Page 1 :
www.mheducation.co.in, ISBN-13: 978-93-5260-529-3, ISBN-10: 93-5260-529-2, , Inorganic chem _IIT JEE, 09 March 2017 12:48:15 PM
Page 5 :
McGraw Hill Education (India) Private Limited
Page 6 :
It gives me immense pleasure to present the first edition of this book for JEE aspirants. This is an outcome of teaching, experience gained through years of interaction with students preparing for JEE., The objective of this book is to provide proper guidance and relevant material to the JEE aspirants. The topics and, problems of this book are framed in a way that they touch the required level of depth for each topic., All the chapters of this book have key concepts, solved examples, three levels of problems and previous years’, questions to provide a quick revision to the aspirants., y, y, y, y, y, y, , The details of the salient points are given below:, KEY CONCEPTS – Efforts have been made to highlight the important theories in short form., SOLVED EXAMPLES – Improve the problem-solving capacity of the aspirants in a short span of time., LEVEL-I– are the problems based on basic concepts useful for JEE Main Exam., LEVEL-II– are the conceptual problems with wide application of topics which are useful for JEE Main Exam., LEVEL-III– are the problems based on comprehension (passage), integer answer types, column matching type and, one or more than one correct answer types to make the students familiar with JEE ADVANCED pattern., PREVIOUS YEARS’ QUESTIONS FOR JEE (Main & Advanced) –covers previous years’ questions asked in IITJEE, AIEEE and JEE Main Exam., , I have tried my best to keep this book free from errors. Last but not the least, constructive criticism and valuable, suggestions from the readers will be highly appreciated to make this book more precise, accurate and useful., – Author
Page 8 :
Preface�v, Chapter 1, , Periodic Table, , 1.1 – 1.31, , Chapter 2, , Chemical Bonding, , 2.1 – 2.36, , Chapter 3, , Coordination Compounds, , 3.1 – 3.32
Page 9 :
viii, , Chapter 4, , Metallurgy, , 4.1 – 4.18, , Chapter 5, , Hydrogen and Its Compounds and S-Block elements, , 5.1 – 5.24, , Chapter 6, , d-and f-block elements, , 6.1 – 6.22, , Chapter 7, , p-Block Elements, , 7.1 – 7.26, , Chapter 8, , Salt Analysis, , 8.1 – 8.26
Page 10 :
Chapter, , Key Concepts, , 1., 2., 3., 4., 5., 6., 7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., , Lowest electronegativity, :, Highest electronegativity, :, Highest ionization potential, :, Lowest ionization potential, :, Lowest electron affinity, :, Highest electron affinity, :, Least electropositive element, :, Lowest melting point metal, :, Highest melting point and, :, boiling point metal, Lowest melting point and, boiling point non-metal, :, Notorious element, :, Lightest element, :, Smallest atomic size, :, Largest atomic size, :, Largest anionic size, :, Smallest cation, :, Most electropositive element, :, Element with electronegativity, :, next to Fluorine, Group containing maximum number :, of gaseous elements in the , periodic table, , Cs, F, He, Cs, Noble gases, Chlorine, F, Hg, W (Tungsten), , He, Hydrogen, Hydrogen, H, Cs, I–, H+, Cs, Oxygen, Zero, group(18th), , 20. Total number of gaseous elements : 11(H, N, O,, in the periodic table F, Cl, He, Ne,, Ar, Kr, Xe,, Rn), 21. Total number of liquid elements in : 6 (Ga, Br, Cs,, the periodic table Hg, Fr, Unb), 22. Smallest anion, : F–, 23. Liquid element of radioactive nature : Fr, 24. Total number of radioactive, : 25, elements in the periodic table, 25. Volatile d-block elements, : Zn, Cd, Hg,, Unb, 26. Element containing no neutron, : H, 27. Most abundant element in earth’s, : Oxygen, crust, 28. Rarest element on earth, : At (astatine), 29. Most abundant metal in crust earth : Al, 30. Element having maximum tendency : Carbon, for catenation in periodic table, 31. Non-metal having highest melting : Carbon, point, boiling point (diamond), 32. Metals showing highest oxidation : Os (+8), state, 33. Most electrovalent compound, : CsF, 34. Most stable carbonate, : Cs2CO3, 35. Strongest base, : CsOH, 36. Strongest basic oxide, : Cs2O, 37. Best electricity conductor among, : Ag, metals
Page 11 :
1.2, , 38. Best electricity conductor among, : Graphite, non-metals, 39. Most poisonous element, : Pu, (Plutonium), 40. Liquid non-metal, : Br, 41. Element kept in water, : Phosphorous, 42. Elements kept in kerosene, : IA group, element, (except Li), 43. Elements sublime on heating, : I2, 44. Noble metals, : Au, Pt etc., 45. Amphoteric metal, : Be, Zn, Al,, Sn, Pb, 46. Amphoteric metalloid, : Si, 47. Metalloids elements, : Si, As, Te,, At, Ge, Sb, 48. Non-metals having metallic lusture : Graphite,, Iodine, 49. Heaviest naturally occurring element : Uranium, 50. Poorest conductor of electricity, , 66. Most abundant element in the, universe, , i. It was proposed by Henry Moseley., ii. Modern periodic table is based on atomic number., iii. Moseley did an experiment in which he, bombarded high speed electron on different metal, surfaces and obtained X-rays. He found out that, v µ Z where v = frequency of X-rays, From this experiment, Moseley concluded that, the physical and chemical properties of the, elements are periodic function of their atomic, number. It means that when the elements are, arranged in the increasing order of their atomic, number elements having similar properties after a, regular interval. This is also known as ‘Modern, periodic Law’., iv. Modern periodic Law – The physical and, chemical properties of elements are a periodic, function of the atomic number., , : Diamond, , 51. Hardest naturally occurring element : Diamond, 52. Lightest solid metal, , : Li, , 53. Amphoteric oxides, : BeO, Al2O3,, ZnO, PbO,, SnO, SnO2,, Sb2O3,, As2O3, etc., 54. Neutral oxides of non metals, : NO, CO,, H2O, N2O, 55. Dry bleacher, : H2O2, 56. Dry ice, : Solid CO2, 57. First man-made element, : 43Te, (Technicium), 58. Smallest period, : Ist, (2 elements), 59. Largest period in periodic table, , : 6, , : Hydrogen, , i. It consist of 7 horizontal periods and 18 vertical, columns (groups), ii. According to IUPAC 18 vertical columns are, named as 1st to 18th group., iii. The co-relation between the groups in long form, of periodic table and in modern form of periodic, table are given below., IA IIA IIIB IVB VB VIB VIIB, 1, , 2, , 3, , 4, , 5, , 6, , 7, , VIII, 8, , 9, , IB IIB IIIA IVA VA VIA VIIA 0, 10 11 12 13 14 15 16 17 18, , iv. Elements belonging to same group having same, number of electrons in the outermost shell so their, properties are similar., , th, , (32 elements), 60. Largest group in periodic table, : IIIB, (32 elements), 61. Most abundant d-block metal, , : Fe, , 62. Most abundant s-block metal, , : Ca, , 63. Highest density (metals), , : Os, Ir, , 64. Highest density (non-metals), , : Boron, , 1, , 1 1s, , 2, , 1H, 2He, , 2, , 2 2s, 2p, , 8, , 3Li-10Ne, , I Short, , 65. Most abundant gas in atmosphere, , : Nitrogen, , 3, , 3 3s, 3p, , 8, , 11Na-18Ar, , II Short, , Period, , n, , Sub-shell, , No.of, elements, , Element, , Name of period, Shortest
Page 12 :
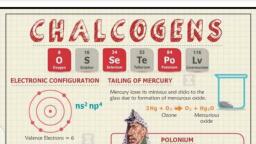
1.3, 4, , 4 4s, 3d, 4p, , 18, , 19K-36Kr, , 5, , 5 5s, 4d, 5p, , 18, , 37Rb-58Xe, , 6, , 6 6s, 4f, 5d, 6p, , 32, , 55Cs-86Rn, , 7, , 7 7s, 5f, 6d, 7p, , 26, , 87Fr-112Unb, , I Long, II Long, Longest (very, long), Incomplete, , 1st/IA/Alkali metals:, , General electronic configuration = ns1, (n = Number of shell), Number of valence shell e- = 1, 2nd/IIA/Alkaline earth metals:, General electronic configuration = ns2, Number of valence shell e- = 2, 13th/IIIA/Boron family:, General electronic configuration = ns2np1, Number of valence shell e- = 3, 14th/IVA/Carbon family:, General electronic configuration = ns2 np2, Number of valence shell e- = 4, 15th/VA/Nitrogen Family/Pricogens: (Used in fertilizer as, urea), General electronic configuration = ns2 np3, Number of valence shell e- = 5, 16th/VIA/Oxygen family/Chalcogens: (Ore forming), General electronic configuration = ns2 np4, Number of valence shell e- = 6, 17th/VIIA/Halogen family/Halogens: (Salt forming), General electronic configuration = ns2 np5, Number of valence shell e- = 7, 18th/Zero group/Inert gases/Noble gases:, General electronic configuration = ns2 np6 (except, He), Number of valence shell e- = 8, , (i) 2nd period elements (Li, Be, B) show diagonal, relationship with 3rd period elements (Mg, Al, Si)., Due to almost similar ionic potential (Ionic potential, = charge/Radius) value they show similarily in, properties., Li, Be, B, , (iii) In 6th period all types of elements are included (s, p, d, and f), (iv) No inert gas in 7th period., (v) Normal elements present in all periods., (vi) Atomic number of last inert gas element is 86., (vii) Long form modern periodic table can be divided into, four portions:, 1. Left portion (IA and IIA) – s-block., 2. Right portion (IIIA to VIIA + zero group) –, p-block., 3. Middle portion (IIIB to VIIB + VIII + IB and IIB), – d-block., 4. Bottom portion (IIIB) – f-block elements, , i. The elements in which ultimate orbit is incomplete, while penultimate orbits are complete are called, as normal elements., ii. Their general electronic configuration is:, IA, 1, , ns, , IIA, 2, , ns, , IIIA, 2, , IVA, , ns np, , 1, , ns1-2, , 2, , VA, , ns np, , 2, , 2, , VIA, 3, , ns np, , 2, , VIIA, 4, , ns np, , ns2np5, , ns2 np1-5, , The elements in which both ultimate (n) as well, penultimate shells (n-1) are incomplete either in atomic, state or in some oxidation state are called as transition, elements., Note: According to this concept Zn, Cd, Hg and Unb, are not transition elements because they do not have, incomplete penultimate shell either in atomic state or in, some oxidation state., Group number: IIIB to VIIB + VIII + IB and IIB, Periods: 4th to 7th, i. General electronic configuration is (n-1)d1-10, ns1 or 2, ii. Total number of d-block elements = 40, , Na, Mg, Al, Si, rd, (ii) 3 period elements (Na, Mg, Al, Si, P, S, Cl) are called, typical elements because they represent the properties, of other element of their respective group., , Total number of transition elements = 36 (Except, Zn, Cd, Hg and Unb), , Note: All transition elements are d-block but all, d-block elements are not transition elements.
Page 13 :
1.4, , 2. Metallic radius (rm): Such type of radius is, determined if atoms are bonded with metallic bond., The elements in which all the three shells, i.e. ultimate (n),, penultimate (n-1) and pre or antipenultimate (n-2) shells,, are incomplete are called as inner-transition elements., i. General electronic configuration is:, , , , da-a, , rm= ––––, d, 2, 3. van der Waal’s radius (rv): Such type of radius is, determined if molecules are bonded with van der, Waal’s force of attraction., A2., A2, a-a, , (n-2)f1-14 (n-1)d0 or 1 ns2, ii. These are 28 in number., iii. Group – IIIB, iv. Period – 6thand 7th, v. Inner transition elements are divided into two, series:, (a) Lanthanoid, series/Rare, earth, elements/, Lanthenones (Ce58–Lu71 14 elements), (b) Actinoid series/Man-made elements/Actinones, (Th90–Lw103 14 elements), , d, , , dA2 - A2, , rv = –––––––, , 2, A2–A2, , rv > rm > rc, , 1. Atomic radius of an element cannot be determined, because atoms never exist in their free state and, position of their outermost e- is uncertain., 2. Atomic radius is determined in bonded state, , 1. Covalent Radius (rc), a. Such type of radius is determined if a single, covalent bond is present between two similar, atoms., , , r =, c, , da – a, ––––––, 2, , , , , Internuclear distance(da-a), , b. If a single bond is present between two different, atoms,, da – b = ra + rb - 0.09 (DEN), Å, (Bond length), , Note: Noble gases are monoatomic molecules bonded, with van der Waal’s force of attraction hence, for noble, gases, van der Waal’s radius is considered., 4. Ionic radius (radius of ions):, a. A cation is always smaller than its parent atom, because it has greater Zeff than its parent atom., As positive oxidation state increases, radius, decreases., Mn > Mn+2 > Mn+7, b. An anion is always larger than its parent atom, because the anion has lower Zeff than its parent, atom. As negative oxidation state increases,, radius increases., O < O- < O-2, 1. Species (atoms, molecules or ions) having same, number of electrons are known as isoelectronic., e.g, Si, N2, CO, CNNO+, 14e, 14e, 14e, 14e, 14e2. Order of radius in monoatomic isoelectronic species:, N-3 > O-2 > F- > Na+ > Mg+2 > Al+3, In isoelectronic species, as atomic number increases,, radius decreases. It is due to increment in Zeff., , ra = covalent radius of A, rb = covalent radius of B, , DEN = difference in electronegativity of A and B, , 1. In periods:, a. As we move left to right in a period, when Zeff
Page 14 :
1.5, , increase in the atomic radius decrease except in, noble gases., Li > Be > B > C > N > O > F < < Ne, Na > Mg > Al > Si > P > S > Cl < < Ar, b. Order of radius in 3d- series:, Sc > Ti > V > Cr < Mn > Fe ~ Co ~ Ni < Cu < Zn, 2. In groups:, a. As we move top to bottom in, number of shell increase the, increases, Li < Na < K < Rb < Cs, F < Cl < Br < I, b. Exception (in d-block):, 4d series ~ 5d series (due, contraction), c. Exception(in p-block): Al > Ga, Note: Radius mainly depends, shells., Some exceptions are:, Li+, > Mg+2, 0.76Å, 0.72Å, H- >, F 1.40Å, 1.33Å, , a group, when, atomic radius, , b. In p-block:, , , , In ~ Tl, Sn ~ Pb, , (a) It is the energy required to remove an e- from, outermost shell of isolated (free) gaseous atom., (b) This process is endothermic., M (g) + IE of M " M+(g) + e-; DH = I E, , (i) Zeff: IE µ Zeff, to lanthanoid, , (ii) Atomic size: IE µ 1, At. Size, , (iii) Penetration power of orbitals: s > p > d > f, , on number of, , (iv) Electronic configuration of outermost subshell:, a. Elements having fully filled or half filled, outermost subshell have greater IE than, expected., (b) Such elements in a period have greater IE, than adjacent elements., 1, 2, 13 14 15 16 17 18, 1, 2, ns, ns, np1 np2 np3 np4 np5 np6, , 1. In lanthanoid series, as atomic number increases,, atomic and ionic radius gradually decreases. It is, called as lanthanoid contraction., 2. Cause: As me move from Ce to Lu, nuclear, charge (Z) increases and 1e- is successively added, into inner 4f-subshell. Since f-e- produces almost, negligible screening effect hence, screening, coefficient (s) remains almost constant and Zeff, increases thus, radius decreases. (due to poor, screening of 4f-e- on outer e-), 3. Effect of lanthanoid contraction is also present, from 72Hf to 82Pb. It is also called as post, lanthanoid contraction or lanthanoid contraction., Due to this, these element have greater Zeff than, expected (its due to poor screening by 14e- present, in 4f-subshell)., Order of radius (along the group), a. In d-block: , 4d series ~ 5d Series, , (Zeff high), , Zr ~ Hf, , Pd ~ Pt, , Y < La (No lanthanoid, contraction), , (fully filled) (half filled) , , , , , , , (fully filled), , 1 < 2 > 13 < 14 < 15 > 16 < 17 < 18, 1 < 13 < 2 < 14 < 16 < 15 < 17 < 18, Order of IE in second period:, Li < B < Be < C < O < N < F < Ne, , Order of IE in third period:, Na < Al < Mg < Si < S < P < Cl < Ar, Periodicity in IE, 1. In periods: as we move from left to right, in, general, IE increases. (except for fullyfilled and, half filled elements), 2. In groups: as we move top to bottom in a group,, in general, IE decreases. (it is due to increase in, atomic size), Exception: (a) due to lanthanoid contraction,, In d-block:, 4d series < 5d series, , Zeff high, Zr < Hf, Pd < Pt, Y > La (No lanthanoid contraction)
