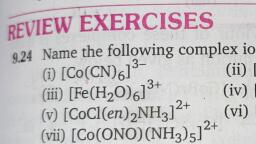Page 1 :
WNIT 8 @-and £Block Elements, , \ Tansition and Inner, | Tansition Elements), , , , ES, , “CT IV’ . 7, , Bye ing this Unit thoroughly, th, or, study onic configuration © reader shoulg, fe elect and, , . be able to 1, vite ties of d-block elements; Ysicochemical Hettcete 5, ro ends in the chemi Configuration and ;, ’ Fe general * try of first TOW transition : Of F-block elements; nome important Properties, jements; . ‘anthanoid contract;, e istry of some important compo, , unds of traneis: * the similarities i ;, pe such as KCr207 and KMno,; *ransition a atferences in t, , , , , elements in which the differentiati, i 1) d subs! aaa ly — to as transition Series called, genents De the periodic aie at ns . 45,6,7,8, 9, are placed 4 ane, ip Land ae kin the soit cetitute a separate shown in Meaty i, poxealled d loc! a = ; ic table (Fig, 8.1). Thisis why talled A 3.1. These series co}, ae clements are also referred to as 4-block elements. They aia . in the, i metals and show several interesting Properties. They n elements, - useful elements and find a Variety of applications not ._I" the forth, oplyin our day to day life but also in industry ipportant charac, The elements in which the differentiating electron 59 Study some i, , ean : and actinoids,, FO —, number [2 —, , (n-2) f-subshell are called inner-transition, , - These elements have been $rouped into two, , Series constitute a separate block, Periodic table, Therefore, the inner, are also referred to as f-block elements,, , Atomic ~, number, , , , vo | tu, 1!, , | ur, , , , Gace tt “so | 60 | 6 | 2 | os] os Te 66 | er | 6x | 69, santhanals [ee fm | he | pn | ca | te | by [iu | & [Tm, os [2 Te Te leleletelels le [i Te, Fig. 8.1 Position of transition (d-block) and inner-transition (f-block) elements in the periodic table., , "8.1 THE TRANSITION ELEMENTS OR d-BLOCK ELEMENTS, , &1.1 Position in the Periodic Table table are usually called transition elements. The d-block is, situated in the middle of the periodic table between group, The elements belonging to the d-block of the periodic 2 and group 13 as shown in Fig. 8.1. It is actually flanked by, , , , Scanned with CamScanner
Page 2 :
430, , s- and p-blocks in the periodic table, The name ‘transition’, given to the elements of d-block is because of their position, between s-and p-block elements. The elements of s-block, are electropositive and have a tendency to form tonic, compounds, On the other hand, the elements belonging, to the p-block are electronegative and have a tendency to, form covalent compounds. The d-block elements show a, transitional behaviour between the two, Le., between, highly electropositive s-block elements and weakly, electropositive p-block elements. This is why the elements, of d-block are termed as transition elements., , 8.1.2 Classification of Transition Elements, , The transition elements or d-block elements are, classified on the basis of (n - 1) d-subshell which gets, filled up in a particular class of these elements. On, this basis, transition elements have been classified, into four horizontal series called transition series., Each series corresponds to the filling of a particular, (n - 1) d-subshell. These transition series are as follows., , (i) First transition series or 3d-series : This series, corresponds to the filling of 3d-subshell and consists of ten, elements from Sc (At. No. = 21) to Zn (At. No. = 30). These, elements belong to the 4" period of the periodic table., , (ii) Second transition series or 4¢-series : This series, involves the filling of 4d-subshell and consists of ten, elements from Y (At. No. = 39) to Cd (At. No. = 48).. These, elements lie in the 5 period of the periodic table., , (iii) Third transition series or 5d-series : This series, involves the filling of 5d-subshell and consists of