pressure, temperature,, , , , specific enthalpy etc., specific volume, define as volume pe:, , density, , , , define as mass per uni, , , , {b) Extensive Property, , , , Definition: Extensive Property is, which depends on mass, , os the orcrerey, , , , , , , , , , Definition : Property is defined as any observable or, measurable characteristic of the system or the variables, , , , , , which determine the state are properties or parameter., , , , - The basic properties of the system are pressure,, volume, temperature, enthalpy, entropy, internal, energy., , 1.2.4 Classification of Properties, (MSBTE - S-08, S-12, W-14, S-15, S-16, S-18, S-19), , Q. Define ‘Intensive property’ and ‘Extensive ee, , Give two example,, , Q. Define intensive property. Give two exampls. ra, , , , , , e.g. Total volume, enthalpy, entropy, etc., , , , Note: The ratio of any extensive crocerty of 2 sistem |, the mass of the system is caifed as an xem |, specific value of the propery (aso own as /, , intensive property)., , , , , , , , , , 1.2.5 State (MSBTE ~ SOS, Sst) WES tT?, , Q. Define State. SSS, , Q. Explain the term retated to thermocynames . Say., Yee, , Scanned with CamScanner
Page 4 :
Thermal Engineering (MSBTE) !, , , , Definition : It is defined as the exact condition of a|, substance, to define it ot least two properties are known., Even if one property is changes then the state of the, Substance changes., , , , OR, Definition : It is the condition of the system (when the, System is in thermodynamic equilibrium) at any particular!, moment which can be identified by the statement of its, Properties such as pressure, temperature, volume, etc., , , , , , , , , , 1.2.6 Process, (MSBTE - S-05, S-15, W-15), , E2Stz8), , Q. Explain the term related to thermodynamics : Process., Sab, , , , Q. Define process., , , , Fundamentals of Thermodynaming, , — Process is assumed to be occurs at such a Sufficieny, slow rate such that the property of the intermediarg, state is infinitesimally small, and then every State, passed through by the system will be in equilibrium, condition can be describe and fixed., , 1.2.10 Non Flow Process, , , , Definition : The process occurring in closed systems Whieh, do not permit the transfer of mass across their boundary, , , , are known as non flow processes. |, ee, , — In this system energy crosses the system boundary j,, the form of heat and work, but there is no mass floy,, into or out of the system., , 1.2.11 Flow Process, , , , , , , , Definition Process is defines as a_ transition, (transformation) in which change from one initial state to}, final state or onother state., , , , , , , , OR, , — It is a path joining succession of the state passed, through by a system. Process is said when one of the, Properties is constant like value of pressure is constant, then called constant pressure process., , 1.2.7 Cycle (MSBTE - W-17), , Definition : Cycle is defined os a process or combination of, processes so conducted that the initial and final state of|, the system are the same., , , , Q. Define thermodynamic cycle ., , , , , , , , , , , , Definition : The process occurring in open systems which, permits the transfer of mass to and from the system are, known as flow process., , , , — In these processes mass enters the system and leaves, after enhancing energy., , — The flow process may be steady flow and non steady, flow processes., , 1.2.12 Point-Function, (MSBTE - W-09, S-11, S-12, S-14, S-17), Cen, , Q. Define point function., , Q. Explain point function., , , , OR, Thermodynamic cycle, , , , Definition : When the process or processes performed on|, the system are in such a way that the final state is, identical with initial state, it is known as thermodynamic, cycle or cyclic process., , 1.2.8 Path, , Thermodynamic system passing through a series of, state constitute a path., , 1.2.9 Quasi-static Process, (Reversible Process), , , , , , , , Definition : The fixing of the path followed by the process, such a process is reversible is known as quasl-static, process., , , , , , The each Property has a single value at each state. ie., Properties of system depend upon state of system, therefore function. €.g. Internal energy, Flow work,, Enthalpy, Volume, Entropy,, volume,, , 2, , Jav =, , 1, , Pressure, temperature, , V2—V, (an exact differential), , Refer Fig. 1.2.4. Let the system undergoes a chans®, from a point A to B. This change can take place ¥®, path A or path B. Hence a Property does not depe™d, , on the path. So it is a point function or a state, function,, , , , Scanned with CamScanner
Page 5 :
Thermal Engineering (MSBTE), , , , , , Fig. 1.2.4, 1.2.13 Path Function, , (MSBTE - W-09, S-11, S-12, S-14, W-14, S-15, S-17), , , , Q. Define path function., , , , , , , , , , A property can be defined as a quantity whose integral, , , , , , is zero,, Work transfer = J Pdv, 2 2, Jap = Jap=P,-p,, 1 1, P, 1, c, A, 2, v, Fig. 1.2.5, , — The thermodynamic quantities which are dependent, on path followed between the two end state of the, process and independent on the two end states are, called path functions., , - Heat and work transfer processes are the path, function., , - Heat and work are inexact differential. Their change, , cannot be written as difference between their end, state., , 2, a # Q-Q, , 2, Jaw « w,-w,, 1, , Difference, Function, , between Point Functlon and Path, , (MSBTE - W-12, W-16, S-17), , , , , , , , Q. Differentiate between point function and path function., , , , , , , , , , , , , Fundamentals of Therm, , , , Q. Define point function and path function with two, examples af each ., , , , , , , , , , , , , , , , , , , , , , , , Q. Dofino path function and point function., , Sr., , No Point function Path function, , 1, | If fora given state, there | Properties which are, Is a definite value for | not thermodynamic are, each property then the | called path functions, property Is known as a, point function., , 2. | Point function are exact | Path functions are, differentials. inexact differentials,, , 3. | Fore.g. pressure For e.g. work and heat., temperature, volume, entropy and enthalpy, etc., , 4. | These type of These are dependent, thermodynamic on the path taken from, properties only depend | one state to the other, on end states, but are Independent of, , other states., 1.2.14 Definitions and Unit Conversion, Volume, Definition : Volume is defined as the amount of space!, that a substance or steam is occupied., , The Volume is the quantity of three dimensional space, , enclosed by a closed surface and it is expressed in m’ or, , 3, cm, also volume express in liter., , Hiter = 107'm’, , Specific volume, , , , Definition :, , , , Specific volume is defined as ratio of}, , substance volume to its mass or volume per unit mass of a}, , , , The standard unit Is the m /kg, , v, , Tomporature, , It Is the measure of hotness or coldness of a body, define as “Measure of the ability of the system” to transfer, , energy by, temperature can determine in which dire, , conduction or, , radiation, , process, The, tion heat flow, , , , , , Scanned with CamScanner