Page 15 :
1.6, , In p-block:, In < Tl, Sn < Pb, (b) Al < Ga, (Zeff high ), General order of IE is:, s-block < f-block < d-block < p-block, , 1. It is the energy released when an e- is added to, outermost shell of an isolated gaseous atom., 2. This process in generally exothermic. (DH = -ve), X(g) + e- " X-(g) + EA of X ; DH = -EA, , , e- gain enthalpy, , DH = -EA, , 1. Successive IE always increases because during, successive removal of e- zeff increases and size, decreases., M(g) + IE1 " M+(g) + e-; DH = + IE1, M+(g) + IE2 " M+2(g) + e-; DH = + IE2, M+2(g) + IE3 " M+3(g) + e- ; DH = + IE3, IE1 < IE2 < IE3 < - - - - - - Energy required to remove nth e- = IEn, Energy required to remove ne- = (IE1 + IE2 + ------------ IEn), 2. IE2 of M = IE1 of M+, IE3 of M = IE2 of M+ = IE1 of M+2, 3. Successive IE always increases but if during, successive removal of e- electronic configuration, becomes stable than rate of increment in, successive I.E. is much more than expected., Mg = (Ne) 3s2, IE1 < IE2 << IE3 (because third electron is removed, from fulfilled electronic configuration), Order of IE2 in second period:, IE2 of M = IE1 of M+, +, , Li, 1s2, , +, , Be, 2s1, , +, , B, 2s2, , +, , C, 2p1, , +, , N, 2p2, , +, , O, 2p3, , +, , F, 2p4, , +, , Ne, 2p5, , Be < C < B < N < F < O < Ne < Li, (in a particular period alkali metal has highest IE2 because, it has very high Zeff), Order of IE3 in second period:, N+2, 2p1, , O+2, 2p2, , negative e- gain enthalpy = EA, EA of X = IE of X3. (a) Elements having fully filled or half filled, outermost sub-shell do not add another ehence, their EA is generally zero., (b) If we still add e- to such elements, process, becomes endothermic and formed anion, becomes unstable., –, , Y(g) + e- " Y(g), – EA; DH = + EA, (group 2,18 and N ), 4. EA1 process is generally exothermic while all, higher EA processes are always endothermic, because anions resist addition of another e-., X(g) + e- " X-(g) + EA1. ; DH = -EA1, (Except group 2, 18, N), , -2, X (g) + e " X (g) - EA2. ; DH = EA2, X-2(g) + e- " X-3(g) - EA3. ; DH = EA3, , 1. In periods: In general as we move from left to, right EA increases., In period (2):, Ne < Be < N < B < Li < C < O < F, , fully fully half, filled filled filled, , IE3 of M = IE1 of M2+, Li+2 Be+2 B+2 C+2, 1s1, 1s2, 2s1, 2s2, , -DH = EA, , F+2, 2p3, , Ne+2, 2p4, , fully fully half, filled filled filled, , B < N < C < O < Ne < F < Li < Be, (in a particular period alkaline earth metal has highest IE3, because it has very high Zeff), , In period (3):, Ar < Mg < Al < Na < P < Si < S < Cl, 2. In groups: In general as we move from top to, bottom in a group EA decreases., Note: Second period elements have lower EA than, expected. They have exceptionally small size. Hence,, incoming e- feels more repulsion than expected and net, attraction becomes less than expected so their EA becomes, less than expected., Order of EA in various groups:, Cl > F > Br > I, S > Se > Te > O
Page 16 :
1.7, , N < P < As < Bi < Sb, Si > C > Ge > Sn > Pb, Al > Ga > In > Tl > B, 1. Tendency of an atom to attract bonded e- pair, towards itself in a bond is known as EN of that, atom., 2. Noble gases do not form interatomic bond hence, their EN is assumed as zero., Factors affecting EN:, 1. Zeff:, , EN µ Zeff, 2. Atomic size:, EN µ 1, atomic size, , 3. Oxidation state:, (a) As positive oxidation state increases, EN, increases., A < A+ < A+2, +7., +4, KMnO4., >, MnO2, (b) As negative oxidation state increases, EN, decreases., B > B- > B-2, -2, -1, +2, H2O < H2O2 < OF2, 4. % s-character: As % s-character increases, EN, increases., sp, >, sp2, >, sp3, 50%, 33.33%, 25%, , 1. Mulliken’s scale:, Xm = IE + EA (both are in eV/atom), 2, , 2. Pauling’s scale:, , DEN = | XA–XB | = 0.208 (EA-B – (EA-A× EB-B)), Bond energies in kcal/mol, , , , , OR,, , , , = 0.1017 (EA-B – (EA-A × EB-B)), , , , Xp ~ Xm, , , Bond energies in kJ/mol, , 2.8, , 3. Allred-Roshow scale:, X = 0.359 Zeff + 0.744 (r = covalent radius (in A°)), r2, , , 1. Metallic and Non-metallic properties:, , Metallic property µ 1, EN, , Non-metallic property µ EN, Nonmetals, Metalloids, Metals, , 1. In periods: As we move from left to right in, a period, Zeff increases hence, EN increases., (Except Noble gases), Li < Be < B < C < N < O < F, Na < Mg < Al < Si < P < S < Cl, 2. In groups: As we move top to bottom in a group,, atomic size increases hence, EN decreases., F > Cl > Br > I, a. Exception:, Al < Ga , (High Zeff ), b. Exception: Due to lanthanoid contraction,, d-block:, 4d-series < 5d-series (high Zeff ), , Zr < Hf, Y > La (No lanthanoid contraction), p-block:, In < Tl, Sn < Pb, , N, O, B, L, E, G, A, S, , Metalloids or semi-metals: elements which can, both gain or loose e-., , B, , C, Si, Ge, , N, P, As, Sb, , O, S, Se, Te, Po, , H, F, Cl, Br, I, At, , Non-metals, , Metalloids, , s-block " Metals, d and f-block " Metals, p-block " Non-metals, metalloids, metals and, noble gases.
Page 17 :
1.8, , 2. Nature of bond:, Nature of interatomic bond depends on DEN., DEN, , Nature of bond, , 0, , Pure covalent, , 0.1 – 0.8, , Covalent, , 0.9 – 1.6, , Polar Covalent, , 1.7, , 50% ionic and 50% covalent, , 1.8 or more, , Ionic, , Covalent, , % ionic character = 16 (DEN) + 3.5 (DEN)2, (Henery – Smith formula), 3. Nature of hydride:, Hydrides: Binary compounds having one element, H., eg. CH4, H2S, HCl etc., (along the group), C N O F, i. Size of central atom (M) increases, Si P, S, Cl ii. Bond length of M-H bond increases, Ge As Se Br iii. H+ loosing tendency increases, Sn Sb Te I, iv. Acidic strength increases, , i., ii., iii., iv., v., , (along the period), EN of Central atom (M) increases, DEN of M-H bond increases, Bond polarity(or ionic character) of M-H, bond increases, Tendency to loose H+ in water increases, acidic strength increases, , Order of Acidic Strength:, HF < HCl < HBr < HI, CH4 < NH3 < H2O < HF, , , Na2O < MgO < Al2O3 < SiO2 < P4O10 < SO3 < Cl2O7, +2, +4, +7, , MnO, <, MnO2 < Mn2O7, , +1, , N2O, , <, , +2, NO, , <, , +3, N2O3, , <, , +5, N2O5, , c. Non-metallic oxides are generally acidic. (Some, are neutral), Neutral oxides are these which do not react, with both acid and base, eg. CO, NO, N2O, H2O, d. Metallic oxides are generally basic. (Some are, amphoteric), Amphoteric oxides are those which react, with both acid and base., eg. s-block: BeO, , d-block: TiO2, VO2, CrO2, Cr2O3, MnO2,, Mn3O4, ZnO etc., p-block: Al2O3, Ga2O3, SnO, SnO2, PbO,, PbO2, As2O3, Sb2O3 etc., Some metallic oxides like CrO3, Mn2O7 etc, are acidic in nature., , 1. Atomic density:, (a) In groups: Down the group both atomic mass, and atomic volume increases but increment, in mass is much more than volume. Hence,, atomic density increases., Exception: Density of Na > K, , Mg > Ca, (b) In periods:, S block, , <, , d-block, , >, , p-block, , CH4 < H2S < HI, , 4. Nature of hydroxides and oxides:, a. oxides form hydroxides in water hence, the nature, of oxides and hydroxides of an element is similar., b. Acidic strength of oxides and hydroxides µ EN of, central atom, , Order of acidic strength:, ClOH > BrOH > IOH, MgO > CaO > SrO > BaO, , Lighter metal, , Heavy metal, , 2. Melting point and boiling point:, (a) In periods: The general order is,, s-block < d-block > p-block, (b) In groups: Down the group the general order, is:, s-block, decreases, , d-block, increases, , groups 13 and 14, decreases, , groups15 to18, increases
Page 18 :
1.9, , Solved Examples, 1. Which of the following is incorrect match?, (a) Z = 48, group = IIB , period No. = 5th, (b) (Xe) 4f7 5d1 6s2, group = IIIB , period = 6th, (c) (Rn) 6d2 7s2, group = IVB, period = 7th, (d) Z = 56, group = IIA , period = 6th, , (a) Co+2, Cr+3, V+3, (b) Mn+2, Fe+3, Cr+, (c) Ni+2, Mn+2, Co+2, (d) Fe+2, Mn+2, Co+2, , Sol.(c) Element, having Z = 48, is Cd, , Ion , Electronic configuration, , , It is member of group 12 or IIB and period 6, , th, , Element having electronic configuration (Xe) 4f7, 5d1 6s2 is a lanthanoid. All lanthanoids belong to, group IIIB and period 6th., Element having electronic configuration (Rn) 6d2, 7s2 is an actinoid. All actinoids belong to group, IIIB and period 7th., Element, having Z = 56, is Ba. It is member of, group 2 or IIA and period 6th., 2. Which of the following metals give inflammable, gas with both acid and base?, (a) Na and Zn, , (b) Mg and Al, , (c) Mg and Be, , (d) Zn and Al, , Sol.(d) Amphoteric metals like Be, Zn, Al, Sn, Pb etc, give H2 gas (inflammable) with both acid and, base., 3. Which of the following have an incorrect order of, ionization energy:, (a) Pb (IE) > Sn (IE), (b) Na+ (IE) > Mg+ (IE), (c) Li+ (IE) < O+ (IE), (d) Be+ (IE) < C+ (IE), Sol.(c) Due to lanthanoid contraction Pb has greater, effective nuclear charge (zeff) than Sn hence,, Pb (IE) > Sn (IE), Na+ has electronic configuration of noble gas, hence,, Na+ (IE) > Mg+ (IE), +, Li has electronic configuration of noble gas, hence,, Li+ (IE) > O+ (IE), +, C has greater effective nuclear charge (zeff) than, Be+ hence,, Be+ (IE) < C+ (IE), 4. Which set of ions have same magnetic moment?, , Sol.(b), , Co+2, Cr+3, V+3, Mn+2, Fe+3, Cr+ , Ni+2, Fe+2, , No. of, unpaired e-, , (Ar) 4s0 3d7, (Ar) 4s0 3d3, (Ar) 4s0 3d2, (Ar) 4s0 3d5, (Ar) 4s0 3d5, (Ar) 4s0 3d5, (Ar) 4s0 3d8, (Ar) 4s0 3d6, , 3, 3, 2, 5, 5, 5, 2, 4, , Ions, having similar number of unpaired e- , have, same magnetic moment., 5. The correct order of acidic strength of the, following is:, (a) SO2 > P2O3 > SiO2 > Al2O3, (b) P2O3 > SO2 > SiO2 > Al2O3, (c) P2O3 > Al2O3 > SO2 > SiO2, (d) Al2O3 > SiO2 > P2O3 > SO2, Sol.(a) Acidic strength of oxides depends on, electronegativity, of, central, atom. As, electronegativity of central atom increases acidic, strength also increases., Correct order of acidic strength is:, SO2 > P2O3 > SiO2 > Al2O3, 6. Ionization energy of F- is 320 kJ mol-1. The, electron gain enthalpy of fluorine would be:, (a) – 320 kJ mol-1, (b) – 160 kJ mol-1, (c) + 320 kJ mol-1, (d) + 160 kJ mol-1, Sol.(a) Ionization energy of F- is 320 kJ mol-1, F- (g) + 320 kJ mol-1 " F (g) + e-;, mol-1, , H = 320 kJ, , Equation for electron gain enthalpy of F is:, F(g) + e- " F- (g) + 320 kJ mol-1; H = –320 kJ, mol-1
Page 19 :
1.10, , 7. The value of IE1, IE2, IE3, and IE4 of an atom are, 7.5 eV, 25.6 eV, 48.6 eV and 170.6 eV respectively., The electronic configuration of the atom will be:, (a) 1s2 2s2 2p6 3s1, (b) 1s2 2s2 2p6 3s2 3p1, (c) 1s2 2s2 2p6 3s2 3p3, (d) 1s2 2s2 2p6 3s2, , For 1 mol, energy needed is 495 kJ, , Sol.(b) The biggest jump occurs from IE3 to IE4, , (c) O > F > N > C (d) F > O > N > C, , IE3 < < IE4, , Sol.(c) IE2 of neutral element is IE1 of cation having, single positive charge. Hence, for order of IE2,, first put +1 charge to each element then write, electronic configuration., , (IEn), , (IEn+1), , n(Valence e-) = 3, Hence, the electronic configuration of the atom, will be 1s2 2s2 2p6 3s2 3p1., 8. The correct order increasing radii is:, (a) Be2+, Mg2+, Na+ (b) K+, Ca2+, S2 (c) O2-, F- , N3-, , (d) S2-, O2-, As3-, , Sol.(a) Correct order of increasing radii are: (a) Be+2 < Mg+2 < Na+, (b) Ca+2 < K+ < S-2, (c) F- < O-2 < N-3, (d) O-2 < S-2 < As39. What will be the distance between H and Cl atom, in HCl. The radius of hydrogen is 0.37 Å and the, radius of chlorine is 1.67 Å?, (According to the concept of covalent radius), (a) 2.04Å, , (b) 1.96Å, , (c) 2.12Å, , (d) 1.0Å, , Sol.(b) Bond length of single covalent bond = rA + rB –, 0.09 ( EN), Bond length (dH - cl) = rH + rcl – 0.09 ( EN), rH = 0.37Å; rcl = 1.67Å and EN = 3.0 – 2.1 = 0.9, dH – cl = 0.37 + 1.67 – 0.09 (0.9), or, dH – cl = 2.04 – 0.08 = 1.96Å, 10. The ionization energy of sodium is 495 kJ mol–1., How much energy is needed to convert atoms, present in 2.3 mg of sodium into sodium ions?, (a) 4.95 J, , (b) 49.5 J, , (c) 495 J, , (d) 0.495 J, , Sol.(b) Ionization energy of Na = 495 kJ/mol, No. of moles of Na in 2.3 mg, 2.3×10, = ––––––– = 10-4 moles, , 23, , , Hence, for 10-4 mol, energy, 495 × 103 × 10-4 J = 49.5 J, , needed, , is, , 11. The correct order of the second ionization, potential of carbon, nitrogen, oxygen and fluorine, is, (a) C > N > F > O (b) O > N > F > C, , C+ N+, , O+ , , 1s2 2s2 2p6 1s2 2s2 2p2 1s2 2s2 2p3, , Half filled, , , F+, , 1s2 2s2 2p4, , From left to right in a period, IE1 increases and, fulfilled or half filled elements have greater IE1, than adjacent elements. Hence, correct order of, IE2 is: C > N > F > O, 12. The electronegativity of the following elements, increases in the order:, (a) S < P < N < O (b) P < S < N < O, (c) N < O < P < S (d) N < P < S < O, Sol. (b), , Group 15, , Group 16, , Period (II), , N, , O, , Period (III), , P, , S, , Correct order of electro negativity is:, P<S<N<O, 13. The formation of the oxide ion, O2-(g), from, oxygen atom requires first an exothermic and, then an endothermic step as shown below :, O(g) + e– " O-(g); egH = -141 kJmol-1, O-(g) + e- " O2-(g);, , egH, , = +780 kJmol-1, , Thus process of formation of O2- in gas phase is, unfavorable even O2- is isoelectronic with neon. It, is due to the fact that :, (a) Oxygen is more electronegative., (b) Addition of electron in oxygen results in, larger size of the ion., (c) Electron repulsion outweighs the stability, gained by achieving noble gas configuration.
