Page 1 :
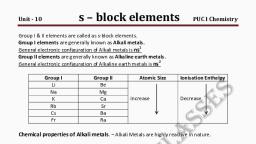
S-BLOCK ELEMENTS, , , , It includes elements of group 1 and 2., General outer electronic configuration:ns1-2, , GROUP 1 ELEMENTS: ALKALI METALS, Trends in Properties, 1. Electronic Configuration: ns1, 2. Atomic and Ionic Radii:, The atomic and ionic radii of alkali metals increase on moving down the group., Reason: The electrons are added to higher shells on moving down the group., 3. Ionization Enthalpy:, The ionisation enthalpy decreases down the group., Reason: This is due to the increase in size down the group., The alkali metals have low ionisation enthalpies., 4. Hydration Enthalpy:, The hydration enthalpies of alkali metal ions decrease with increase in ionic, sizes., Li+ > Na+ > K+ > Rb+ > Cs+, Reason: The smaller the ion, the more is the extent of hydration and higher is the, enthalpy of hydration., Li+ has maximum degree of hydration and so lithium salts are mostly hydrated,, e.g., LiCl· 2H2O., 5. Physical Properties:, All the alkali metals are silvery white, soft and light metals., Because of the large size, alkali metals have low density. The Density generally, increases down the group., Exception: Potassium is lighter than sodium., They have low melting and boiling points., Reason: Weak metallic bonding due to the presence of only a single valence, electron in them.
Page 2 :
Flame Colouration: The alkali metals and their salts impart characteristic colour, to a flame., Reason: This is because the heat from the flame excites the outermost electron, to a higher energy level. When the excited electron comes back to the ground, state, they emit energy in the form of radiations., , This property is used for the detection of alkali metals and is called Flame Test., , Question: Why alkali metal shows flame coloration?, An: This is because the heat from the flame excites the outermost electron, to a higher energy level. When the excited electron comes back to the, ground state, they emit energy in the form of radiations., , Elements like K, Rb &Cs shows photoelectric effect. This is due to their low, ionisation enthalpies. So they are used as electrodes in photoelectric cells., , Question: Why alkali metal like K, Rb &Cs shows photoelectric effect?, An: This is due to their low ionisation enthalpies., Question: Alkali metal like K, Rb &Cs is used as electrodes in, photoelectric cells. Give reason., An: Due to their low ionisation enthalpies, they shows photoelectric effect.
Page 3 :
Chemical Properties, (i) Reactivity towards air:, Alkali metals tarnish in dry air., It is due to the formation of their oxides., These oxides in turn react with moisture to form hydroxides., They burn vigorously in oxygen forming oxides., o Li forms monoxide., o Sodium forms peroxides., o K, Rb and Cs form superoxides., , Note: The superoxide O2– ion is stable only in the presence of large cations, such as K, Rb, and Cs., The oxides and the peroxides are colourless when pure, but the, superoxides are yellow or orange in colour., The superoxides are also paramagnetic., Li reacted with nitrogen of air to form the nitride, Li3N., , (ii) Reactivity towards water:, The alkali metals react with water to form hydroxide and dihydrogen., , The hydroxides are white crystalline solids., The alkali metal hydroxides are the strongest of all bases., (iii) Reactivity towards dihydrogen:, The alkali metals react with dihydrogen at high temperature to form ionic, hydrides., They have high melting point.
Page 4 :
(iv) Reactivity towards halogens:, Alkali metals react with halogens to form halides of the general formula, MX., They are high melting, colourless crystalline solids., The melting and boiling points always follow the trend: fluoride > chloride >, bromide > iodide., All these halides are soluble in water., The low solubility of LiF in water is due to its high lattice enthalpy whereas, the low solubility of CsI is due to smaller hydration enthalpy of its two ions., (v) Reducing nature:, The alkali metals are strong reducing agents., Lithium, being the most and sodium, the least powerful., The reducing character increases down the group from Na to Cs because, of the decrease in ionisation energy, , (vi) Solutions in liquid ammonia:, The alkali metals dissolve in liquid ammonia giving deep blue solutions, which are conducting in nature., o The blue colour of the solution is due to the ammoniated electrons., o Ammoniated electrons absorb energy in the visible region of light., The solutions are paramagnetic., In concentrated solution, the blue colour changes to bronze colour and, become diamagnetic.
