Page 1 :
QUESTIONS, 1. Hydrogen molecules differs from chlorine molecule in the following respect, a) Hydrogen molecule cannot participate in coordination bond formation butchlorine, molecule can, b) Hydrogen molecule can form intermolecular hydrogen bonds but chlorinemolecule, does not, c) Hydrogen molecule is polar while chlorine molecule is non-polar, d) Hydrogen molecule is non-polar but chlorine molecule is polar, 2. The property of hydrogen which distinguishes it from alkali metals is, a) its non-metallic character, b) its reducing character, c) its affinity for non metal, d) its electropositive character, 3. Hydrogen accepts an electron to form inert gas configuration. In this itresembles, a) halogen, b) chalcogens, c) alkali metals, d) alkaline earth metals, 4. Which of the following statements is correct ?, a) It has oxidation number of –1 and +1, b) It will not be liberated at anode, c) Hydrogen has same electronegativity as halogens, d) Hydrogen has same IP as alkali metals, 5. Why does H+ ion always get associated with other atoms or molecules?, a) Loss of an electron from hydrogen atom results in a nucleus of very small sizeas compared, to other atoms or ions. Due to small size it cannot exist free., b) It resembles both alkali metals and halogens, c) Its reactivity is similar to halogens, d) Ionisation enthalpy of hydrogen resembles that of alkali metals, 6. Which one of the following is not an isotope of hydrogen ?, a) Ortho hydrogen, b) Deuterium, c) None of these, d) Tritium, 7. Number of neutrons in three isotopes of hydrogen, protium, deuterium and tritium respectively, is, a) 0, 1, 2, b) 2, 1, 0, c) 2, 0, 1, 1
Page 2 :
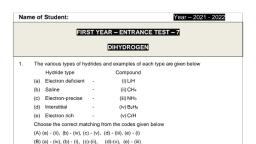
d) 1, 1,1, Why, is, water, gas (mixture of CO and H2) also called ‘syn gas’?, 8., a), Because it is used in the synthesis of methanol and a number ofhydrocarbons, b), None of these, c), Because it is synthesised from methane gas, d), Because it is synthesised from sewage, saw – dust, scrap wood etc., 9. Pure H2O2 is :, (a) Semi – solid, (b) Liquid, (c) Solid, (d) Gas, 10. The freezing point of heavy water is, (a) 0°C, (b) 3.8°C, (c) 4°C, (d) 1°C, 11. Which of the following hydrides are generally nonstochiometric in nature?, (a) Ionic Hydrides, (b) Molecular Hydrides, (c) Interstitial Hydrides, (d) All of the Above., 12. Hydrogen is the most abundant element on earth after, (a) Oxygen, (b) Carbon, (c) Sulphur, (d) None of the Above, 13. Hydrogen is a good, (a) Oxidizing, (b) Reducing, (c) Acidic, (d) Basic, 14., , Hydrogen is most, (a) Abundant, (b) None, , ., , agent., , element in the universe., , (c) Both, (d) Consumer, , 15. Permutit is:, a), b), , hydrated sodium aluminium silicate, sodium hexa meta-phosphate, 2
Page 3 :
c), d), , sodium silicate, sodium meta-aluminate, , 16. H2O2 used in rocket has the concentration:, (a) 50%, (b) 90%, (c) 70%, (d) 30%, 17. What is the product of the reaction of H2O2 with Cl2?, (a) O2 + HOCl, (b) HCl + O2, (c) H2O + HCl, (d) HCl + H2, 18. Which of the following statements regarding hydrogen peroxide is/ are incorrect?, (a) As aerating agent in production of sponge rubber, (b) As an antichlor, (c) For restoring white colour of blackened lead painting, (d) All of the above, 19. Atomic hydrogen is called, (a) Protium, (b) Deutrium, (c) Nascent Hydrogen, (d) Tritium, 20. _________________ on water decolourises H2O2, (a) O3, (b) Acidic KMnO4 solution, (c) Black Suspension of Lead Sulphide(PbS), (d) None of these., 21. The volume of 10 volume H2O2 required to liberate 500 ml of O2 at S.T.P. is :, (a) 25 ml, (b) 50 ml, (c) 100 ml, (d) 125 ml, 22. Hydrogen is evolved by action of dil.HNO3 on, a) Fe, b) Mg or Mn, c) Cu, d) Al, 23. A molten ionic hydride on electrolysis gives, a) H+ ions moving towards cathode, b) H+ ions moving towards anode, c) H2 gas evolved at cathode, d) H2 gas evolved at anode, KCET-2019, 1) Which of the following is not true regarding hydrogen as fuel?, 3
Page 4 :
a), b), c), d), , The combustible energy of hydrogen can be directly converted to electrical energy in fuel cell, High calorific value, Hydrogen gas can be easily liquefied and stored, Combustible product is eco-friendly, , KCET-2018, 2) H2O2 is, a) An oxidising agent, c) Both oxidising and reducing agent, , b) A reducing agent, d) Neither oxidising nor reducing agent, , 3) Which of the following will cause hardness in water, a) Ca+2, b) Na+, c) Cld) K+, KCET-2017, 4) In the manufacture of hydrogen from water gas (CO+ H2), which of the following is correct, statement?, a) CO is oxidized to with steam in the presence of a catalyst followed by absorption of in alkali., (b) CO and are separated based on difference in their densities., (c) Hydrogen is isolated by diffusion., (d) H2 is removed by occlusion with Pd., KCET-2016, 5) Water softening in clark’s process uses, a) CaHCO3 b) NaHCO3, c) Na2CO3, , d) Ca(OH)2, , 6), , a), b), c), d), , H2O2 acts as oxidising agent in both equations., H2O2 acts as reducing agent in both equations., H2O2 acts as reducing agent in (B) and oxidising agent in (A)., H2O2 acts as reducing agent in (A) and oxidising agent in (B)., , JEE MAINS-2020, 7) In comparison to the zeolite process for the removal of permanent hardness, the synthetic resin, method is:, a) less efficient as the resins cannot be regenerated, b) more efficient as it can exchange only cations, c) less efficient as it exchanges only anions, d) more efficient as it can exchange both cations as well as anions, , 4