Page 20 :
1.11, , (d) O- ion has comparatively smaller size than, oxygen atom., Sol.(c) Process of formation of O2- ion in gaseous phase, is unfavorable because O- ion (anion) resists, addition of another e- due to repulsion hence,, electron repulsion outweighs the stability gained, by achieving noble gas configuration., 14. Which is the correct in the following (a) Radius of Cl atom is 0.99 Å, while that of Cl+, ion is 1.54 Å, (b) Radius of Cl atom is 0.99 Å, while that of Na, atom is 1.54 Å, (c) Radius of Cl atom is 0.99 Å, while that of Clion is 0.81 Å, (d) Radius of Na atom is 0.95 Å, while that of, Na+ ion is 1.54 Å, Sol.(b) The atomic radius decreases along the period., Also cations are always smaller than their parent, atom and anions are always larger than their, parent atom., 15. Which oxide of ‘N’ is isoelectronic with CO2:, (a) NO2, (b) NO, (c) N2O, (d) N2O2, Sol.(c) N2O is isoelectronic with CO2. Both have 22, electrons., 16. Arrange Ce3+, La3+, Pm3 and Yb3+ in increasing, order of their size (a) Yb3+ < Pm3+ < Ce3+ < La3+, (b) Ce3+ < Yb3+ < Pm3+ < La3+, (c) Yb3+ < Pm3+ < La3+ < Ce3+, (d) Pm3+ < La3+ < Ce3+ < Yb3+, Sol.(a) Lanthanide contraction is observed in these ions,, i.e., ionic radius decreases as atomic number, increases., 17. In which of the following compounds chromium, shows maximum radius: (a) K2Cr2O7, , (b) CrO2Cl2, , (c) Cr2(SO4)3, , (d) CrCl2, , Sol.(d) In CrCl2, oxidation state of chromium is +2, (minimum). Thus it will have maximum radius., As positive oxidation state increases, radius, decreases., 18. The IP1, IP2, IP3, IP4, and IP5 of an element are, 7.1, 14.3, 34.5, 46.8, 162.2 eV respectively., The element is likely to be (a) Na, , (b) Si, , (c) F, , (d) Ca, , Sol.(b) The jump in IP values exist in IP5 and thus, removal of fifth electron occurs from inner, shell. Thus element contains four electrons in its, valence shell. It means the element belongs to the, group 14., 19. Following are ground state, configuration of some neutral atoms:, (a) 1s2 2s2 2p3, , electronic, , (b) 1s2 2s2 2p5, , (c) 1s2 2s22p6 3s1 (d) 1s2 2s2 2p6, (i) Which of the following would have lowest, IE?, (ii) Arrange them in increasing order of IE, Sol. (i) Three electrons in 2p subshell (i.e. half, filled) indicate for its greater stability while, 6 electrons in 2p indicate for its maximum, stability. Thus electronic configuration, (c) having 1 electron in 3s would require, minimum IE, (ii) c < a < b < d (increasing order of IE), 20. The atomic number of three elements A, B and, C are a, a+1 and a+2, C is an alkali metal. In a, compound of A and C, the nature of bonding is (a) Co-ordinate, , (b) Covalent, , (c) Ionic, , (d) Metallic, , Sol.(c) If C is alkali metal, A should be halogen (nonmetal). Between metal and non-metal ionic bond, is present.
Page 21 :
1.12, , Exercise, (C) Transition elements, 2-, , +, , 1. X is isoelectronic with “O2 ” and has Z + 1, neutron (Z is atomic number of X2-) then:, (a) Mass number of X2- is 27, (b) Mass number of X2- is 57, (c) Atomic number of X2- is 28, (d) Number of proton X2- is 15, 2. Which of the following statements is not correct, regarding hydrogen atom ?, (a) It resembles halogens in some properties, (b) It resembles alkali metals in some properties, (c) It can be placed in 17th group of periodic, table, (d) It can not be placed in first group of periodic, table, 3. If an atom has electronic configuration 1s2 2s2 2p6, 3s2 3p6 3d3 4s2, it will be place in:, Second group, (c) Fifth group, , Third group, (d) Sixth group, , 4. Among the following, the element with the lowest, atomic number that has a ground-state electronic, configuration of (n-1) d5 ns1 is located in the:, (a) Fifth period, (b) Sixth period, (c) Fourth period (d) Third period, 5. In species X2+ the mass number is 20 and number, of neutrons are 10 then calculate the number of, electrons in species X2+:, 4, , (b) 7, , (c) 6, , (d) 8, , 6. The elements which are characterised by the, outer shell configuration ns1, ns2 and ns2 np1to, ns2np5are collectively called as:, (a) Transition elements, (b) Representative elements, (c) Lanthanides, (d) Inner-transition elements, 7., Column - I, (Type of element), (A) Inert gas elements, (B) Representative elements, , Column – II, (Outer electronic, configuration), (i) ns1-2 and ns2 np1 to ns2np5, 2, , 2, , (ii) 1s and ns np, , 6, , (iii) (n-2) f1-14 (n-1)d0-1 ns2, , (D) Inner- transition elements (iv) (n-1) d1-10 ns1 or 2, , (a), (b), (c), (d), , A- i, B-ii,C-iii, D-iv, A-ii, B-i, C-iii, D-iv, A-ii, B-i, C-iv, D-iii, A-i, B-ii, C-iv, D-iii, , 8. Which of the following is an incorrect match?, (a) Z = 65, group = IIIB, period – 6th, (b) Z = 46, group = VIIIB, period – 5th, (c) Z = 108, group = XB, period – 8th, (d) Z = 57, group = IIIB, period – 6th, 9. The element with atomic number 56 is likely to, have the same outer shell configuration as the, element with atomic number:, (a) 12, , (b) 18, , (c) 14, , (d) 24, , 10. Electronic configuration of species M2+ is 1s2,, 2s2, 2p6, 3s2,3p6,3d6 and its atomic weight is 56., The number of neutrons in the nucleus of species, M is:, (a) 32, , (b) 26, , (c) 30, , (d) 28, , 11. Which is correct order of ionic mobility in, aqueous medium?, , (a) Li+ < Na+ < K+, (b) Na+ < Mg2+ < Al3+, (c) Al3+ < Na+ < Mg2+, (d) Li+> Na+ > K+, 12. Which one of the following is not a characteristic, of p-block elements?, (a) The last electrons in them enters into a, p-orbital, (b) They mostly form acidic oxides, (c) Down the group, stability of lower oxidation, state increases, (d) They mostly form basic oxides, 13. Which of the following species has a value of, magnetic moment, m = 35?, (a) Cr3+, (c) Fe2+, , (b) Mn2+, (d) Cu2+
Page 22 :
1.13, , 14. The paramagnetic species among the following, is:, , 22. Which of the following has the largest ionic, radius?, , (a) Na+, , (b) Zn2+, , (a) Be2+, , (b) Mg2+, , (c) Cu+, , (d) Fe3+, , (c) Ca2+, , (d) Sr2+, , 15. All of the following possess complete d-subshells, except:, +, , (b) Cu, , 3+, , (d) Zn2+, , (a) Ag, (c) Ga, , 2+, , 16. Calculate the ‘X’ in Mnx+ if µ=3.87 BM, , 23. The correct order of the size of C, N, P and S is:, (a) N < C < P < S, , (b) C < N < P < S, , (c) N < C < S < P, , (d) C < N < S < P, , 24. Which of the following pair of elements have, almost similar atomic radii?, , (a) 2, , (b) 3, , (a) Zr, Hf, , (b) Cu, Ag, , (c) 4, , (d) 5, , (c) Sc, Ti, , (d) Pd, Ni, , 17. The first element of a group in many ways differs, from the other heavier members of the group., This is due to:, (a) the small size, (b) the high electronegativity and high ionization, potential, (c) the unavailability of d-orbitals, (d) all of the above, 18. Be and AI show diagonal relationship hence, both, have:, (a) almost same of electronegativity, (b) amphoteric nature of oxides, (c) approximately same polarizing power of, respective cations, (d) all the properties above, 19. Which of the following set contains pair of, elements that do not belong to same group but, show chemical resemblance?, (a) Hf, Zr, , (b) K, Rb, , (c) Be, Al, , (d) B, Al, , 20. Which of the following set of magic numbers is, not correct for given group?, (a) 18, 18, 32 & IIIB, (b) 8, 8, 18, 18, 32 & VIA, (c) 18, 32, 32 & IB, (d) 8, 8, 18, 18, 32 & IIA, 21. Correct order of ionic radius of following, isoelectronic species is:, (a) Se-2 > Br- > Kr > Rb+ > Sr+2, (b) S-2 > Cl- > K+ > Ar > Ca+2, (c) N-3 > O-2 > Ne > F- >Ca+2, (d) F- > Ne > Na+ > Al+3 > Mg+2, , 25. In which of the following compounds, manganese, shows maximum radius?, (a) MnO2, , (b) KMnO4, , (c) MnO, , (d) K3 (Mn(CN)6), , 26. Ionization enthalpies tend to decrease going, down any column of main group elements, because------------ going down the column., (a) Nuclear charge increases, (b) Number of shielding electrons increases, (c) Atomic size increases, (d) Effective nuclear charge increases, 27. The ionization potential of nitrogen is more than, that of oxygen because of:, (a) Greater attraction of electrons by the nucleus, (b) Extra stability of the half-filled p-orbitals, (c) Smaller size of nitrogen, (d) More penetration effect, 28. Which of the following transition involve, maximum amount of energy?, (a) M-(g) " M(g) (b) M-(g) " M+(g), (c) M+(g) " M2+(g) (d) M2+(g) " M3+(g), 29. Which of the following process refers to IE2 of, X?, (a) X(g) " X2+(g), (b) X+(g) " X2+(g), (c) X+(aq) " X2+(g) (d) X(g) " X+(g), 30. Which of the following statement concerning, ionization energy is not correct?, (a) The IE2 is always more than the first., (b) Within a group, there is a gradual increase, in ionization energy because nuclear charge, increases., (c) Ionization energy of Be is more than B., (d) Ionization energy of noble gases are high.
Page 23 :
1.14, , 31. Lanthanide contraction is related with:, (a) Sharp decrease in atomic size in lanthanide, series, (b) Slow or gradual decrease in atomic size in, lanthanide series, (c) Constancy in atomic size, (d) All the above, 32. Relation between electron gain enthalpy and, electron affinity is:, (a) EA = DHe.g., , (b) EA = 2DHe.g., , (c) EA = –2DHe.g. (d) EA = –DHe.g., 33. The process requiring absorption of energy is:, (a) N " N-, , (b) F " F-, , (c) Cl " Cl-, , (d) H " H-, , 34. Second and successive electron gain enthalpy of, an element, (a) is always negative (energy is released), (b) is always positive (energy is absorbed), (c) can be positive or negative, (d) is always zero, 35. Of the following pairs, the one containing, examples of metalloid elements is:, (a) B and Al, (b) Ga and Ge, (c) Al and Si, (d) As and Sb, 36. The group in the periodic table that contains the, elements in all the different physical states at, room temperature is:, (a) VA, (b) IA, (c) VIIA, (d) IVA, 37. Elements of which group form anions most, readily?, (a) Oxygen family (b) Nitrogen family, (c) Halogens, (d) Alkali metals, 38. What is the percentage of ionic character in CsF:, (according to Henry-Smith formula), {EN of Cs = 0.7 and EN of F = 4.0}, (a) 100%, (b) 10%, (c) 90.9%, (d) 99%, 39. In halogens, which of the following decrease, from iodine to fluorine?, (a) Bond length, (b) Electronegativity, (c) The ionization energy of the element, (d) Oxidizing power, , 40. As we proceed from top to bottom in the periodic, table:, (a) hydroxides are more basic, (b) oxyacids are less acidic, (c) neither (a) and (b) of the above, (d) Both (a) and (b) of the above, 41. Among the following oxides, which is least, acidic?, (a) Al2O3, , (b) B2O3, , (c) CO2, , (d) NO2, , 42. Which of the following oxides is neutral?, (a) SiO2, , (b) CO, , (c) ZnO, , (d) SnO2, , 43., , , , , , What is the nature of Al2O3 and B2O3?, (a) Acidic, Acidic, (b) Acidic, Amphoteric, (c) Amphoteric, Amphoteric, (d) Amphoteric, Acidic, , 44. Correct order of acidic strength is:, (a) SiH4 > PH3 > CH4 > HCl, (b) HCl > PH3 > CH4 > SiH4, (c) HCl > SiH4 > PH3 > CH4, (d) HCl > PH3 > SiH4 > CH4, 45. Which of the following oxide is acidic?, (a) N2O5, , (b) Mn2O7, , (c) CrO3, , (d) All, , 1. An element X belongs to fourth period and, fifteenth group of the periodic table. Which one of, the following is true regarding the outer electronic, configuration of X? It has:, A. Partially filled d-orbitals and completely, filled s-orbital, B. Completely filled s-orbital and completely, filled p-orbitals, C. Completely filled s-orbital and half filled, p-orbitals, D. Half filled d-orbitals and completely filled, s-orbital, (a) A,B & C, , (b) Only A & B, , (c) A, B & D, , (d) Only C
Page 24 :
1.15, , 2. Vishal Thakur went to meet his friend Sumit,, Where he saw that his friend was doing the study, of a particular chemistry book. But he could not, find the theoretical value of bond length in H-F, but he found that rH and rF are 0.37 Å and 0.72 Å, respectively and eletronegativity of F and H are, 4.0 and 2.1 respectively. What is bond length of, H-F bond?, (a) 1.09, , (b) 1.784, , (c) 0.92, , (d) 0.46, , 3. Choose the correct order of ionic radius for the, following species:, (a) Cl- > I- > Te2- > Ar+, (b) Te2- > I- > Cl- > Ar+, (c) I- > Te2- > Cl- > Ar+, (d) I- > Cl- > Ar+ > Te2-, , (c) General outermost shell e- configuration of, d-block element is ns 1-2 (n -1)d1-10, (d) All actinoids are man made elements, 9. A°/2 atoms of X(g) are converted into X+(g), by absorbing energy E1. A°/2 ions of X+(g) are, converted into X-(g) with release of energy E2., Hence ionization energy and electron affinity of, X(g) are:, (a), , 2E1, ,, A∞, , 2(E1 – E1 ), A∞, , (b), , 2E1, ,, A∞, , 2(E 2 – E1 ), A∞, , 2E 2, (E1 – E 2 ), , (c), ,, A∞, A∞, (d) None of these, , 4. Which statement is correct?, (a) Tl+ ion is more stable than Tl3+, (b) Pb4+ salts act as good oxidizing agents, (c) Bi5+ salts act as good oxidizing agents, (d) All of these, , 10. Which represents correct order of acidic strength?, (a) NH3 > PH3 > AsH3 > SbH3 > BiH3, (b) K2O > ZnO > NO2, (c) NaOH < KOH < RbOH < CsOH, (d) CH4 < NH3 < H2O < HF, , 5. Among the elements Ca, Mg, P and Cl, the order, of increasing atomic radii is:, (a) Mg < Ca < Cl < P, (b) Cl < P < Mg < Ca, (c) P < Cl < Ca < Mg, (d) Ca < Mg < P < Cl, , 11. Which of the following statements is incorrect?, (a) Cesium is the most electropositive element, while F is the most electronegative element, (b) Cl has the highest -ve electron gain enthalpy, out of all the elements, (c) Electron gain enthalpy of N as well as that of, noble gases is positive, (d) In any period, the atomic radius of the noble, gas is lowest, , 6. Element X belongs to 4th period. It contains 18, and 1 electron in the penultimate and ultimate, orbit. The element X should be:, (a) Normal element, (b) Transition element, (c) Inert gas, (d) Inner-transition element, 7. General electronic configuration of outermost, and penultimate shell is (n-1)s2 (n -1)p6 (n -1)dx, ns2 . If n = 4 and x = 5, then number of protons in, the nucleus will be :, (a) > 25, , (b) < 24, , (c) 25, , (d) 30, , 8. Select correct statement:, (a) La and Ac belong to f-block, (b) An element having atomic number 31 belongs, to 3rd period, , 12. Which of the following is correct order of, decreasing acidic character?, (i) ClO2 > SO2 > SiO2 > CO2, (ii) ClO2 > SO2 > SiO2 > SnO2, (iii) N2O3 > P2O3 > As2O3 > Bi2O3, (iv) N2O5 > P2O5 > As2O5 > Bi2O5, (a) i, ii, iii, , (b) ii, iii, iv, , (c) i, iii, iv, , (d) i, ii, iv, , 13. Which of the following conclusions are correct, regarding the element having atomic number, equal to 113?, (i) It is present in the 8th period of the modern, periodic table, (ii) It is present in the group 13 in the periodic, table
Page 25 :
1.16, , (iii) It is a p-block element, (iv) Oxidation states of this element may be +1 or, +3., (a) i, iii, iv, , (b) ii, iii, iv, , (c) i, ii, iv, , (d) i, iv, , 14. Which of the following statement(s) is(are), correct?, (a) The electronic configuration of Cr is (Ar) 3d4, 4s2 (Atomic number of Cr = 24), (b) Cr is a representative element., (c) In silver atom, 23 electrons have a spin of one, type and 24 of the opposite type., (d) The oxidation state of nitrogen in HN3 is –3., 15. Find the formula of halide of a metal whose, successive ionization energies are x, 2x, 5x, 20x,, 25x kJ mol-1 respectively., (a) MX, , (b) MX2, , (c) MX3, , (d) M2X, , 16. Which is/are true statement(s) about s-block, elements?, (a) Metals are obtained by the electrolysis of, fused chlorides, (b) Only one type of valency, +1 for IA and +2, for IIA, is shown, (c) Oxides are basic except BeO, (d) all of the above are correct statements, 17. Which of the following statement(s) is/are, correct?, (i) Vander waal’s radius of iodine is more than, its covalent radius, (ii) All isoelectronic ions belong to the same, period of the periodic table, (iii) IE1 of N is higher than that of O while IE2 of, O is higher than that of N, (iv) he 1st electron gain enthalpy of Cl is negative, while second is positive, (a) i, ii, , (b) i, ii, iii, , (c) i, iii, iv, , (d) i, ii, iii, iv, , 18. Consider the following electronic configuration, of an element (P):, (Xe)4f145d16s2, Then correct statement about element (P) is:, (a) It belongs to 6th period and 1st group, (b) It belongs to 6th period and 2nd group, , (c) It belongs to 6th period and 3rd group, (d) None of these, 19. The set representing the correct order of ionic, radius is:, (a) Na+ > Mg2+ >Al3+ > Li+ > Be2+, (b) Na+ > Li+ > Mg2+ > Al3+ > Be2+, (c) Na+ > Mg2+ > Li+ > Al3+ > Be2+, (d) Na+ > Mg2+ > Li+ > Al3+ > Be2+, 20. In the compound M-O-H, the M-O bond will be, broken in water if:, (a) D (EN) of M and O < D (EN) of O and H, (b) D (EN) of M and O = D (EN) of O and H, (c) D (EN) of M and O > D (EN) of O and H, (d) Cannot be predicated according D (EN) data, 21. Consider the following changes:, M(s) " M(g), ........................ (i), 2+, M(s) " M (g) + 2e ........................ (ii), M(g) " M+(g) + e- ........................ (iii), M+(g) " M2+(g) + e- ........................ (iv), M(g) " M2+(g) + 2e- ........................ (v), The second ionization energy of M could be, calculated from the energy values associated, with:, (a) i+iii+iv, (b) ii-i+iii, (c) i+v, (d) v-iii, 22. Consider the following conversions:, (i) O(g) + e- " O-(g), DH1, (ii) F(g) + e- " F-(g), DH2, (iii) Cl(g) + e- " Cl-(g), DH3, (iv) O-(g) + e- " O2-(g), DH4, That according to given information the incorrect, statement is:, (a) DH3 is more negative than DH1 and DH2, (b) DH1is less negative than DH2, (c) DH1, DH2 and DH3 are negative whereas DH4, is positive, (d) DH1 and DH3 are negative whereas DH2 and, DH4 are positive, 23. Ionic radii of:, (a) 35Cl- > 37Cl (c) K+ > Cl-, , (b) Mn7+ > Ti4+, (d) P3+ > P5+, , 24. The correct order of relative stability of half filled, and completely filled sub-shell is:, (a) p3 > d5 < d10 < p6 (b) d5 > p3 < d10 < p6, (c) d5< p3 < d10 < p6, , (d) p3 > d10 < d5 < p6
Page 26 :
1.17, , 25. The five successive ionization energies of an, element are 800, 2427, 3658, 25024 and 32824, kJ Mol-1 respectively. The number of valence, electron is:, (a) 3, , (b) 5, , (c) 1, , (d) 2, , 26. What is the order of ionization energies of the, coinage metal?, (a) Ag > Cu > Au, , (b) Cu > Ag > Au, , (c) Cu < Ag < Au, , (d) Au > Cu > Ag, , 27. IE2 for an element is invariably higher than IE1, because:, (a) It is difficult to remove electron from cation, (b) The size of the cation is smaller than its atoms, (c) Zeff is more for cation, (d) All the above, 28. Two p-block elements x (outer configuration ns2, np3) and z (outer configuration ns2 np4) occupy, neighbouring positions in a period. Using this, information which of the following is correct with, respect to their ionization potential Ix and Iz?, (a) Ix> Iz, (b) Ix< Iz, (c) Ix = Iz, (d) Relation between Ix and Iz is uncertain, 29. Fluorine has the highest electronegativity among, the group on the pauling scale, but the electron, affinity of fluorine is less than that of chlorine, because:, (a) The atomic number of fluorine is less than, that of chlorine, (b) Fluorine being the first member of the family, behaves in an unusual manner, (c) Chlorine can accommodate an electron better, than fluorine by utilising its vacant 3d orbital, (d) Small size, high electron density and an, increased electron repulsion make addition, of an electron to fluorine less favourable than, that in the case of chlorine, 30. Select correct statement about radius of an atom:, (a) Values of vanderwaal’s radii are larger, than those of covalent radii because the, vanderwaal’s forces are much weaker than, the forces operating between atoms in a, covalently bonded molecule., (b) The metallic radii are smaller than the vander, , waal’s radii, since the bonding forces in the, metallic crystal lattice are much stronger than, the vander waal’s forces., (c) Both (a) & (b), (d) None of these, 31. Which represents alkali metals (i.e. 1st group, metals) based on IE1 and IE2 values in kJ mol-1?, , (a), (b), (c), (d), , IE1, X 500, Y 600, Z 550, M 700, , IE2, 1000, 2000, 7500, 1400, , 32. Match the correct atomic radius with the element:, S.No., , Element, , Code Atomic radius (pm), , (i) Be (p) 74, (ii) , C (q) 88, (iii) O (r) 111, (iv) B (s) 77, (v) N (t) 66, (a), (b), (c), (d), , (i)–r, (ii)–q, (iii)–t, (iv)–s, (v)–p, (i)–t, (ii)–s, (iii)–r, (iv)–p, (v)–q, (i)–r, (ii)–s, (iii)–t, (iv)–q, (v)–p, (i)–t, (ii)–p, (iii)–r, (iv)–s, (v)–q, , 33. Electronic configurations of four element A, B, C, and D are given below:, (i) 1s2 2s2 2p6, , (ii) 1s2 2s2 2p4, , (iii) 1s2 2s2 2p6 3s1 (iv) 1s2 2s2 2p5, Which of the following is the correct order of, increasing tendency to gain electron ?, (a) i < iii < ii < iv, , (b) i < ii <iii < iv, , (c) iv < ii < iii < i, , (d) iv < i < ii < iii, , 34. Which of the following is the wrong statement?, (a) All the actinoid elements are radioactive., (b) Alkali and alkaline earth metals are s-block, elements., (c) Pnictogens and halogens are p-block elements., (d) The first member of the lanthanoid series is, lanthanum, 35. Which is true statement(s)?, (a) Larger the value ionization enthalpy, easier is, the formation of cation., (b) Larger the value of electron affinity, easier is, the formation of anion.