Page 5 :
(vii)Salts of Oxo-Acids:, Oxo-acids are those in which the acidic proton is on a hydroxyl group, with an oxo group attached to the same atom., , The alkali metals form salts with all the oxo-acids., E.g:Na2CO3,NaHCO3,K2CO3,Na2SO4, They are generally soluble in water., They are thermally stable. Thermal stability increases down the group., Question: Why Li2CO3 is not stable to heat?, An: Due to smaller size, lithium ion could not stabilize a larger anion like, carbonate (CO32-)., , ANOMALOUS PROPERTIES OF LITHIUM, The anomalous behaviour of lithium is due to the:, (i) Smaller size, (ii)High ionisation enthalpy., (iii) High charge/ radius ratio (polarising power), Difference between Lithium and other Alkali Metals, , , , , , , , , Lithium is much harder., The melting point and boiling point are higher than the other alkali metals., Lithium is least reactive but the strongest reducing agent among all the alkali, metals., Lithium reacts with nitrogen to form a nitride, Li3N., LiCl is deliquescent and crystallises as a hydrate, LiCl.2H2O., Li reacted with air to form oxide while others form peroxide or superoxide., LiF and Li2O are comparatively much less soluble in water than the, corresponding compounds of other alkali metals.
Page 6 :
DIAGONAL RELATIONSHIP BETWEEN Li and Mg, The diagonal relationship between Li and Mg is due to their similarity in size., , , , , , , , Both Li and Mg are harder than other elements in the respective groups., Both Li and Mg form a nitride by direct combination with nitrogen., Both Li and Mg react with oxygen to form normal oxides Li2O and MgO, respectively., Lithium and magnesium react slowly with water., Both LiCl and MgCl2 are deliquescent and crystallise as LiCl·2H2O and, MgCl2·8H2O., The carbonates of lithium and magnesium are not stable., , Uses of Alkali Metals, , , , , , , , , , , Lithium metal is used to make useful alloys., i. White metal: It is an alloy with lead which is used to make bearings for, motor engines., ii. Alloy with aluminium to make aircraft parts., iii. Alloy with magnesium to make armour plates., Lithium is also used to make electrochemical cells., Lithium is used in thermonuclear reactions., Liquid sodium metal is used as a coolant in nuclear reactors., Sodium and Potassium has a vital role in biological systems., Potassium chloride is used as a fertilizer., Sodium Hydroxide and Potassium hydroxide is used in soaps., Caesium is used in photoelectric cells.
Page 7 :
SOME IMPORTANT COMPOUNDS OF SODIUM, 1. Sodium Carbonate (Washing Soda), Na2CO3·10H2O, Preparation (SOLVAY PROCESS):, Step 1:CO2 is passed through a solution of ammonia in water to form ammonium, carbonate., , Step 2: CO2 is passed through a solution of ammonium carbonate to form ammonium, hydrogencarbonate., , Step 3: It is then treated with NaCl to form Sodium Hydrogencarbonate., , Step 4: Sodium Hydrogencarbonate formed on heating gives sodium carbonate., , OR, [Prepared by passing CO2 gas under pressure into a strong sodium chloride solution, saturated with ammonia. Sodium Hydrogen Carbonate precipitated on heating gives, sodium carbonate], In this process NH3 is recovered when the solution containing NH4Cl is treated, with Ca (OH) 2., , Question:K2CO3 cannot be prepared by Solvay process. Give reason., An: This is because the solubility of KHCO3 is fairly large. Hence it does not precipitate, easily., Properties, Sodium carbonate is a white crystalline solid., It is readily soluble in water., It exists as a decahydrate, Na2CO3 .10H2O. This is also called washing soda., On heating, the Decahydrate loses its water of crystallization to form, monohydrate.
Page 8 :
Above 373K, the monohydrate becomes completely anhydrous and changes to a, white powder called Soda ash., The solution of Na2CO3 is alkaline due to hydrolysis., , Uses:, (i) It is used in water softening, laundering and cleaning., (ii) It is used in the manufacture of glass, soap, borax and caustic soda., (iii) It is used in paper, paints and textile industries, (iv) It is an important laboratory reagent., , 2. Sodium Chloride, NaCl (Common salt or Table salt), The most abundant source of sodium chloride is sea water., Preparation, 1. Common salt is generally obtained by evaporation of sea water., 2. It is also prepared by the crystallisation of brine solution. The crude salt is dissolved, in minimum amount of water and filtered to remove insoluble impurities. The solution is, then saturated with hydrogen chloride gas. Crystals of pure sodium chloride separate, out., Properties, High Melting point (1082K)., High solubility in water (36g in 100g of water)., Uses:, (i)It is used as a common salt or table salt for domestic purpose., (ii) It is used for the preparation of Na2O2, NaOH and Na2CO3.