a total of, 10 elements namely La (At. No. = 57), and those from Hf, (At. No. = 72) to Hg (At. No. = 80). These elements lie in, the 6 period of the periodic table., , (iv) Fourth transition series or 6d-series : This series, corresponds to the filling of 6d-subshell. The series is, incomplete-and consists of elements Ac (At. No. = 89),, and those having atomic number 104 and above.These, elements belong to the 7 period of the periodic table., , 8.1.3 Electronic Configuration of Transition, Elements, , As mentioned earlier, the transition elements are, characterised by the filling of (n - 1) d sub-shell. They have, electronic configurations of the type (n - 1) q'-10 ns! ~ 2, The first transition series involves the filling of 3d-subshell,, Thus on moving from left to right in this series, the valence, electrons enter into 3d-orbitals and fill them according, to the common rules for filling of orbitals, Similarly, the, second, the third and the fourth transition series involve, , the filling of 4d-, 5d- and 6d-subshells Tespectively., The electronic configurations of transition elements, belonging to the above mentioned series are listed in Table 8.1,, , Nootan Chen, Table 8.1. Electronic Configurations of Transition my, 1. First Transition Serles (3d-series) Ma,, , , , , , , , , , , , , , , , , , , , , , Atomic | Electronic Reprene,, Element |SyMbOl) timber | Configuration i, C, 24.2 64.2, 25 xin, Scandium | Sc} 21 at ae (naa, 25.2 642, 2s, Titanium Ti “2 , Pat = {ANI 3¢ ga, 25.2 64,2, 2s? p® 3, Vanadium | V | 23 ee 4 (Ar) 30 4a, 2, Chromium | Cr 24 ° Pub # (An) af aa, 26, Manganese | Mn 3 tad a (Ar) 36 4g, 2 9,2 64.2, ton | | 28 | 283A nat, Cobalt Co | 27 ey 39° ta 30 42, P, . 2526, Nickel Ni | 28 ° Pat 36) tan) 32, 25.2 6 |, Copper cu 29 Pao at Ar] 3¢0 yr |, 52 653, Zinc Zn | 30 ° je aa {Ar] 3d! 42 |, 2. Second Transition Series (4d-series) y, Atomic | Electronic | pe Bre, Element | Symbol} i oher Configuration ofa, C, 5 39 | 157 2s? p® 3,7 oe, Yttrium Y gio ba S| Ukr) 4d gr, P SP, a 2, Zirconi ar 40 12228 ? |, irconium 6 gO a6 Ukr] 4222 |, P sp, 262 |, Niobium Nb 41 wate 32 |, 6 god. 26 Ue) ais |, s |, P “P, Molybdenum | Mo 42 12 " Poa? Ike 48s) |, Pp 4s° p |, ‘ |, Technetium Te | 43 1S 25° pias mii, Pd 45° p', ; d® ss! |, Ruthenium Ru 44 1S 23% poae (Kr) 4d” s! |, Pp d~ 4s*p |, / d’ 5s!, Rhodium Rh | 45 1a? 25% pas? (kr) 4f's!, ped 4s"p, 1 |, Palladium Pa | a6 fat 242 p62 tre!, d™ 45, P 95,0", 1 1, Silver Ag 47 1 2 Pas (kr) als, ped 4s'p, Cadmi a? ss! 1052, imium Cd] 48 | 1s? 262 p® 362] ued ad, p® qo 4s? p®, d!9 5.2, , , , , , , , , , , , , , Scanned with CamScanner
Page 4 :
fo) |= 90100, , w n » “0 80 eo 0, Atomic Number ———e, Fig. 8.2 Decrease in the energies of subshells with, , increase in atomic number. 2, and calcium has electronic configuration 15? 2s? 2p° 3s, 3p° 43°, Beyond calcium, there is a sharp decrease in, the energy of 3d-orbitals and they lie below 4p-orbitals., Therefore, in the next element scandium (At. No. = 21),, the differentiating electron goes to a 3d-orbital instead ofa, 4p-orbital and scandium has configuration 1s? 2s? 2p” 3s, 3p° 3d! 4s”. In the subsequent elements of first transition, series, ie., upto zine (At. No. = 30), the differentiating, electrons enter into 3d-orbitals. As electrons enter into, 3d-orbitals, they become more effective in shielding, 4s-electrons from the nucleus. Therefore, 3d-orbitals are, further pulled lower as compared to 4s-orbitals., , The similar trends are observed in case of the elements, belonging to the second and the third transition series. In, the second transition series, the differentiating electrons, enter into 4d-orbitals instead of Sp-orbitals because the, former have lower energy (Fig. 8.2). In lanthanum, the, energies of 4f, 5d and 6s-orbitals lie very close to one, another and therefore one electron enters the 