Page 27 :
1.18, , (c) Larger the value of ionization energy as well, as electron affinity, smaller is the Mulliken, electronegativity of atom., (d) Larger the Zeff, larger is the size of atom., 36. The lithium ion (Li+) and hydride ion (H-) are, isoelectronic ions. Which statement about these, systems is true?, (a) Chemical properties of these ions are identical, since they are isoelectronic., (b) Li+ is a stronger reducing agent than H (c) More energy is needed to ionize H- than Li+, (d) Radius of H- is larger than that of Li+, 37. The correct order of increasing first ionization, energy is:, (a) Ca < K < Ne < P < F, (b) F < Ca < Ne < P < K, (c) K < Ca < P< F < Ne, (d) Ne < F < P < Ca < K, 38. The number of d-electrons in Fe2+, (atomic number = 26) is not equal to that of:, (a) p-electrons in 10Ne, (b) s-electrons in 12Mg, (c) d-electrons in Fe, (d) p-electrons in Cl39. Which of the following transition results in, increase in magnetic moment value?, (a) Mn2+ " Mn4+ (b) Ni2+ " Ni4+, (c) Cu2+ " Cu+, , (d) Zr " Zr2+, , 40. The compound of vanadium with chlorine has, magnetic moment 1.73 BM. The vanadium, chloride has the formula:, (a) VCl2, (b) VCl3, (c) VCl4, (d) VCl5, 41. Which of the following order of radius is not, correct?, (a) Yb+3 < Pm+3< Ce+3 < La+3, (b) Mg+2 < Na+ < Al < F (c) K > Ca > Mg > Li, (d) O < O-2 < F < F42. Correct trend of first ionization energy in group-13, is:, (a) B > Al > Ga > In > Tl, (b) B > Al > Ga > Tl > In, (c) B > Tl > Ga > Al > In, (d) B > Ga > Al > In > Tl, , 43. Which has the lowest anion to cation size ratio?, (a) LiF, , (b) NaF, , (c) Csl, , (d) CsF, , 44. Select the incorrect statement:, (a) Size of H- is larger than F (b) Rb is more electropositive compared to Ca, (c) Na+ is more electronegative than the Na, (d) Cl-is more electronegative than that of F, 45. Four elements P, Q, R and S have atomic number, Z-1, Z, Z+1 and Z+2 respectively. If Z is 17, then, bond between which pair of elements will be least, covalent:, (a) S and Q, , (b) P and R, , (c) S and R, , (d) S and P, , ONE OR MORE THAN ONE OPTIONS CORRECT, TYPE, 1. Select the correct statement(s):, (a) Alkali metals have lowest IE in respective, period., (b) Noble gases have highest IE in respective, period., (c) EA1 of N < EA1 of O, (d) F-is the strongest reducing agent among, halide ions., 2. The electronic configuration of given species (X), is 1s2, 2s2, 2p6,3s2,3p6,3d5, 4s1. This can be its:, (a) Cationic form X+ (b) Anionic form X (c) Excited state, , (d) Ground state, , 3. In which of the following arrangements, the order, is according to the property indicated against it?, (a) IE1: O > N > C > B, (b), , egH, , (with – ve sign): Cl > F > Br > I, , (c) Metallic radius: Rb > K > Na > Li, (d) Ionic size: F-> Na+ > Mg2+ > Al3+, 4. In which of the following arrangements, the order, is according to the property indicated against it?, (a) Basic strength: SbH3 > AsH3 > PH3 > NH3, (b) IE1: N > O > C > B, (c) Oxidising power: PbO2 > SnO2 > SiO2 > CO2, (d) Acid strength: HI > HBr > HCl > HF
Page 28 :
1.19, , 5. Which of the following orders is (are) correct for, size?, (a) Al ª Ga, 3+, , 2-, , -, , +, , 2+, , (b) Te > I > Cs > Ba, 6+, , (c) Cr < Cr, , (d) Pd ª Pt, , 6. Which of the following statements is/are correct?, (a) The second ionization enthalpy of oxygen, element is greater than that of fluorine, element., (b) The third ionization enthalpy of phosphorus, is greater than that of aluminium., (c) The first ionization enthalpy of aluminium is, slightly greater than that of gallium., (d) The second ionization enthalpy of copper is, greater than that of zinc., 7. Which of the following is/are correct order(s)of, electron affinity?, (a) N < C < O < F (b) P < Si < S < Cl, (c) Si < P < S < Cl (d) C < N < O < F, 8. Which of the following is correct order of, electronegativity?, (a) Cs > Rb > Na, , (b) Li < Be < B, , (c) C < N < O, , (d) Cl > F > Br, , 9. Poor shielding of nuclear charge by d or f- orbital, electrons is responsible for which of the following, facts?, (a) Atomic radius of Nb (4d- series) is, comparable to that of Ta (5d- series)., (b) The 1st ionization enthalpy of copper is less, than that of zinc., (c) The value of electron gain enthalpy is more, negative for sulphur than for oxygen., (d) The 1st ionization energy for gold is greater, than that of silver., 10. Which of the following is/are true order(s)?, (a) B+ < B < BSize, (b) I < Br < Cl < F, Electron gain enthalpy, (with negative sign), (c) O2-< O < O+, Zeff, (d) Na < Al < Mg < Si Ionization potential, 11. Select the endothermic step(s):, (a) S-(g) + e- " S2-(g), (b) Ne(g) + e- " Ne-(g), (c) N(g) + e- " N-(g), (d) Al2+(g) " Al3+(g)+ e-, , COMPREHENSIONS TYPE QUESTIONS, Read the following passage carefully and answer the, question., Comprehension # 1 (Q. 12 to 14), It is not possible to measure the atomic radius precisely, since the electron cloud surrounding the atom does, not have a sharp boundary. One practical approach to, estimate the size of an atom of a non-metallic element is, to measure the distance between two atoms when they are, bound together by a single bond in a covalent molecule, and then dividing by two. For metals we define the term, “metallic radius” which is taken as half the internuclear, distance separating the metal cores in the metallic crystal., The van der Waal’s radius represents the overall size of, the atoms which includes its valence shell in a non bonded, situation. It is the half of the distance between two similar, atoms in separate molecules in a solid. The atomic radius, decreases across a period and increases down the group., Same trends are observed in case of ionic radius of the, species having same number of electrons depends on the, number of protons in their nuclei. Sometimes, atomic and, ionic radii give unexpected trends due to poor shielding of, nuclear charge by d- and f-orbital electrons., 12. Which of the following relations is correct, if, considered for the same element ?, (a) R Van der Waal > R Covalent > R Metallic, (b) R Covalent > R Metallic > R Van der Waal, (c) R Van der Waal > R Metallic > R Covalent, (d) R Metallic > R Covalent > R Van der Waal, 13. K+, Cl-, Ca2+, S2- ions are isoelectronic. The, decreasing order of their size is:, (a) Ca2+ > K+ > Cl- > S2(b) S2- > Cl- > K+ > Ca2+, (c) K+ > Cl- > Ca2+ > S2 (d) S2- > Cl- > Ca2+ > K+, 14. Select the INCORRECT option regarding atomic/, ionic sizes:, (a) Zn > Cu, , (b) Pb2+ > Pb4+, , (c) Zr ª Hf, , (d) N3- < Al3+, , Comprehension # 2 (Q. 15 to 17), Effective nuclear charge (Zeff) is the net attractive force on, electrons under Consideration and is equal to:, Zeff = Z – s (nuclear charge – screening constant). Zeff or, s is calculated by Slater’s formula, as given.
Page 29 :
1.20, , If one electron is present in the outermost orbit, there will, be no screening in that orbital. Each electron contribute,, 0.35 (total electrons minus one electron) present in the, outermost shell., A contribution of 0.85 for each electron is taken in the, (n-1)th shell., For all other electrons contribution is 1 for each electron., 15. The screening constant (s) for 4s electron of Mn, (Z = 25) will be :, (a) 18.00, , (b) 4.25, , (c) 18.35, , (d) 21.40, , 16. Which of the following statement is wrong?, (a) IE1 of Ga > Al, due to imperfect shielding of, 3d-orbitals in Ga., (b) IE1 of Ga > Al, due to perfect shielding of, 3d-orbitals in Ga., (c) The atomic size of Ga and Al are almost same, because of poor shielding effect of electrons, in d-orbitals as the effective nuclear increases, in Ga., (d) IE1 of group 16 elements is less than that of, group 15 elements., 17. Which of the following statement is wrong?, (a) The number of lobes in d-orbitals are 4., (b) IE1 of element increases along the period., (c) IE1 of the group 3 elements is more than that, of the group 2 elements, (d) IE1, IE2 and IE3 of an element are 9.5, 18.5, and 154.4 eV predict that the element has, either two s-electrons or two p-electrons in, the valence shell., , The energy required to remove an electron from the, outermost shell of an isolated gaseous atom is known as, IE1 of that atom. Similarly, the energy required for the, removal of the electron from the unipositive ion, dipositive, ion and tripositive ion are known as IE2, IE3 and IE4, respectively, and are called successive ionization energies., The magnitude of the charge depends on the size of the, orbital of electron. Electrons in smaller orbitals are on, average close with each other and have more repulsion., Thus for Be (2s2), the IE1 and IE2 are 9.3 and 18.2 eV, atom-1, whereas for Ca (4s2), the vales are 6.1 and 11.9 eV., 18. The correct order of arrangement of the first, ionization energies of C, N, O and F (in decreasing, values) is:, , (a) C > N > O > F (b) O > N > F > C, (c) O > F > N > C (d) F > N > O > C, 19. Four elements have the following first ionization, energies in kJ mol-1 : 762, 709, 59 and 558. The, elements are Ga, Ge, In and Sn (not in order)., Which of these elements has the ionization energy, of 762 kJ mol-1?, (a) In, , (b) Ga, , (c) Sn, , (d) Ge, , 20. Among the following ionization reactions, which, one will have the maximum value of ionization, energy?, (a) Be " Be+, , (b) Be+ " Be2+, , (c) Sr " Sr+, , (d) Sr+ " Sr2+, , Comprehension # 4 (Q. 21 to 23), Energy is released when an electron is added to neutral, isolated gaseous atom in its ground state to give monoanion, and this is known as EA1 or egH1. Greater is the amount, of energy released the greater will be EA. EA is expressed, in eVatom-1 or kJ mol-1, 21. EA values of N and P are exceptionally low,, because:, (a) Both N and P have half-filled p-orbitals in the, valence shell., , (b) The atom is more stable than the, corresponding anion., (c) The electronic configuration of the anion, N- and P- is relatively more stable than the, corresponding atom., (d) Both (b) and (c)., 22. Select the correct statements (More than one, correct):, (a) EA1 and DegH1 of an atom of element have, same magnitude, (b) DegH1(-ve) of Al > B, (c) DegH1(-ve)of P > N, (d) DegH1(-ve) of S > O, 23. Select the correct statements (More than one, correct):, (a) DegH1of noble gases have large positive, values., (b) DegH1 of noble gases have large negative, values., (c) DegH1 if helium (He) is the lowest of all the, noble gases., (d) DegH1 of Ar is lower than that of Ne.