Page 9 :
3. Sodium Hydroxide (Caustic Soda), NaOH, , Preparation, Sodium hydroxide is generally prepared commercially by the electrolysis of sodium, chloride in Castner-Kellner cell., A brine solution is electrolysed using a mercury cathode and a carbon anode. Sodium, metal discharged at the cathode combines with mercury to form sodium amalgam., , The amalgam is treated with water to give sodium hydroxide., , Properties, Sodium hydroxide is a white, translucent solid., It melts at 591K., It is readily soluble in water to give a strong alkaline solution., Crystals of sodium hydroxide are deliquescent., The sodium hydroxide solution at the surface reacts with the CO2 in the atmosphere to, form Na2CO3., , Uses:, (i) In the manufacture of soap, paper, artificial silk and a number of chemicals, (ii) In petroleum refining, (iii) In the purification of bauxite, (iv) In the textile industries, (v) For the preparation of pure fats and oils, (vi) As a laboratory reagent., , Sodium Hydrogencarbonate (Baking Soda), NaHCO3, Sodium hydrogencarbonate is known as baking soda because it decomposes on, heating to generate bubbles of carbon dioxide (leaving holes in cakes or, pastries and making them light and fluffy)., Preparation, It is prepared by saturating a solution of sodium carbonate with carbon dioxide.
Page 10 :
Properties, White crystalline powder., It is less soluble in water., Uses:, As a laboratory reagent., In baking powder., In fire extinguishers., As a mild antiseptic for skin infections., BIOLOGICAL IMPORTANCE OF SODIUM, Sodium ions participate in the transmission of nerve signals., Regulates the flow of water across cell membranes., Helps in the transport of sugars and amino acids into cells., BIOLOGICAL IMPORTANCE OF POTASSIUM, They activate many enzymes., Participate in the oxidation of glucose to produce ATP., With sodium it is responsible for the transmission of nerve signals.
Page 11 :
GROUP 2 ELEMENTS: ALKALINE EARTH METALS, The Group 2 elements (except beryllium) are known as, Alkaline earth metals., Trends in Properties, 1. Electronic Configuration: ns2, 2. Atomic and Ionic Radii:, The atomic and ionic radii of alkali metals increase on moving down the group., Reason: The electrons are added to higher shells on moving down the group., The atomic and ionic radii of the alkaline earth metals are smaller than those of, the corresponding alkali metals in the same periods., 3. Ionization Enthalpy:, The ionisation enthalpy decreases down the group., Reason: This is due to the increase in size down the group., The first ionisation energies of the alkaline earth metals are higher than those of, the corresponding alkali metals. This is due to their small size as compared to, the corresponding alkali metals., The second ionisation enthalpies of the alkaline earth metals are smaller than, those of the corresponding alkali metals., 4. Hydration Enthalpy:, The hydration enthalpies of alkaline earth metal ions decrease with increase in, ionic sizes., Be2+> Mg2+ > Ca2+ > Sr2+ > Ba2+, Reason: The smaller the ion, the more is the extent of hydration and higher is the, enthalpy of hydration., The hydration enthalpies of alkaline earth metal ions are larger than those of, alkali metal ions. Thus, compounds of alkaline earth metals are more extensively, hydrated than those of alkali metals, e.g., MgCl2 and CaCl2 exist as MgCl2.6H2O, and CaCl2· 6H2O while NaCl and KCl do not form such hydrates., 5. Physical Properties:, The alkaline earth meals are silvery white, lustrous and relatively soft, but, harder than the alkali metals., Alkaline earth metals have higher melting and boiling points than alkali metals., Reason: Stronger metallic bonding due to their smaller size., They have high electrical and thermal conductivities
Page 12 :
Flame Colouration: The alkaline earth metals and their salts impart, characteristic colour to a flame., Reason: This is because the heat from the flame excites the outermost electron, to a higher energy level. When the excited electron comes back to the ground, state, they emit energy in the form of radiations., , The flame test for Ca, Sr and Ba is helpful in their detection in qualitative, analysis and estimation by flame photometry., The electrons in beryllium and magnesium are too strongly bound to get excited, by flame. Hence, these elements do not impart any colour to the flame., Question: Why Be and Mg do not show flame coloration?, An: Due to their smaller size, the electrons are strongly bound to the, nucleus.So no excitation with the flame is possible., 6. Chemical Properties:, (i) Reactivity towards air:, Beryllium and magnesium are kinetically inert to oxygen because of the, formation of an oxide film on their surface., Powdered beryllium burns brilliantly on ignition in air to give BeO and Be3N2., Magnesium burns with dazzling brilliance in air to give MgO and Mg3N2., Calcium, strontium and barium are readily attacked by air to form the oxide and, nitride., Be, Mg and Ca forms monoxides., Sr and Ba form peroxides., BeO is essentially covalent in nature, while the oxides of other elements are, ionic in nature., BeO is amphoteric, while oxides of other elements are basic.