5d-orbital, before 4f-orbitals begin to fill. Therefore, lanthanum, (At. No. = 57) has the configuration, [Xe] 5d 6s”. As one, electron enters into a 5d-orbital, the 4f-orbitals are pulled, lower as compared to 5d-orbitals due to the shielding, effect of 5d-electrons. Therefore, in the subsequent, elements, i.e., from Ce (At. No. = 58) to Lu (At. No. = 71),, the differentiating electrons enter into 4f-orbitals. These, 14 elements (Sc - Lu) are termed as lanthanoids and are, regarded as inner transition elements. As soon as all the, 4f-orbitals get filled, the differentiating electrons further, enter into 5d-orbitals in elements hafnium (At. No. = 72), to mercury (At. No. = 80) and the third transition series is, obtained., , Nootan Ch 4, , tlona of ¢ ty, reaptlonal olectrontc configura # Of Cran, the aa configurations given In Mble Bl, r ’ yy, that the sequence of filling of electrons Ind iby) hy, fierurbed In ehromlum and copper and these mh ", postal exceptional configurations. This can by we ms, +, Hows ‘, as fol rhe completely filled or half filled Subshety ., bility. Therefore, the subshells of the typ, We,, tiara filled) or py d’, f” (half filled} v Ps), f he usual trend of the filling of cleat My, , stable, If t, followed, chromium should have the configuration ‘my, 2p° aie? Sp? nd’ 4e2, In this configuration 4s js tea |, , nut Jd ts neither completely nor halg qrP!to,, ie an the inter-electronic repulsion forces one an ?, to enter into Jd -subshell to acquire the configuration tie, one 307 3p? qd° 4s', This makes both 3d and 4g hey, and imparts an extra stability to the atom, Simila, , i}, copper, the expected electronic configuration should Yh, 2s, , 35? 3p’ 3d? 4s, Again, the inter-electronic tg :, 4s-electron to enter into the 3d-subsh ll lon, Ponta filled. Thus, copper acquires the contigy, 15225? 2p® 37 3p® 3d! 4s). In this configuration, subg, is completely filled while 4s is half filled. This impart, extra stability to copper atom. an, The extra stability of half filled and completely fil, subshells can be explained in terms of symmetry illeg, , subshells possess symmetrical distribution of ae, which allows the maximum exchange of electrons Ong, makes the system more stable. This is why ang, such subshells are of lower energy and are Meera, , more stable. ; i, , 8.1.4 Definition of Transition Elements, , Earlier it was mentioned that, in general, the transito,, elements are those elements in which differentitn,, electrons enter into (n - 1)d-subshell. On the basis ¢, electronic configuration and general characteristics, the, transition elements may be defined more precisely as given, below., , Transition elements are those elements which Possess, incompletely filled d-orbitals in their ground state or in their, ions existing in chemically significant oxidation states,, , In the light of above definition, let us examine the, elements of first transition series. Except Cu and 2n, all, other elements have partly filled d-orbitals in their, elementary form (ground state) and are true transition, elements. Cu possesses completely filled d-orbitals in is, elementary form. Its common oxidation states are +1 and+2., The electronic configuration of Cu, Cu* ion (oxidation state, = +1), and Cu* ion (oxidation state = +2) are as follows., , 15? 25? ap® 3s? 3p8 34 4s!, Cu’ 152 25? 2p6 462 ap6 gq!, (Cuprous ion) S° 2s" 2p” 3s° 3p° 3d, , Scanned with CamScanner
Page 5 :