Page 30 :
1.21, , SINGLE AND DOUBLE VALUE INTEGER TYPE, QUESTIONS, 24. Most stable oxidation state of thallium is +n., What is the value of n?, 25. Total number of elements which have more, ionization energy as compare to their next higher, atomic number elements. Li, Be ,B, C, N, O, F,, Ne, 26. How many elements are more electropositive, than Cl?, B, N, O, S, P, At, H, Li, 27. Total number of elements which have only, single oxidation state (other than zero) in their, corresponding stable compounds: Cs, Ba, F, Zn,, Be, Al, Sr, Ga, Pb, 28. How many pairs in their first species have lower, ionization energy than second species?, (a) N and O, (b) Li and Li+, (c) O and S, (d) Ba and Sr, (e) I and I–, (f) Be and B, (g) Br and K, MATCHING THE COLUMN TYPE QUESTIONS, 29., , 1. The correct order of acidic strength is:, (a) Cl2O7 > SO2 > P4O10, (b) CO2 > N2O5 > SO3, (c) Na2O > MgO >Al2O3, (d) K2O > CaO > MgO, (III-JEE, 2000), 2. The correct order of radii is:, (a) N < Be < B, , (b) F-< O2- < N3-, , (c) Na < Li < K, , (d) Fe3+ < Fe2+ < Fe4+, (III-JEE, 2000), , 3. The set representing the correct order of first, ionization potential is:, (a) K > Na > Li, , (b) Be > Mg > Ca, , (c) B > C > N, , (d) Ge > Si > C, (III-JEE, 2001), , 4. Identify the least stable ion amongst the following:, (a) Li-, , (b) Be-, , (c) B-, , (d) C(III-JEE, 2002), , Column I, , Column II, , a. Na > Mg > Al > B p., , Oxidizing nature, , b. F > N > C > B > Si q., , Lowest IE1, , c. F > O > Cl > N, , r., , Metallic character, , d. Out B, C, Al and, Si, C have, , s., , Non-metallic, character, , t., , Highest IE1, , 30., Column I, , Column II, , a., , N2O, , p., , Normal oxide, , b., , Na2O, , q, , Neutral oxide, , c., , Ga2O3, , r., , Suboxide, , d., , C3O2, , s., , Basic oxide, , e., , Mn3O4, , t., , Amphoteric oxide, , f., , SnO2, , u., , Mixed oxide, , 5. Identify the correct order of acidic strengths of, CO2, CuO, CaO, H2O is:, (a) CaO < CuO < H2O < CO2, (b) H2O < CuO < CaO < CO2, (c) CaO < H2O < CuO < CO2, (d) H2O < CO2 < CaO < CuO, (IIT-JEE, 2002), 6. Statement-1: Pb4+ compounds are stronger, oxidizing agents than Sn4+ compounds., Statement-2: The higher oxidation states for the, group 14 elements are more stable for the heavier, members for the group due to inert pair effect., (a) Statement-1 is True, Statement-2 is true,, Statement-2 is a correct explanation for, Statement-1., (b) Statement-1 is True, Statement-2 is true,, Statement-2 is NOT a correct explanation for, Statement-1., (c) Statement-1 is True, Statement-2 is False, (d) Statement-1 is False, Statement-2 is True, (III-JEE, 2008)
Page 31 :
1.22, , 7. Which of the following represent the correct order, of increasing IE1 for Ca, Ba, S, Se and Ar?, (a) S < Se < Ca < Ba < Ar, (b) Ba < Ca < Se < S < Ar, (c) Ca < Ba < S < Se < Ar, (d) Ca < S < Ba < Se < Ar, (III-JEE, 2013), 8. The correct order of ionic radius is:, (a) Ce > Sm > Tb > Lu (b) Lu > Tb > Sm > Ce, (c) Tb > Lu > Sm > Ce (d) Sm > Tb > Lu > Ce, (AIEEE, 2002), 9. Ce3+, La3+, Pm3+ and Yb3+ have ionic radii in the, increasing order as:, (a) La3+ < Ce3+ < Pm3+ < Yb3+, (b) Yb3+ < Pm3+ < Ce3+ < La3+, (c) La3+ = Ce3+ < Pm3+ < Yb3+, (d) Yb3+ < Pm3+ < La3+ < Ce3+, (AIEEE, 2002), 10. According to the modern Periodic Law of, elements, the variation in properties of elements, is related to their?, (a) Nuclear masses, (b) Atomic numbers, (c) Nuclear neutron-proton number ratio, (d) Atomic masses, (AIEEE, 2003), 11. The reduction in atomic size with increase in, atomic number is a characteristic of elements of:, (a) d-block, (b) f-block, (c) Radioactive series, (d) High atomic masses, (AIEEE, 2003), 12. Which one of the following groups represents, a collection of isoelectronic species? (Atomic, number of Cs is 55 and of Br is 35), (a) N3-, F-, Na+, (b) Be, Al3+, Cl (c) Ca2+, Cs+, Br (d) Na+, Ca2+, Mg2+, (AIEEE, 2003), 13. The atomic numbers of vanadium (V), chromium, (Cr), manganese (Mn) and iron (Fe) respectively, 23, 24, 25 and 26. Which one of these may be, expected to have the higher second ionization, enthalpy?, (a) Cr, (b) Mn, (c) Fe, (d) V, (AIEEE, 2003), , 14. Which one of the following sets of ions represents, the collection of isoelectronic species?, (a) K+, Cl-, Mg2+, Sc3+, (b) Na+, Ca2+, Sc3+, F (c) K+, Ca2+, Sc3+, Cl (d) Na+, Mg2+, Al3+, Cl(AIEEE, 2004), 15. Which one of the following ions has the highest, value of ionic radius?, (a) O2(b) B3+, (c) Li+, (d) F(AIEEE, 2004), 16. Among Al2O3, SiO2, P2O3 and SO2 the correct, order of acid strength is:, (a) Al2O3 < SiO2 < SO2 < P2O3, (b) SiO2 < SO2 < Al2O3 < P2O3, (c) SO2 < P2O3 < SiO2 < Al2O3, (d) Al2O3 < SiO2 < P2O3 < SO2, (AIEEE, 2004), 17. The formation of the oxide ion requires first, an exothermic and then an endothermic step as, shown below:, O(g) + e- = O-(g) DH° = –142 kJ mol-1, O-(g) + e- = O2-(g) DH° = 844 kJ mol-1, This is because of:, (a) O- ion will tend to resist the addition of, another electron, (b) Oxygen has high electron affinity, (c) Oxygen is more electronegative, (d) O- ion has comparatively larger size than, oxygen atom, (AIEEE, 2004), 18. Which among the following factors is the most, important in making fluorine the strongest, oxidizing halogen?, (a) Hydration enthalpy, (b) Ionization enthalpy, (c) Electron affinity, (d) Bond dissociation energy, (AIEEE, 2004), 19. Pick out the isoelectronic structure from the, following:, I. +CH3, II. H3O+, III. NH3, IV. CH3 (a) I and II, (b) III and IV, (c) I and III, (d) II, III and IV, (AIEEE, 2005)
Page 32 :
1.23, , 20. Which of the following factors may be regarded, as the main cause of lathanoid contraction?, (a) Poor shielding of one of 4f electron by, another in the subshell., (b) Effective shielding of one of 4f electrons by, another in the subshell., (c) Poorer shielding of 5d electrons by 4f, electrons, (d) Greater shielding of 5d electrons by 4f, electrons, (AIEEE, 2005), 21. In which of the following arrangements the, order is NOT according to the property indicated, against it?, (a) Al3+ < Mg2+ < Na+ < F- - Increasing ionic size, (b) B < C < N < O - Increasing first ionisation, enthalpy, (c) I < Br < F < Cl - Increasing electron gain, enthalpy (with negetive sign), (d) Li < Na < K < Rb - Increasing metallic redius, (AIEEE, 2005), 22. The lanthanide contraction is responsible for the, fact that:, (a) Zr and Y have about the same radius., (b) Zr and Nb have similar oxidation state., (c) Zr and Hf have about the same radius., (d) Zr and Zn have same oxidation state., (AIEEE, 2005), 23. Which of the following oxides is amphoteric in, character?, (a) SnO2, , (b) SiO2, , (c) CO2, , (d) CaO, (AIEEE, 2005), , 24. The increasing order of the first ionization, enthalpies of the elements B, P, S and F (lowest, first) is:, (a) F < S < P < B (b) P < S < B < F, (c) B < P < S < F (d) B < S < P < F, (AIEEE, 2006), 25. Which one of the following sets of ions represents, a collection of isoelectronic species?, (a) N3-, O2-, F-, S2 (b) Li+, Na+, Mg2+, Ca2+, (c) K+, Cl-, Ca2+, Sc3+, (d) Ba2+, Sr2+, K+, Ca2+, (AIEEE, 2006), , 26. Lanthanoid contraction is caused due to:, (a) The same effective nuclear charge from Ce to, Lu, (b) The imperfect shielding on outer electrons, by 4f electrons from the nuclear charge, (c) The appreciable shielding on outer electrons, by 4f electrons from the nuclear charge, (d) The appreciable shielding on outer electrons, by 5d electrons from the nuclear charge, (AIEEE, 2006), 27. Following statements regarding the periodic, trends of chemical reactivity of the alkali metals, and the halogens are given. Which of these, statements gives the correct picture?, (a) Chemical reactivity increases with increase, in atomic number down the group in both the, alkali metals and halogens, (b) In alkali metals the reactivity increases but, in the halogens it decreases with increase in, atomic number down the group, (c) The reactivity decreases in the alkali metals, but increases in the halogens with increase in, atomic number down the group, (d) In both alkali metals and the halogens the, chemical reactivity decreases with increases, in atomic number down the group, (AIEEE, 2006), 28. The set representing the correct order of ionic, radius is:, (a) Na+ > Li+ > Mg2+ > Be2+, (b) Li+ > Na+> Mg2+ >Be2+, (c) Mg2+ > Be2+ > Li+ > Na+, (d) Li+ > Be2+ > Na+ > Mg2+, (AIEEE, 2009), 29. The correct sequence which shows decreasing, order of the ionic radii of the elements is:, (a) Al3+ > Mg2+ > Na+> F-> O2 (b) Na+ > Mg2+ > Al3+ > O2- > F (c) Na+ > F- > Mg2+ > O2- > Al3+, (d) O2- > F-> Na+> Mg2+ > Al3+, (AIEEE, 2010), 30. The outer electronic configuration of Gd (Atomic, number 64) is:, (a) 4f3 5d5 6s2, , (b) 4f8 5d0 6s2, , (c) 4f4 5d4 6s2, , (d) 4f7 5d1 6s2, (AIEEE, 2011)
Page 33 :
1.24, , 31. The correct order of electron gain enthalpy with, negative sign of F, Cl, Br and I having atomic, number 9,17, 35 and 53 respectively is:, (a) F > Cl > Br > I (b) Cl > F > Br > I, (c) Br > Cl > I > F (d) I > Br > Cl > F, (AIEEE, 2011), 32. Which one of the following orders presents the, correct sequence of the increasing basic nature of, the given oxides?, (a) Al2O3 < MgO < Na2O < K2O, (b) MgO < K2O < Al2O3 < Na2O, (c) Na2O < K2O < MgO < Al2O3, (d) K2O < Na2O < Al2O3 < MgO, (AIEEE, 2011), 33. The increasing order of the ionic radii of the given, isoelectronic species is:, (a) Cl-, Ca2+, K+, S2-, , (b) S2-, Cl-, Ca2+, K+, , (c) Ca2+, K+, Cl-, S2-, , (d) K+, S2-, Ca2+, Cl(AIEEE, 2012), , 34. Which of the following presents the correct order, of second ionization enthalpies of C, N, O and F?, (a) O > N > F > C (b) F > O > N > C, (c) C > N > O > F (d) O > F > N > C, (JEE Main Online 2012), 35. Which among the following elements has the, highest ionization enthalpy?, (a) Nitrogen, , (b) Boron, , (c) Carbon, , (d) Oxygen, (JEE Main Online 2012), , 36. Electron gain enthalpy with negative sign of, fluorine is less than that of chlorine due to:, (a) High ionization enthalpy of fluorine, (b) Smaller size of chlorine atom, (c) Smaller size of fluorine atom, (d) Bigger size of 2p orbital of fluorine, (JEE Main Online 2013), 37. The order of increasing sizes of atomic radii, among the elements O, S, Se and As is:, (a) As < S < O < Se, (b) Se < S < As < O, (c) O < S < As < Se, (d) O < S < Se As, (JEE Main Online 2013), , 38. What is the following represents the correct order, of increasing first ionization enthalpy for Ca, Ba,, S, Se and Ar?, (a) Ca < S < Ba < Se < Ar, (b) S < Se < Ca < Ba < Ar, (c) Ba < Ca < Se < S < Ar, (d) Ca < Ba < S < Se < Ar, (JEE Main, 2013), 39. The first ionization potential of Na is 5.1 eV. The, value of electron gain enthalpy of Na+ will be:, (a) -2.55 eV, , (b) -5.1 eV, , (c) -10.2 eV, , (d) +2.55 eV, (JEE Main, 2013), , 40. Similarity in chemical properties of the atoms of, elements in a group of the periodic table is most, closely related to:, (a) Atomic numbers, (b) Atomic masses, (c) Number of principal energy levels, (d) Number of valence electrons, (JEE Main Online 2014), 41. Which of the following arrangements represents, the increasing order (smallest to largest) of ionic, radii of the given species O2-, S2-, N3-, P3-?, (a) O2- < N3- < S2-< P3 (c) N3-< O2-< P3-< S2-, , (b) O2-< P3-< N3-< S2(d) N3- < S2-< O2-< P3(JEE Main Online 2014), , 42. The ionic radii (in Å) of N3- , O2- and F- are, respectively:, (a) 1.36, 1.40 and 1.71, , (b) 1.36, 1.71 and 1.40, , (c) 1.71, 1.40 and 1.36, , (d) 1.71, 1.36 and 1.40, (JEE Main, 2015), , 43. Which of the following atoms has the highest first, ionization energy?, (a) Na, , (b) K, , (c) Sc, , (d) Rb, (JEE Main, 2016)
Page 34 :
1.25, , Answer Key, 1. (a), 11. (a), 21. (a), 31. (b), 41. (a), , 2. (d), 12. (d), 22. (d), 32. (d), 42. (b), , 3. (c), 13. (b), 23. (c), 33. (a), 43. (d), , 4. (c), 14. (d), 24. (a), 34. (b), 44. (d), , 5. (d), 15. (b), 25. (c), 35. (d), 45. (d), , 6. (b), 16. (c), 26. (c), 36. (c), , 7. (c), 17. (d), 27. (b), 37. (c), , 8. (c), 18. (d), 28. (d), 38. (c), , 9. (a), 19. (c), 29. (b), 39. (a), , 10. (c), 20. (b), 30. (b), 40. (d), , 1. (d), 11. (d), 21. (d), 31. (c), 41. (d), , 2. (c), 12. (d), 22. (d), 32. (c), 42. (c), , 3. (b), 13. (b), 23. (d), 33. (a), 43. (d), , 4. (d), 14. (c), 24. (c), 34. (d), 44. (d), , 5. (b), 15. (c), 25. (a), 35. (b), 45. (a), , 6. (b), 16. (d), 26. (d), 36. (d), , 7. (c), 17. (c), 27. (d), 37. (c), , 8. (c), 18. (c), 28. (a), 38. (d), , 9. (b), 19. (b), 29. (d), 39. (b), , 10. (d), 20. (c), 30. (c), 40. (c), , 1. (a,b,c), 2. (a,d), 9. (a,d) 10. (a,c,d), 17. (c), 18. (d), 25. (2), 26. (6), 29. a r; b s; c p;d t, 30. a q,r; b p,s; c t; d, , 1. (a), 11. (b), 21. (b), 31. (b), 41. (a), , 2. (b), 12. (a), 22. (c), 32. (a), 42. (c), , 3. (b), 13. (a), 23. (a), 33. (c), 43. (c), , 3. (b,c,d), 11. (a,b,c,d), 19. (d), 27. (7), r; e, , 4. (b,c,d), 12. (c), 20. (b), 28. (2), , 5. (a,b,d) 6. (a,b,d), 13. (b), 14. (d), 21. (a), 22. (a,b,c,d), , 7. (a,b), 15. (d), 23. (a,d), , 8. (b,c), 16. (b), 24. (1), , u; f t, , 4. (b), 14. (c), 24. (d), 34. (d), , 5. (a), 15. (a), 25. (c), 35. (a), , 6. (c), 16. (d), 26. (b), 36. (c), , 7. (b), 17. (a), 27. (b), 37. (d), , 8. (a), 18. (a), 28. (a), 38. (c), , 9. (b), 19. (d), 29. (d), 39. (b), , 10. (b), 20. (c), 30. (d), 40. (d), , Hints and Solutions, 1., (a), , , , 2., (d), , Number of e- in O2+ = 15 = number of e- in X2Atomic number of X2- is 13 (z), Number of neutrons = Z+1 = 14, Mass number of X2- = 13 + 14 = 27, Hydrogen resembles halogens in some, properties and also resembles alkali metals in, some properties. So, it can be placed in first or, 17th group., , 3., (c) This element belongs to d-block, Group number of d-block = (ns + (n – 1) d), , =2+3=5, 4., (c) Cr belongs to fourth period., 5., (d) Mass number (proton + neutron) of X2+ = 20, Number of neutrons = 10, Hence, Number of protons of X2+ =10, Number of e- in X2+ = 8, 6., (b) Elements of group 1, 2 and 13 to 17 are called, as representative elements.