Page 13 :
(ii) Reactivity water:, Beryllium and magnesium are kinetically inert to water because of the formation, of an oxide film on their surface., Calcium, strontium and barium vigorously reacted with water to form, hydroxides., The solubility, thermal stability and the basic character of these hydroxides, increases down the group., Beryllium hydroxide is amphoteric in nature., (iii) Reactivity towards the halogens:, All the alkaline earth metals combine with halogen at elevated temperatures, forming their halides., BeF2 is prepared by the thermal decomposition of (NH4)2BeF4, BeCl2 is conveniently made from the oxide. BeCl2 is formed by passing chlorine, over a heated mixture of Beryllium oxide and carbon., Beryllium halides are covalent while all other halides of alkaline earth metals are, ionic in nature., Structure of BeCl2, In the solid state, BeCl2 has a polymeric chain structure., , In the vapour phase BeCl2 tends to form a chloro-bridged dimer which, dissociates into the linear monomer at high temperatures of 1200 K., , The tendency to form halide hydrates gradually decreases (for example,, MgCl2·8H2O, CaCl2·6H2O, SrCl2·6H2O and BaCl2·2H2O) down the group. It is, because of the decrease in hydration enthalpy due to the increase in size., (iv) Reactivity towards hydrogen:, All the elements except Be combine with hydrogen upon heating to form their, hydrides, MH2.
Page 14 :
BeH2 can be prepared by the reaction of BeCl2 with LiAlH4., , (v) Reactivity towards acids:, The alkaline earth metals readily react with acids liberating dihydrogen, ., (vi) Reducing nature:, Like alkali metals, the alkaline earth metals are strong reducing agents., They have large negative values of their reduction potentials., Their reducing power is less than those of their corresponding alkali metals., (vii) Solutions in liquid ammonia:, Alkaline earth metals dissolve in liquid ammonia to give deep blue black, coloured solutions., , The blue colour of the solution is due to the ammoniated electrons., Ammoniated electrons absorb energy in the visible region of light., (ix) Salts of Oxoacids:, The alkaline earth metals also form salts of oxoacids like carbonates, sulphates,, nitrates etc., (1)Carbonates:, o Carbonates of alkaline earth metals are insoluble in water. The solubility, of carbonates in water decreases down the group., o The thermal stability increases with increasing cationic size., o All the carbonates decompose on heating to give carbon dioxide and the, oxide., o Beryllium carbonate is unstable and can be kept only in the atmosphere of, CO2., (2) Sulphates:, o The solubility decreases down the group., o BeSO4 and MgSO4 are readily soluble in water.
Page 15 :
(3) Nitrates:, o All of them decompose on heating to give the oxide., , ANOMALOUS BEHAVIOUR OF BERYLLIUM, Be compounds are more covalent in nature., Be does not react with water even at high temperatures., BeO and Be(OH)2 are amphoteric., Maximum coordination number of Be is 4., DIAGONAL RELATIONSHIP BETWEEN Be and Al, Like aluminium, beryllium is not readily attacked by acids because of the, presence of an oxide film on the surface of the metal., The chlorides of both beryllium and aluminium have Cl– bridged chloride, structure., The hydroxides of Be and Al are amphoteric., Beryllium hydroxide dissolves in excess of alkali to give a beryllate ion,, [Be(OH)4]2– just as aluminium hydroxide gives aluminate ion, [Al(OH)4]–., SOME IMPORTANT COMPOUNDS OF CALCIUM, 1. Calcium Oxide or Quick Lime, CaO:, Preparation:, It is prepared on a commercial scale by heating limestone in a rotary kiln at, 1070-1270K., The carbon dioxide is removed as soon as it is produced to proceed the reaction, to completion., Properties:, Calcium oxide is a white amorphous solid., It has a melting point of 2870 K., On exposure to atmosphere, it absorbs moisture and carbon dioxide., , On adding water, it forms Slaked lime [Ca (OH) 2]. This process is called slaking, of lime.