ments (Transition and Inner ‘Transition Rlem, ‘tements), , ,pioek 2 64.2 4,6, aif L182 298308 pa?, , ol" a cu! ion have completely filled d., pot cu sn jon possesses partly filled Cotman,, 5, CH orcan be regarded as a transit okt, yeh oper re, ion element, COPE of Cur fon, ie., in +2 oxidation state,, a consider the case of zinc, Its common, eel +2 in which it forms Zn?" ions. The, gonfigurations of Zn and Zn** ion are as follows, P44? 20? 2p 30? 3p8 aalOiqy? ', é | 132.28? 2p® 367 ap6 al?, fa » configurations it is cle;, a ese config lear that neith, regi possesses partly filled d-orbitals, Hed sind, - 0 regarded as a transition element. ‘, it, in nt ay the. last elements of the second and the, si Mtion series, ie., cadmium and mercury also lack, wird wailed d-orbitals both in their elementary form and, yn ‘common oxidation state of +2 as is clear from the, jn ne, wing, follo¥, , Jet, , east 2p® as? 3p® 3d" 4s? 4p 4d! 542, a 2 2s? 2p® 357 3p° 3d! 452 4p° 4d!, i 2s? 2p® 35? 3p® 3d" 432 4p® 4a? af 14 562 596, , Hg 5d! 6s”, , a: 2s? 2p® 3s? 3p® 3d! 4s? ap® 4d! 4p 552 598 sq!, ‘Thu:, , ents., , elem ence, it may be concluded that the end-members of, 1 the three transition series, Le., Zn, Cd and Hg are not, rransition elements irt the true sense because they possess, oo mpletely filled d-orbitals in their elementary form as well, ain their commonly existing ions. Infact, Zn, Cd and Hg, donot exhibit the general characteristic properties of the, transition elements and behave quite differently from, the rest of the elements belonging to the d-block of the, , eriodic table. However, they are generally studied with, the d-block elements to maintain a rational classification, , of elements., , 8.1.5 General Properties of Transition Elements, , The transition elements belonging to a particular, series differ only in the number of (n — 1) d-electrons. They, possess the same number of electrons in their valence shell, (ns*). Therefore, the elements belonging to a particular, series do not differ so much from one another in their, physical and chemical properties as the representative, elements belonging to the same period do., , Transition elements show several interesting physicochemical properties. The important trends in the chemistry of, first row transition elements are discussed below., , 1, Atomic Radii, ‘The atomic radii of transition elements lie in between, te of s- and p-block elements. They are listed in Table, , Hg, 5, Cd and Hg can not be regarded as transition, , , , , ‘Table 8.2, Atomic Radii of d-B, , , , , , , , , , , , , , , , , , , , , , , , First Transition Series, Element | Atomic Radius, . (pm), Se 164, 1 147, v 135, cr 129, Mn 197, Fe 126, Co 125, Ni 125, cu 128, un 197, Third Transition Series, ‘Aomie Radius, , Element (om), , la 168, , uf 156, , Ta 143, , w 137, , Re 137, , Os 134, , Ir 136, , Pr 139, , Au 144, , Hg 151, , , , The atomic radii of transition elements exhibit, , following trends., , (@ In general, the atomic radii of transition elements, belonging to a particular series decrease with*increase in, atomic number; the decrease becomes small aftenmidway., , Explanation : In the beginning, the decrease in, atomic-radii with-increase in the atomic number'is due to, an increase in the. nuclear charge. As-the atomic number, increases, the added electronsenter into (n — 1) d-subshell, and shield the outermost electrons. The shielding effect, increases with increase in the number-of d-electrons, Le.,, with increase in the atomic number. Thus, the effect of the, increased nuclear charge due to increase in atomic number, is counterbalanced by the increased shielding effect of the, (n - 1) d-electrons. This is why, the atomic radii remain, almost constant after mid-way in each series., , (ii) Near the end of each series, there is a slight increase, in the atomic radii, , Explanation : Towards the end of a series, the electron, repulsions between the added electrons in the similar orbitals, predominate over the attractive forces due to the increase in, nuclear charge. This results in the expansion of the electron, cloud and consequently the atomic radii increase., , This can be understood by taking the example of the, elements of first transition series. Upto chromium (3, , , , Scanned with CamScanner