Page 35 :
1.26, , 7., (c) Inert gas elements " 1s2 and ns2 np6, Representative elements " ns1-2 and ns2 np1 to, ns2 np5, Transition elements " (n – 1) d1-10 ns1or2, Inner – transition elements " (n – 2) f1-14, (n – 1) d0-1 ns2, 8., (c) Z = 108, group number Viii B , period – 7th, 9., (a) The element, with atomic number 56, belongs, to group 2 (alkaline earth metal). The element,, with atomic number 12, also belongs to group, 2., 0., (c) e- configuration of M2+=1s2 2s2 2p6 3s2 3p6 3d6, e- configuration of M = 1s2 2s2 2p6 3s2 3p6 4s2, 3d6, , = atomic number is 26, Atomic weight of M = 56, Number of neutrons = 56 – 26 = 30, 1, 11., (a) Ionic mobility µ, Size in aqueousmedium, Order of size in aqueous medium:, Li+ (aq) > Na+(aq) > K+(aq), Order of ionic mobility:, Li+ (aq) < Na+(aq) < K+(aq), 12., (d) p-block elements mostly form acidic oxides, not basic oxides., 13., (b) Ion, e- configuration Number of unpaired e- m, Cr+3, (Ar) 4s° 3d3, 3, 15, +2, Mn, (Ar) 4s° 3d5 , 5, 35, Fe+2, (Ar) 4s°3d6 , 4, 24, +2, 9, Cu, (Ar) 4s°3d , 1, 3, +3, 5, 14., (d) e configuration of Fe = (Ar) 4s° 3d, It has unpaired e- hence, it is a paramagnetic, species., 15., (b) Ag+ = (Kr) 5s° 4d10, Cu+2 = (Ar) 4s° 3d9, Ga+3 = (Ar) 4s° 3d10 4p°, Zn+2 = (Ar) 4s° 3d10, 16., (c) m = 3.87 BM, Hence, number of unpaired e- in Mn x+ = 3, Mn = (Ar) 4s° 3d5, Mn4+ = (Ar) 4s° 3d3, 17., (d) The first element of a group generally belongs, to second period. It has a small size, high, ionization potential and electronegativity. It, does not have d – orbitals., 18., (d) Oxides of Be and Al are amphoteric. They have, , almost similar electronegativity and polarizing, power., 19., (c) Be and Al show diagonal relationship., 20., (b) Correct set of magic numbers for group VIA is, 8,18,18,32., 21., (a) For a given series of isoelectronic species, as, atomic number increases, radius decreases., Se-2 > Br- > Kr > Rb+ > Sr+2, 22., (d) Order of ionic radius is:, Be+2 < Mg2+ < Ca2+ < Sr2+, 23., (c) Their relative positions in periodic table,, C, N , (II period), , P, S, (III period), The correct order of size is:, N < C < S < P, 24., (a) Due to lanthanoid contraction Zr and Hf have, almost similar atomic radii., 25., (c) As positive oxidation state increases, radius, decreases., +4, +7, +2, +3, MnO2 KMnO4, MnO, K3 [Mn(CN)6], 26., , (c) Down the group, ionization enthalpy decreases., It is due to increment in atomic size., , 27., , (b) Due to extra stability of the half filled p-orbitals,, N has greater ionization potential than that of, O., , 28., (d) Order of ionization energy is:, M- < M+ < M2+, M+2has smallest size and highest effective, nuclear charge., 29., , (b) In IE2 process, 1e- is removed from X+(g), , 30., , (b) Down the group, IE decreases. It is due to, increment in atomic size., , 31., , (b) In lanthanide series, as atomic number, increases, atomic radius gradually decreases It, is called as lanthanide contraction., , 32., , (d) Electron affinity (EA) = - D e.g. (e- gain, enthalpy), , 33., , (a) Outermost sub shell of N is half filled. In, process, N " N-, absorption of energy takes, place., , 34., , (b) Second and successive electron gain enthalpy, of an element is always positive because anions, resist addition of another e‑., , 35., , (d) As and Sb are metalloids.
Page 36 :
1.27, , 36., (c) In group VIIA (Halogens),, F2 and Cl2 " gas, Br2 " liquid, I2 " solid, 37., (c) Halogens have highest electro negativity., 38., (c) percentage ionic character, , = 16 (DEN) + 3.5 (DEN)2, , = 16 (3.3) + 3.5 (3.3)2, , = 90.9 %, 39., (a) In halogens, bond length decreases from iodine, to fluorine., 40., (d) Acidic strength of µ Electro negativity of, oxides and hydroxides, central atom, 41., (a) Order of acidic strength:, Al2O3 < B2O3 < CO2 < NO2, 42., (b) Neutral oxides are CO, NO, N2O, H2O, 43., , (d) Al2O3, , Amphoteric, , B2O3, Acidic, 44., (d) In order of acidic strength:, HCl > PH3 > SiH4 > CH4, 45., (d) They all are acidic., , 1., , d) The elements ‘X’ is ‘As’. Its electronic, configuration is (Ar) 4s2 3d10 4p3., 2., (c) Bond length of H – F = rH + rF – 0.09 (D EN), , = 0.37 + 0.72 – 0.09 (1.9), , = 0.92 Å, 3., (b) Order of ionic radius:, I- > Cl Te2- > I(They are isoelectronic), 4., (d) Due to inert pair effect the more common, oxidation state for Tl, Pb and Bi are +1, +2 and, +3 respectively., 5., (b) The relative positions of these elements in, periodic table:Mg, , P, , Cl, , Ca, Order of atomic radius: , Ca > Mg, , Mg > P > Cl, 6., (b) The element ‘X’ is ‘Cu’. Its electronic, configuration is (Ar) 4s1 3d10. In third shell, 18e- and in fourth shell 1e- is present., , (c) The electronic configuration of element is 3s2, 3p6 3d5 4s2. This element is ‘Mn’., 8., (c) La and Ac belong to d - block, Element having atomic number 31 belongs to, 4th period., Elements after 92U are man-made elements., 9., (b) X(g) + E1 " X+(g) + eA, ( 0 atoms), 2, A0, atoms absorbs E1 energy, 2, 2E1, So, 1 atom absorbs, energy, A0, 2E1, Hence, ionization energy of X(g) is, A0, 2E1, X(g) +, " X+(g) + e-……………eq (1), A0, X+(g) + 2e " X-(g) + E2, A, ( 0 ions), 2, A0, ions release E2 energy, 2, 2E2, So, 1 ion releases, energy, A0, 2E2, Hence, X+(g) + 2e-" X-(g) +, ….....eq(2), A0, , (1 ion), 7., , , , , , Equation for electron affinity of X(g) is,, X(g) + e-" X-(g) + EA of X(g)., We can get this equation by adding equation, (1) and (2),, 2(E 2 - E1 ), X(g) + e-"X-(g) +, A0, 2(E 2 - E1 ), , Hence, electron affinity of X(g) is, ., A0, 10., (d) In covalent hydrides, as we move left to right, in a period acidic strength increases., 11., , (d) In any period, noble gas has largest atomic, radius because for noble gases vander waal’s, radius is considered., , 12., , (d) Acidic strength of Oxides µ Electronegativity, of central atom., , CO2 is more acidic than SiO2 hence, option ‘i’, is incorrect., 13., , (b) The elements having atomic number 113, belongs to group 13 and 7th period., , 14., , (c) The electronic configuration of Cr is (Ar) 3d5, 4s1 and it is a representative element. In HN3,, oxidation state of nitrogen is -1/3.
Page 37 :
1.28, , 15., , (c) The biggest jump in successive ionization, energy is from IE3 to IE4. Hence, this element, has 3 valence e-., 16., (d) In s-block, all oxides are basic except BeO., BeO is an amphoteric oxide. They are obtained, by the electrolysis of fused chlorides., 17., (c) Na+ and F- are isoelectronic but Na belongs to, 3rd period while F belongs to 2nd period., 18., (c) The element (P) is Lu, which is a lanthanoid., All lanthanoids belong to the 3rd period., 19., (b) Ionic radius of Li+ (0.76Å) is larger than that, of Mg2+ (0.72Å)., 20., (c) The bond having greater polarity (or, greater, DEN) has greater chance of dissociation in, water., 21., (d) The equation for second ionization energy of, M is,, M+(g) " M2+(g) + e This equation will be obtained by (V)–(iii), M(g) "M2+(g) + 2e– (M(g) "M+(g)+ e-), M+(g) " M+2 + e22., (d) EA1 process is generally exothermic while, EA2 process is always endothermic. Hence,, DH1, DH2 and DH3 are negative whereas DH4, is positive., 23., (d) As positive oxidation state increases, radius, decreases., 24., (c) Completely filled sub-shell is more stable than, half filled sub-shell., 25., (a) The biggest jump in successive ionization, energy is from IE3 to IE4. Hence, the number, of valence electron is 3., 26., (d) Due to lanthanoid contraction 5d-series, elements have greater effective nuclear hence,, they have higher ionization energy., 27., (d) IE2 for an element is higher than IE1 because, after removal of 1st electron, 2nd electron is, removed from the cation. The cation is smaller, than its parent atom and it has greater effective, nuclear charge (Zeff) than its parent atom., 28., (a) Due to extra stability of half-filled p-subshell,, elements of group 15 have higher IE than, elements of group 16., 29., (d) Fluorine has small size, high electron density, and an increased electronic repulsion., 30., (c) The order of radius is:, rvander waal > rmetallic > rcovalent, , 31., , (c) Z has biggest jump from IE1 to IE2 hence, it, has 1 valence e-., 32., (c) The order of radius is:, , Be > B > C > N > O, 33., (a) Elements A, B, C and D are Ne, O, Na and F, respectively. Their correct order of EA is:, Ne < Na < O < F, 34., (d) The first member of the lanthanoid series is Ce, (cerium). Pricogens are group 15 elements., 35., (b) Smaller the value of IE, easier is the formation, of cation. Larger the value of EA, easier is the, formation of anoin., IE+EA, Electronegativity on Mulliken’s scale =, 2, 1, Size µ, Z eff, 36., (d) For isoelectronic species, as atomic number, increases radius decreases., 37., (c) The relative order of these elements in periodic, table is:, F, , Ne, , P, K, Ca, The correct order of IE1 is:, , K < Ca < P < F < Ne, 38., (d) The, number, of, d-electrons, in, Fe2+ ([Ar] 4s03d6) are 6., p-electrons in Ne (1s2 2s2 2p6) are 6., s-electrons in Mg (1s2 2s2 2p6 3s2) are 6., d-electrons in Fe ([Ar] 4s23d6) are 6., p-electrons in Cl- (1s2 2s2 2p6 3s2 3p6) are 12., 39., (b) Ni+2= [Ar] 4s0 3d8 (2 unpaired electrons), Ni+4= [Ar] 4s0 3d6 (4 unpaired electrons), 40., (c) Magnetic moment = 1.73BM, The number of unpaired e- = 1, V = [Ar] 4s2 3d3, V+4 = [Ar] 4s° 3d1, , In VCl4, oxidation state of ‘V’ is ‘+4’, 41., (d) The correct order of radius is:, , F < O < F-< O-2, 42., (c) The correct trend of first ionization energy is:, , B > Al < Ga > In < Tl, , , Higher Higher, , Zeff, Zeff, B > Tl > Ga > Al > In (Based on practical, values)
Page 38 :
1.29, , 43., , 44., , 45., , (d) Cs+ is largest cation and F- is smallest anion, hence, CsF has the lowest anion to cation size, ratio., (d) F is more electronegative than that of Cl-., Anions are less electronegative than neutral, atoms., (a), Elements Atomic number, , , , P, , 16, , Q, , 17, , R, , 18, , S, , 19, , The bond between S (alkali metals) and Q, (halogens) will be most ionic (least covalent), , 1. (a, b, c) Value of ionization energy increases from left, to right in a period., Hence, alkali metals have lowest IE and, noble gases have highest, IE in respective period., Due to half filled outermost 2p-subshell, N, has lower EA1 than O, (a, d), If ‘X’ Mn then this e- configuration represent, cationic form X+ (Mn+), , If ‘X’ is ‘Cr’ then this e- configuration, represents ground state., 3.. (b, c, d) The correct order of IE1 is:, N > O > C > B, The remaining orders are correct., (b, c, d) The correct order of basic strength is:, SbH3 < AsH3 < PH3< NH3, The remaining orders are correct., 5. (a, b, d) As positive charge increases, radius, decreases. Hence, Cr+3 is larger than Cr+6., 6. (a, b, d) Due to higher effective nuclear charge, Ga, has greater first ionization enthalpy than Al., 7. (a, b), The correct orders of electron affinity are:, , N<C<O<F, , P < Si < S< Cl, 8. (b, c), Down the group, electronegativity decreases., As we move from left to right in a period, electronegativity increases., 9. (a, d), Due to poor shielding of nuclear charge by d, or f-orbital electrons, 5d-series elements have, greater elective nuclear charge., , 10. (a, c, d) The correct order of DHeg(With negative, sign) is:, , Cl > F > Br >I, , The remaining orders are correct., 11. (a, b, c, d), , , , , , S-(g) + e-" S2-(g); EA2 of S, Ne (g) + e-" Ne-(g); EA1 of Ne, N (g) + e-" N-(g); EA1 of N, Al2+(g) + e-" Al3+(g); IE3 of Al, These all steps are endothermic., , 12. (c) , , rVan der Waal > rMetallic > rCovalent, , 13. (b) , , In isoelectronic series, as atomic number, increases, radius decreases., , Both N3- and Al3+ are isoelectronic. The, correct order of radius is:, N3- > Al3+, 15. (d), Mn = 1s2 2s2 2p6 3s2 3p6 3d5 4s2, 14. (d) , , , Other, (n-1), ns, , s = 1 × 0.35 + 13 × 0.85 + 10 × 1, = 21.40, 16. (b) , , IE1 of Ga > Al, due to imperfect shielding of, 3d-orbitals in Ga., , 17. (c), , IE1 of the group 2 elements is more than that, of the group 3 elements., , 18. (d), Order of the first ionization energy is:, , C<O<N<F, 19. (d) , Relative positions of these elements in, periodic table is:, Ga, , Ge, , In, , Sn, , , , Among these four elements, Ge has highest, first ionization energy., 20. (b) , Correct orders of ionization energy:, , Be > Sr, Be+ > Sr+, Be+ > Be, 21. (a) , , Both N and P have stable half-filled p-orbitals, in the outermost shell., , 22. (a, b, c, d), , | EA1 | = |DegH1 |, , , , Second period elements have lower EA1 than, third period elements., , 23. (a, d), , Noble gases have stable outermost shell econfiguration hence, DegH1 of noble gases, have large positive values.
Page 39 :
1.30, , 24. , 25., , 26., 27., , Due to inert pair effect, the most stable, oxidation stable of Tl is +1., Order of ionization energy is:, Li < Be >B < C < N > O < F < Ne, B, S, P, At, H, Li, Cs, Ba, F, Zn, Be, Al, Sr, of ionization, } Order, energy, , 28., Li and Li+ (Li < Li+), , Ba and Sr (Ba < Sr), 29., 30., , a" r, a "q, r, e" u, , 1., , (a) Acidic strength of oxides µ electronegativity, of central atom., (b) In isoelectronic species, as atomic number, increases radius decreases., (b) Down the group ionization potential decreases., (b) Alkaline earth metals anion are unstable, because they have fully filled outermost subshell., (a) Acidic strength of oxides µ electronegativity, of central atom., (c) The lower oxidation states for the group, 14 elements are more stable for the heavier, members for the group due to inert pair effect., (b) Relative positions of these elements in periodic, table,, , 2., 3., 4., , 5., 6., , 7., , b"s, b " p, s, f"t, , c" p, c" t, , S, Ca, , d" t, d" r, , Ar, , Se, , Ba, The correct order of increasing IE1:, , Ba < Ca < Se < S < Ar, 8., (a) In lanthanoids, as atomic number increases, radius decreases., 9., (b) In lanthanoids, as atomic number increases, ionic radius decreases., 10., (b) The variation in properties of elements is, related to their atomic numbers., 11., (b) It is due to lanthanoid contraction., 12., (a) N-3, F- and Na+ all have 10 electrons., , (a) Electronic configuration of Cr is (Ar) 4s1 3d5, after removal 1e-, next will be removed from, half filled d-subshell., 14., (c) K+, Ca+2, Sc+3 and Cl- all have 18 electrons., 15., (a) Correct order of radius:, B3+ < Li+ < F- < O-2, 16., (d) Acidic strength of oxides µ electronegativity, of central atom., 17., (a) O-(g) + e–"O-2 (g). This process is endothermic because anion will tend to resist the, addition of another electron., 18., (a) Due to very high hydration enthalpy of F-, F2 is, strongest oxidizing halogen., 19., (d) H3O+, NH3 and CH3- all have 10e-., 20., (c) The main cause of lanthanoid contraction, is poor shielding by 4f electrons on outer, electrons., 21., (b) The correct order of first ionization enthalpy:, , B<C<O<N, 22., (c) Due to lanthanoid contraction, 4d and 5d-series, elements have almost similar radius., 23., (a) SnO2 is an amphoteric oxide., 24., (d) The correct order of first ionization enthalpy:, , S<P, , B<F, +, 25., (c) K , Cl-, Ca+2 and Sc+3 all have 18 electrons., 26., (b) Cause of lanthanoid contraction is the, imperfect shielding on outer electrons by 4f, electrons from the nuclear charge., 27., (b) Order of reactivity in alkali metals:, , Li < Na < K < Rb < Cs, Order of reactivity in halogens:, F2 > Cl2 > Br2 > I2, 28., (a) The correct order of ionic radius is:, Na+ > Li+, Li+ > Mg+2, Mg+2 > Be+2, 29., (d) In isoelectronic species, as atomic number, increases ionic radius decreases. The correct, order of radius is:, O-2 > F- > Na+ > Mg2+> Al3+, 30., (d) The electronic configuration of Gd (atomic, number 64) is:, , [Xe] 6s2 4f7 5d1, 31., (b) The correct order of negative e- gain enthalpy, (electron affinity) is:, , Cl > F > Br > I, 13.