Page 16 :
Mixture of quick lime with caustic soda is called Soda Lime., It is a basic oxide and so it combines with acidic oxides at high temperature., , Uses:, It is used for manufacturing cement., It is the cheapest form of alkali., It is used in the manufacture of sodium carbonate from caustic soda., It is used in the purification of sugar., It is used in the manufacture of dye stuffs., 2. Calcium Hydroxide (Slaked lime), Ca (OH)2:, Preparation:, It is prepared by adding water to quick lime., , Properties:, It is a white amorphous powder., It is sparingly soluble in water., The aqueous solution slaked lime is known as lime water., The suspension of slaked lime in water is known as milk of lime., When CO2 is passed through lime water, it turns milky due to the formation of, CaCO3., When excess of carbon dioxide is passed, milky colour disappears to form, calcium hydrogencarbonate., , Milk of lime reacts with chlorine to form hypochlorite, a constituent of bleaching, powder., , Uses:, It is used in the preparation of mortar, a building material., It is used in white wash due to its disinfectant nature., It is used in glass making., It is used in tanning industry., It is used for the preparation of bleaching powder., It is used for purification of sugar.
Page 17 :
3.Calcium Carbonate, CaCO3, It occurs in nature in several forms like limestone, chalk, marble etc., Preparation:, It is prepared by passing carbon dioxide through slaked lime., It is prepared by the addition of sodium carbonate to calcium chloride., Excess of carbon dioxide should be avoided since this leads to the formation of, water soluble calcium hydrogencarbonate., Properties:, It is a white fluffy powder., It is almost insoluble in water., When heated to 1200 K, it decomposes to form carbon dioxide., It reacts with dilute acid to liberate carbon dioxide., , Uses:, It is as a building material in the form of marble., It is used for the manufacture of quick lime., It is used as an antacid., It is used as mild abrasive in tooth paste., It is used in chewing gums., It is used in cosmetics., Calcium carbonate along with magnesium carbonate is used as a flux in, metallurgy., Specially precipitated CaCO3 is extensively used in the manufacture of high, quality paper., 4.Calcium Sulphate (Plaster of Paris), CaSO4·½ H2O, It is a hemihydrate of calcium sulphate., Preparation:, It is prepared by heating gypsum to 393K., Above 393 K, no water of crystallization is left and anhydrous calcium sulphate,, CaSO4 is formed. This is known as dead burnt plaster.
Page 18 :
It has a remarkable property of setting with water. When mixed with water it, forms a plastic mass that gets into a hard solid in 5 to 15 minutes., Uses:, It is used as plaster for bone fracture or sprain., It is used in dentistry., It is used in ornamental work., It is used for making statues and busts., CEMENT, Cement is an important building material., It was first introduced in England in 1824 by Joseph Aspidin., It is also called Portland cement.It resembles the natural lime stone quarried in, the Isle of Portland, England., Cement is a product obtained by combining a material, rich in lime, CaO with, other material such as clay which contains silica (SiO2) along with the oxides of, aluminium, Iron and Magnesium., Manufacture of Cement:, The raw materials for the manufacture of cement are limestone and clay., When clay and lime are strongly heated together, they fuse and react to form, cement clinker., This clinker is mixed with 2-3% by weight of gypsum to form cement., Thus important ingredients present in Portland cement are dicalcium silicate, (Ca2SiO4), tricalcium silicate (Ca3SiO5 ) and tricalcium aluminate (Ca3Al2O6 )., Setting of Cement:, When mixed with water, the setting of cement takes place to give a hard mass., This is due to the hydration of the molecules of the constituents and their, rearrangement., The purpose of adding gypsum is to slow down the process of setting of the, cement so that it gets sufficiently hardened., Uses:, It is used in concrete., It is used in plastering., It is used in the construction of bridges, dams and buildings.
Page 19 :
BIOLOGICAL IMPORTANCE OF MAGNESIUM AND CALCIUM, An adult body contains about 25g of Mg, 1200g of Ca, 5g of Iron and 0.06g of, copper., All enzymes that utilize ATP in phosphate transfer require magnesium as the, cofactor., About 99% of Ca is present in bones and teeth., Calcium also plays important roles in neuromuscular function, Interneuronal, transmission, blood coagulation etc., The main pigment for the absorption of light in plants is chlorophyll which, contains Mg.