Page 40 :
1.31, , 1, 32., (a) Basic strength of oxides µ, Electronegativity of, , central atom, , 33., (c) In isoelectronic species, as atomic number, increases ionic radius decreases. The order of, radius is:, Ca2+ < K+ < Cl-< S234., (d) IE of M = IE1 of M+, C+, N+, O+, F+, 2p1, 2p2, 2p3, 2p4, , The correct order of second ionization enthalpy, is:, , C<N<F<O, 35., (a) The correct order of ionization enthalpy is:, , B<C<O<N, 36., (c) F-atom has smaller size and incoming e- feels, more repulsion from already present e- in, F-atom hence, electron gain enthalpy with, negative sign (electron affinity) of fluorine is, less than that of chlorine., 37., , (d) The relative position of elements in periodic, table is:, O, S, As, , Se, , The correct order of atomic radius is:, , O < S < Se < As, , 38., , (c) The relative position of elements in periodic, table is:, Se, Ca, , Ar, , Se, , Ba, , , The correct order of first ionization enthalpy, is:, , Ba < Ca < Se < S < Ar, 39., (b) Na (g) + 5.1ev " Na+ (g) +e Na+ (g) + e- "Na + 5.1ev; DHeg = - 5.1ev, , (e- gain enthalpy), 40., (d) All elements in a group have similar number of, valence electrons., 41., (a) P-3 and S-2 are isoelectronic hence, their order, of radius is S-2 < P-3, 42., (c) N-3, O-2and F- are isoelectronic hence, their, order of radius is, N-3 > O-2, >, F , (1.71 Å) (1.40 Å), (1.36Å), 43., (c) Order of first ionization energy is:, , Sc > Na > K > Rb
Page 42 :
Chapter, , Key Concepts, INTRODUCTION, The formation of a chemical bond between two atoms, implies that the system consisting of these two atoms at, stable internuclear distance is energetically more stable, than the two isolated atoms. A general study on the, reactivity of different elements revealed that noble gases, have little tendency to combine with other elements. This, leads to the fact that the noble gases have stable outer, configuration (ns)2 (np)6 (octet configuration). All other, atoms combine to achieve the stable octet configuration, either by mutual sharing of electrons (covalent bond) or, by complete transfer of electron(s) from one atom to other, (ionic bond)., , In Lewis structure, a bond between the two atoms is shown, by Lewis electron-dot symbols in which valence electrons, are shown by dots around the letter symbol of the atom., The dots are placed as follows., Place a single dot on the four sides of the letter symbol, followed by the second dot till all the valence-electrons, have been accounted for., Illustrations:, Lithium Beryllium, (2s1) , (2s)2 , Be, Boron Carbon, B, (2s)2(2p)1, (2s)2(2p)2, , C, , Nitrogen Oxygen, N, (2s)2(2p)3, (2s)2(2p)4, , O, , According to this theory, atoms can combine either, by transfer of outer-shell electrons, known as valence, electrons, from one atom to another or by sharing the, valence electron(s) in order to achieve octet configuration, (i.e., a total of eight electrons) in their respective valence, shells., , Fluorine Neon, F, (2s)2(2p)5, (2s)2(2p)6, , Ne, , The sharing of electron(s) leads to the formation of, covalent bond while transferring of electron(s) leads to, the formation of ionic bond between the two involved, atoms., , A covalent bond involves mutual sharing of valence, electrons between two atoms. The sharing of two, four and, six electron leads to the formation of a single, double and, triple bond, respectively., , KÖSSEL, LEWIS THEORY, OF CHEMICAL COMBINATION
Page 43 :
2.2, , A covalent bond is formed if the atoms have lesser number, of valence electrons as compared to the nearby noble gas, which has octet configuration. Such elements are known, as electronegative elements. Thus, the criterion of the, formation of covalent bond is:, Electronegative element + Electronegative element, , Covalent bond, Exception to the octet rule is the hydrogen atom which can, accommodate only two electrons which corresponds to the, electronic configuration of nearby helium (1s2) atom., Illustrations:, , If a pair of electrtons shared between two atoms comes, exclusively form one of the atoms, the bond formed is, said to be a coordinate covelent (or dative) bond. To keep, track of electrons, a coordinate covalent bond may be, represented by an arrow (, ). Once a coordinate bond is, formed, it behaves like a covalent bond., H3N – BF3, F, H, F, H, H–N, , Cl2 , , Cl + Cl, , Cl - Cl, , or, , Cl Cl, , O + O, , O, , O , , or, , O=O, , N, , or, , N∫N, , Formation of Triple Bond(s), N2 , , N + N, , N, , The octet rule is generally obeyed by the elements of, second and third periods with the following exceptions:The Incomplete Octet, BeCl2, Cl, , Cl, , Cl Be Cl, 4 electrons, , Odd-Electron Molecules, , All atoms of a compound containing odd number of, electrons will not satisfy octet rule as even number of, electrons are required for pairing of electrons., NO, N + O, , N + O, , H, , H–N, , F, , H, , B–F, F, , Writing a Lewis Structure, , Formation of Double Bond(s), O2 , , + B–F, , N, , O, , 7 electrons, , The Expanded Octet, Elements of third period and beyond can accommodate, more than 8 electrons due to the availability of vacant d, orbitals., PCl5, Cl, Cl, Cl, P, 5 Cl + P, 5 Cl + P, Cl, Cl, (P has 10 electrons), , The structure of a molecule or ion may be written by, following the steps listed below:, 1. Calculate the total number of valence electrons of the, atoms in the molecule. For an anion, add the number, of negative charges and for a cation, subtract the, number of positive charges., 2. Write the skeleton structure of the molecule or ion, connecting every bonded pair of atoms by a single, bond, i.e., a pair of electron dots., 3. Assign a total of eight electrons in each atom (except, hydrogen) surrounding the central atom., 4. Distribute the remaining electrons (if any) as pairs to, the central atom., If there are fewer than eight electrons on the central atom,, move one or two pairs of electron from a surrounding, atom to form double or triple bond between the two atom., Atoms that often form multiple bond are C, N, O and S., Lewis structure of COCl2, Step 1 Valence electrons are 4 + 6 + 2 × 7 = 24, Step 2 Carbon being the most electropositive atoms, occupies the central position to which other atoms, are bonded., O, , Cl : C : Cl, Step 3 Assign 8 electrons each to surrounding atoms, , , O, Cl : C : Cl, , There were 24 valence electrons and all of them, have been distribuled. However, the central C, atom has only 6 electrons. In order that this atom, also has 8 electrons, move one pair of elctrons
Page 44 :
2.3, , from O to the bond connecting C atom, thus, forming a double bond., O, O, O, ||, Cl : C : Cl, Cl : C : Cl or Cl – C – Cl, , The formal charge an atom is the difference between the, valence electrons in an isolated atom and the number of, electrons assigned to that atoms in a Lewis structure. The, equation for computing formal charge is,, Formal charge = Valence electrons on free atom –, 1, Number of (Nonbonding + bonding) electrons in, 2, a Lewis structure, The sum of the formal charges of atoms in a Lewis, structure is equal to the charge on the molecular species., , Formal, , The polarization of bonded pair of electrons between two, atoms is expressed in terms of physical quantity known as, dipole moment (symbol : µ). It is defined as, µ = ( q) (r), where q is the partial charge separation between two, atoms and r is the distance between the two atoms., , Charge, , Representation of Dipole Moment, , Illustration, COCl2 molecule Lewis structure Cl : C : Cl, O, , Atom, , Valence Electrons in Lewis, electrons, structure, in a free Non- Bonding, atom, boding, , Each atom in a molecule has its own ability to attract, the bonded pair of electrons. This ability is known, as electronegativity. The bonded pair of electrons in, homonuclear diatomic molecules (such as H2, O2, F2, Cl2,, etc.) is shared equally by both atoms. This is not correct, in the case of heteronuclear diatomic molecules (such as, HCl, HF, NO, etc.) The bonded pair of electrons is closer, to the atom having larger electronegativity. Consequently,, this atom acquires a partial negative charge while the other, atom acquires equal partial positive charge. Because of the, charge separation, the covalent bond between these two, atoms is said to be a polar covalent bond., , Dipole moment is a vector quantity, i.e., it has magnitude, as well as direction. In chemistry, dipole moment is, indicated by the crossed arrow as shown in the following., positive end, negative end, , Cl, , 7, , 6, , 2, , 1, 7 − (6 + × 2) = 0, 2, , O, , 6, , 4, , 4, , 1, 6 − ( 4 + × 4) = 0, 2, , that is, it is directed from positive end to the negative end., , 8, , 1, 4 − ( 0 + × 8) = 0, 2, , In SI system, unit of dipole moment = (unit of q), (unit of ) = Cm, In CGS system, unit of dipole moment = (esu), (cm), Most molecules have dipole moment of the order of 10–18, esu cm. This value of dipole moment is known as 1 debye, (written as 1 D)., , 1.6 × 10 −19 C , 1D = 10–18 esu cm = (10–18) (1esu ) , 4.8 × 10 −10 esu , , , , –2, –30, (10 m) = 3.33 × 10 cm, , C, , 4, , 0, , Computing formal charge of atoms in a molecule or ion, helps deciding a possible Lewis structure of the species., The guiding principles are as follows:, having the lowest magnitude of formal charge is the, preferred structure., of formal charges, the one having negative formal, charges one the more elecronegative atoms is the, preferred structure., , Unit of Dipole Moment, , Each bond in a molecule has a dipole moment, known, as bond moment. The dipole moment of a molecule is, obtained by the vector addition of these bond moments.
Page 45 :
2.4, , Illustration, The bond moment of O – H bond is 1.52 D. The bond, angle of H2O is 105º. The dipole moment of H2O molecule, will be, µH2O = 2µOH cos (105º/2) = 2 (1.52 D) (0.609) = 1.85 D, , , H, , 52.5º, , The dipole moment of a nonpolar polyatomic molecule, is zero inspite of the fact that the bond moments of the, molecule is not zero. This is due to the fact that the, individual bond moments in the molecule is symmetrically, placed so that their vector additions is zero., Illustration:, F, , , , B, , Percent ionic character =, , Illustration:, The bond moment of O – H bond is 1.52 D. If bond length, O – H is 95 pm, its percent ionic character will be, µ, Percent ionic character = OH ×100, e rOH, , O, H, , F, , µ AB, ×100, µ ionic, where µionic = (e rAB) corresponds to 100% ionic character, of the bond., , , O == C == O, , F, , (1.52 D)(3.33 × 10 −30 Cm / 1D), , , , =, , , , = 33.3 %, , (1.6 × 10 −19 C)(95 × 10 −12 m ), , × 100, , Sometimes, one can write more than one equivalent Lewis, structures differing in the distribution if electrons over a, given skeleton of atoms in a molecule. None of the individual, structures adequately explains the characteristics of the, molecule. However, these can be explained if the actual, structure of the molecule is considered as the superposition, of individual structures. This phenomenon is known as, resonance and the individual structures are known as, resonating structures. It is represented by a double-headed, arrow ( ) inserted between the resonating structures., Illustrations:, , Both NH3 and NF3 have pyramdial shapes with lone pair, of electrons on nitrogen atom., , || S, , O, , O, , S, | S, O written as O, O, O, , The resonance hybrid of the two Lewis structures makes, both the S – O bond lengths equal in size., , , N, H, , H, H, , N, F, , F, F, , In NH3, orbital dipole acts in the same direction as the sum, of bond vectors of the three N – H bond bonds. In NF3,, orbital dipole acts in the opposite direction to the sum of, bond vectors of the three N – F bond bonds. These facts, make the dipole moment of NH3 (µ = 1.57 D) larger than, that of NH3 (µ = 0.24 D)., , The percent ionic character of a polar bond A – B is, defined as:, , written as, , The transfer of valence electron(s) from one atom of an, element to the valence shell of the atom of some other
Page 46 :
2.5, , element leads to the formation of positive and negative, ions, respectively. The electrostatic attraction between the, positive and negative ions results in the formation of an, ionic bond between the involved ions., –, , : :, , : :, , +, Na + :Cl:, 2, 6, (3s)0 (3s) (3p), , Na + Cl:, 2, 5, (3s)1 (3s) (3p), , + . 2F : 5, (2s) (2p), , –, , : :, , : :, , : :, , :F ., + . Mg ., 5, 2, (2s)2 (2p), (3s), , 2+, Mg + : F :, 2, 6, 0, (3s) (2s) (2p), , Energies Involved in the Formation of One Molecule of, Sodium Chloride, The formation of Na+Cl–(g) from Na(g) and Cl(g) involves, the following steps., (i) Na(g), (ii) Cl(g) + e–, , Na+(g) + e–, , Ei = 8.24 × 10–19 J, , Cl–(g), , (iii) Na+(g) + Cl–(g), , Eea= –5.78 × 10–19 J, , Na+Cl–(g) PE, , where PE is the potental energy in the formation of ionic, bond. This is evaluated by the expression, QQ, PE = 1 2, (4πε0 )r, where Q1 = Q2 = 1.60 × 10–19C and r = rNa+ + rCl–, = 95 pm + 181 pm = 276 pm. Considering Q1 and Q2 as, point charges, we have, (1.60 × 10 −19 C)(−1.60 × 10 −19 C), PE =, (4)(3.14)(8.854 × 10 −12 C2 N −1m −2 )(276 × 10 −22 ), = –8.34×10–19J, Hence, for the reaction Na(g) + Cl(g), , Na+Cl–(g), , we have E = E1 + Eea + PE, , = (8.24 – 5.78 – 8.34) × 10–19 J, , = – 5.88 × 10–19 J, The negative value of E indicates that the, formation of an isolated ionic bond Na+Cl–(g) is feasible, as the combination is energetically more stable than Na(g), and Cl(g) taken together., For E in Equation (3) to be negative, we must have, (i) Low value of Ei. This is shown by electropositive, element(s)., (ii) High value of Eea. This is shown by electronegative, element(s)., Hence, Electropositive + Electronegative, element , , element, , Ionic, Bond, , (eg, elements of Gp.1) (eg, elements of Gp. 17), , Formation of 1 mol of Solid Ionic Compound from, Constituent Elements, Taking an example of sodium chloride, we have the, following steps in the formation of solid compound., (i) Na(s), Na(g), H1 = 108 kJ mol–1, 1, (ii), Cl2(g), Cl(g), H2 = 120 kJ mol–1, 2, (iii) Na(g), Na+(g) + e–, H3 = 496 kJ mol–1, (iv) Cl(g) + e–, Cl–(g), H4 = –349 kJ mol–1, Na + (g) + Cl − (g) → Na + Cl − (s) ∆H 5 = −788 kJ mol −1, −1, 1, Na(s) + Cl2 (g) → Na + Cl(s) ∆H = −313kJ mol, 2, Since H is negative, the formation of solid NaCl is, energetically favorable. From the values of H’s listed, above, it is obvious that the step (v) is the most favourable, step since its highly exothermic nature counter acts the, endothermic steps (i) to (iii)., , (v), , The enthalpy involved in the reversal of step (v), i.e.,, NaCl(s), Na+(g) + Cl–(g) is known as lattice energy of, the ionic solid., By definition, lattice energy of an ionic solid is the energy, required to completely separate one mole of solid ionic, compound into gaseous constituent ions. Larger the value, of lattice energy, more stable the ionic compound., The lattice energy is determined indirectly through the use, of Born-Haber cycle. The latter involves the steps (i) to (v), listed above for the formation of solid ionic compound. In, this cycle, H is determined experimentally. Subtraction, of H1, H2, H3 and H4 from the value of H gives the, value of H5. The lattice energy is negative of the value, of H5., Fajan Rules: An ionic compound has partial covalent, character and vice versa. The partial covalency in an ionic, compound my be explained qualitalively with the help of, Fajan rules described in the following., High charge and small size of the cation: Such an ion, will exert a greater effect in polarizing anions causing, cationic electronic charge to penetrate partially into the, anionic electronic cloud resulting into the partial covalent, bond character to the ionic bond., The elecronic, cloud of such an anion is most easily polarized by the, cation because the anionic charge cloud is less influenced, by the nuclear charge of the anion., Electron configuration of the cation: For two cations of, the same size and charge, the cations of electronic, configuration (n–1)dxns0 (i.e., transition metal ions) have
Page 47 :
2.6, , more polarizing power than the cation of electronic, configuration (n – 1)s2(n–1)p6 ns0 (i.e., alkali and alkaline, earth metal ions). This is due to less shielding of nucleus by, the electronic cloud of transition metal ions as compared, to that in the alkali and alkaline metal ions., Hg2+ ion has larger polarizing effect than Ca2+ ion. Lithium, salts have more covalent chracter than the alkali salts. I–, ion is more easily polarized than Cl– ion by Ag+ ion., , The covalent-bonded molecules have definite shapes, which cannot be accounted for by Lewis structures., , A simple theory, known as VSEPR theory, was proposed, by Sidgwick and Powell in 1940 and was developed and, refined by Nyholm and Gillespie in 1957. The guiding, rules of this theory are as follows., The number of electrons pairs in the valence shell of, the central atom of a molecule decides the shape of the, molecule. These pairs of electrons occupy the specific, positions so as to minimize the mutual electronic repulsion., A multiple bond is treated as if it is a single electron pair., The repulsive interaction of electron pairs decrease in the, order, Lone pair (lp) - Lone pair (lp) > Lone pair (lp) - Bond pair, (bp) > Bond pair (bp) > Bond pair (bp), The shapes of molecules as predicted by VSEPR theory, are shown in Table., , Shapes of some molecules on the basis of VSEPR model, Molecules, , Number of valence, Electrons, around the, central atom, , Basic shape, , Electron, pairs, , Bonding, pairs, , Lone pairs, , (i) BeCl2, , 4, , 2, , 2, , 0, , Linear, , (ii) BCl3, , 6, , 3, , 3, , 0, , Triangular, planar, , CH4, (iii) NH3, H2O, , 8, 8, 8, , 4, 4, 4, , 4, 3, 2, , 0, 1, 2, , Tetrahedron, , PF4, (iv) SF4, CIF3, , 10, 10, 10, , 5, 5, 5, , 5, 4, 3, , 0, 1, 2, , Trigonal, bypramid, , 12, 12, , 6, 6, , 6, 5, , 0, 1, , Octahedron, , (iv), , SF4, IF5
Page 48 :
2.7, , A few examples of molecules containing lone pair, electrons along with their geometry are described in the, following, , .., , .., , S, , O, , N, O, , Shape : Bent, Bond angle : 109.5°, , H, , H, H, Shape : Trigonal pyrmidal, Bond angle : 107°, , s, In this, overlapping, the electronic charge is concentrated between, the nuclei of bonding atoms., p, In this, overlapping, the electronic charge is concentrated above, the plane of nuclei of the bonding atoms., End to end overlap, , .., , .., , O, , H, , H, , 2pA, , 2pB, , Shape : Bent, Bond angle : 104.5°, sideways, overlap, , The quantitative description of chemical bond is provided, by the quantum mechanical theories. Two theories,, namely, valence bond (VB) and molecular orbital (MO), have been developed., The essential guidelines of VB method are as follows., (i) A molecule is considered to be a collection of, atoms with electrons occupying their respective, atomic orbitals., (ii) The formation of molecule is analysed in terms, of interactions amongst electrons-electrons,, electrons-nuclei and nuclei-nuclei., (iii) For a molecule to be stable, the electrostatic, attractions must predominate over the electrostatic, repulsion. The difference in these two is released in, the form of heat. Thus, a molecule is energetically, more stable than the individual atoms., , The electronic configuration of oxygen, atom is (1s)2 (2s)2 (2px) 2(2py)1 (2pz) 1. There are two 2p, atomic orbital, each containing one electron. Thus it can, form two bonds — and bonds., z, , z, , y, , Ob, �-bond, , �-bond, , The electronic configuration of, nitrogen atom is (1s)2(2s)2(2px)1(2py)1(2pz)1. There are, three 2p orbitals, each containing one electron. Thus it can, form three bonds—one and two -bonds., z, , z, , y, , y, , x, , In a molecule, two of overlapping of orbitals having, directional chracteristics may be distinguished., , y, , Oa, , Electron associated with atom HA can go to the atom HB, and vice versa through the overlap region. Also in the, overlap region, there will be some probability of finding, both the electrons and thus according to Pauli’s exclusion, principle, these two electrons must have opposite spins., , The intervening electronic charge between the two, nulcei has an affect of decreasing nuclear repulsion and, maximises electron-nuclei attractions. This lead to the, stale H2 molecule., , 2pB, , 2pA, , x
Page 49 :
2.8, �-bond, , �-bond, , According to the valence bond theory,, 2, 2, Should form no chemical bond, 4Be (1s , 2s ), as it does not contain any, unpaired electron., 2, 2s2, 2p1), B, (1s, Should form a single bond as, 5, it contains only one unpaired, electron., 2, 2, 1, 1, 6C (1s , 2s , 2px , 2p y) Should form two bond as it, contains two unpaired electron., Experimentally it is found that Be is divalent, B is trivalent, and C is tetravalent., To explain this, the concept of hybridisation is introduced., In this concept we have,, Two or more atomic orbitals of the same atom mix, each other to provide a new set of indentical number of, degenerate orbitals. These orbitals, known as hybrid, orbitals, are completely identical in size, shape and, orientations., sp3 Hybridisation: In sp3 (pronounced as ‘s-p three’), hybridisation, one s orbital and three p orbitals of the same, valence shell of an atom combine to give four degenerate, equivalent sp3 hybrid orbitals. These four orbitals are, directed towards the four corners of a regular tetrahedron, making an angle of 109°28' with respect to each other., , Three main hybridisation involving d orbitals are as, follows :, sp2d or dsp2 Hybridisation: The resultant four hybrid, orbital lie in a plane with bond angle of 90° with respect, to each other., In dsp2, d orbital belongs to penultimate shell while in, sp2d, it belong to the valence shell., sp3d or dsp3 Hybridisation: The resultant five hybrid, , orbitals are directed to the corners of trigonal bipyramid, three are in the same plane making an angle 120° with, each other, fourth and fifth are directed perpendicular to, the plane (one above and the other below)., sp3d2 or d3sp3 Hybridisation: The resultant six hybrid, orbitals are directed to the corners of regular octahedron, four are in the same plane making an angle 90° with each, other, fifth and sixth are directed perpendicular to the, plane (one above and the other below)., sp3d3 or d3sp3 Hybridisation: The resultant seven, orbitals are directed to the corners of a regular pentagonal, bipyrimide five are in the same plane and sixth and seventh, are directed perpendicular to the plane (one above and the, other below)., , Molecular orbital theory provides the explanation for the, formation of bond in a molecule on the lines very similar, to those of atomic orbitals. The essential guidelines of this, theory are as follows., Like atomic orbitals in an atom, there exists molecular, orbitals in a molecule. The only difference is that an, atomic orbital is a monocentric (i.e., exists around a single, nucleus) while a molecular orbitals is a polycentric (i.e.,, exists around more than one nucleus and thus belongs to, the molecule as a whole)., Each molecular orbitals in a molecule describes different, electronic behaviour and has a fixed energy., Electrons in a molecule occupy molecular orbitals in, accordance with aufbau principle, Pauli’s exclusion, principle and Hund’s rule., The square of molecular orbital (which a mathematical, expression) evaluated at a point around the nuclei of the, molecule gives the probability of finding electron at that, point., The shape of a molecular orbital is the region around the, nuclei where there exists 90–95% probability of finding, the electron., The designation of, a molecular orbital starts by starting its, or nature, followed by the atomic orbitals into which it separates at, larger distance. The antibonding orbital is designated by, placing an asterisk on the symbol or ., The effective combinations of atomic orbitals of atoms A, and B are as follows:
Page 51 :
2.10, , 2. The number of bonds in a molecule is defined by a, physical quantity, known as bond order. It is defined, as one half of the net excess of bonding electrons, i.e.,, Bond order=, , Number of (bonding antibonding) electrons, 2, , 3. The strength of a bond depends on the bond order of, the molecule. The larger the bond order, the stronger, the bond, the larger the dissociation energy of the, molecule., , 4. Addition of an electron in the bonding orbital or, removal of an electron form the antibonding orbital, increases bond order and hence increases stability of a, molecule., 5. Removal of an electron form a bonding orbital or, addition of an electron in the antibonding orbital, decreases bond order and hence decreases stability of, a molecule., 6. Paramagnetism in a substance is due to the presence, of unpaired electrons in the molecules., , Solved Examples, 1. An element (X) forms compounds of the formula, XCl3, X2O5 and Ca3X2 but does not form XCl5., Which of the following is the element(X)?, (a) B, , (b) Al, , (c) N, , (d) P, , Sol.(c) ‘N’ can form NCl3, N2O5 and Ca3N2 but can not, form NCl5. Due to absence of d – orbital’s, ‘N’can, not expand its valency to 5., 2. Which of the following anions can not be formed, by boron?, (a) BF63-, , (b) BH4-, , (c) B(OH)4-, , (d) BO2-, , Sol.(a) Any second period element can form maximum 4, bonds (covalent and co-ordinate). After formation, of 3 covalent bonds, ‘B’ can form only one coordinate bond because it has only one vacant, orbital., 2S 2P, , (c) d – orbital taking part in sp3d2 are d X2– Y2 and, dZ2, (d) d – orbital taking part in sp3d3 are dXY, dZ2, and dX2– Y2, Sol.(b) dsp2 (s + pX + pY + dX2–Y2), , sp3d (s + pX + pY) (pZ + dZ2), sp3d2 (s + pX + pY + dX2–Y2) (pZ + dZ2), sp3d3 (s + pX + pY + dXY + dX2–Y2) (pZ + dZ2), 5. Which of the following set is not correct?, (a) N2O, O3, NH4+ all have co – ordinate bonds., (b) H2O, NO2, ClO2 all are ‘V’ shape molecules., (c) I3–, ICl2–, NO2+ all are linear molecules., (d) SF4, SiF4, XeF4 all are tetrahedral in shape., Sol.(d) N∫ N, , O, , , O, O, , 3. Which of the following would result in the, formation of strongest p- bond if the molecular, axis is x – axis?, (a) 2px + 2px, , (b) 2py + 2py, , (c) 2py + 3dxy, , (d) 2pz + 4pz, , Sol.(b) 2px + 2px will form s – bond, 2py+2py and 2px+3dxy will form p-bond but 2pp +, 2pp bond is stronger than 2pp + 3dp, 4. Which of the following statement is wrong?, (a) d – orbital taking part in dsp2 is dX2– Y2, (b) d – orbital taking part in sp3d is dXY, , H+ (all have co– ordinate bond), , O, , O, , B(I excitation), , H3N, , CI, , N, , , H, , H, , O, , O, , O, , O, , ('V' shape), , I3-, ICl2-, NO2+ (all are linear), SF4 (See-saw shape) SiF4, XeF4 (square, , (Tetrahedral) planar shape), 6. The incorrect order of bond dissociation energy, will be :, (a) H – H > Cl – Cl > Br – Br, (b) Si – Si > P – P > Cl – Cl, (c) C – C > N – N > O – O, (d) H – Cl > H – Br > H – I
Page 52 :
2.11, , Sol.(b) Bond length depends on size of atoms order of, bond length is: Si – Si < P – P < Cl – Cl, 1, Bond energy µ, Bond length, , Hence, correct order of bond energy is:, 7. The incorrect order of bond dissociation energy, will be :, (a) CO < CO2 < CO32- (C – O bond), (b) CN- < NCN2- < R-CO-NH2 (C – N bond), (c) ClO- < ClO2- < ClO3- < ClO4- (Cl – O bond), (d) SO2 < SO42- < SO32- (S – O bond), CO2, (2), , CO32(1.33), , CN (3), , NCN2(2), , R-CO-NH2, (1.5), , ClO (1), , ClO2(1.5), , ClO3(1.67), , SO2, (1.5), , SO42(1.5), , SO32(1.33), , ClO4(1.75), , In bracket bond order is given., 8. In which of the following change, adjacent bond, angle increases?, -, , (a) BeF2 + 2F, , BeF42-, , (b) SiF4 + 2F-, , SiF62-, , (c) BF3+ F, , -, , BF4-, , (d) NH3+ H+, -, , Sol.(d) BeF2+ 2F, sp, (180°), , , SiF4 + 2F-, , NH4+, , sp2, (120°), , NH3+ H+, sp3, (107°48'), , N2-2, , (a) B2, , B2-2, , (c) O2+, , O2-, , (d) O2-, , O2-2, , N2, (0), , N2-2, (2), , B2, (2), , B2-2, (0), , O2+, (1), , O2(1), , O2(1), , O2-2, (0), , 10. Which of the following statement(s) is not true, for the given species?, N2, CO, CN- and NO+, (a) All species have linear shape, (b) All species have some dipole moments, (c) All species are isoelectronic, (d) All species have identical bond order and, they are diamagnetic in nature., Sol.(b) All diatomic species are linear., Dipole moment of N2 is zero but remaining, species have some dipole moments., All species have 14 e-, bond order is 3 and, diamagnetic in nature., x, , 11. Dipole moment of, , is 1.5 D. Calculate, x, , Dipole moment of, , x, , x, x, , 2-, , BeF4, sp3, (109° 28'), SiF62-, , sp3, sp3d2, (109° 28') (90°), , BF3 + F-, , (a) N2, , Sol.(c) By using molecular orbital theory, we can calculate, number of unpaired e-in all these species., , Si – Si > P – P > Cl – Cl, , Sol.(a) CO, (3), , 9. In which of the following processes, the value of, magnetic moment does not change?, , BF4sp3, (109° 28'), NH4+, sp3, (109° 28'), , (a) 1.5 D, , (b) 1 D, , (c) 2.35 D, , (d) 3 D, , x, , Sol.(c), x, , x, x, , Three bond moments are equal in magnitude and, they are 120° apart hence, their result is zero. Net, dipole moment of this molecule equals to the, x, , that of
Page 53 :
2.12, , 12. Which of the following pair of compounds are, polar, planar and sp2 hybridisation?, (a) H2CO3, SO2, , (b) HClO2, H2CO3, , (c) BFClBr, ClF3, , (d) SO3, O3, , Solid form of PBr5 is PBr4+ Br–, , sp3, , Sol.(a) H2CO3 and SO2, (Both are polar, planar and have sp2 hybridisation), In HClO2, hybridisation of Cl is sp3, In ClF3, hybridisation of Cl is sp3d, SO3 is non – polar, 13. Back bonding in BF3 does not affect:, (a) Planarity, Lewis acidic strength and bond, angle, (b) Bond length, hybridization and bond strength, (c) Bond angle, planarity and geometry, (d) Lewis acidic strength, bond length and bond, order (B-F), Sol.(c) Due to back bonding in BF3, it’s bond length ,, bond energy, bond strength and lewis acidic, strength changes but there is no change in bond, angle, hybridization, geometry and planarity., 14. Which of the following molecule has 2pp - 3pp, back bonding?, (a) OCl2, , (b) BF3, , (c) CCl2, Sol.(c), , (d) CCl3-, , Molecule, , Type of back bonding, , OCl2, , 2pp – 3dp, , BF3, , 2pp - 2pp, , CCl3-, , 2pp – 3dp, , :CCl2, , 2pp – 3pp, , Solid form of XeF6 is XeF5+ XeF7–, , sp3d2 sp3d4, 17. In (B4O5(OH)4)2- the number of boron atoms, having on octet of electron is :, (a) 0, (b) 1, (c) 2, (d) 4, Sol.(c) Structure of (B4O5(OH)4)2- is:OH, , O, , 15. In which of the following molecule 2C – 2e- bond, is absent?, , , B, HO, , (d) B2H6, , B, , O, , O, , OH, O, , B, OH, , In this structure 2 Boron atoms have octet of, electrons in their outermost shell., 18. Which of the following statement is not correct?, (a) The maximum number of atoms in one plane, in B3N3H6 are 12., (b) There is no S – S bond in S3O9., (c) Maximum number of identical bond angles, in ClO4- are 4., (d) Number of bridging oxygen in P4O10 are 6., H, B, , Sol.(c), , , , H, , N, , N, , B, , B., N, H, , H, , The complete structure is, Planar hence, all 12 atoms, are in same plane., H, , Structure of S3O9 is:o, , o, , (b) Al2Cl6, , (c) BeH2 in solid state, , O, , B, , H, , In :CCl2, vacant 2p-orbital of ‘C’ and paired, 3p-orbital of ‘Cl’ form back bond., , (a) BeCl2 in Vapor state, , Solid form of PCl5 is PCl4+ PCl6–, , sp3, sp3d2, , s, , o, , o, , There is no S – S bond, , -, , Sol.(c) In BeH2 all bonds are 3C-2e ., 16. In which of the following d – orbital’s are not, used by central atom in hybridization?, (a) PF5 (s), , (b) PCl5 (s), , (c) PBr5 (s), , (d) XeF6 (s), , Sol.(c) Solid form of PF5 is PF4+ PF6–, , sp3 sp3d2, , s, , o, , o, , s, o, , o, , o, , Structure of ClO4- is:O–, , , CI, , , In this tetrahedral structure, identical bond angles are 6., O, , O, O
Page 55 :
2.14, , 26. Which of the following interaction lies in the, range of 8-42 kJ/mol?, -, , (a) F …..HF, +, , (b) Xe…..H2O, -, , (c) Cs …..OH, , (d) O2N–, , –OH…H2O, , Sol.(d) The interaction present in O2N– –OH…H2O, is intermolecular H-bond. It’s bond energy lies, between 8-42 kJ/mol., The interaction in F-…..HF is very strong H-bond., It’s strength almost equals to the covalent bond., +, , -, , The interaction in Cs …..OH is an ionic bond., The interaction in Xe…..H2O is dipole – induced, dipole interaction which is weaker than H-bond., 27. Which of the following compound has highest, lattice energy?, (a) AlF3, , (b) Na2S, , (c) Al2O3, , (d) CaF2, , /Z +/ /Z -/, r, Here, /Z+/ and /Z-/ are magnitude of charge of, cation and anion respectively., Sol.(c) Lattice energy (Uo) µ, , r ª r+ + r Al2O3 has highest lattice energy among these, four because it has highest value of /Z+/ /Z-/, 28. What is the DH of the following reaction?, Mg (g) + 2F (g), , Mg2+ (g) + 2F- (g), , If DegH of F = -328 kJ/mol, First ionisation energy of Mg = 737.7 kJ/mol, Second Ionisation energy of Mg = 1451 kJ/mol, (a) 1532.7 kJ/mol, , (b) 1860.7kJ/mol, , (c) 2516.7kJ/mol, , (d) 1451kJ/mol, , Sol.(a) Given that,, F (g) + e- F- (g); DH1 = -328 KJ/mol, Mg (g) Mg+ (g) + e-; DH2 = 737.7 KJ/mol, Mg+(g) Mg+2 (g) + e-; DH3 = 1451 KJ/mol, Mg (g) + 2F(g) Mg+2 (g) + 2F- (g); DH, , , DH = DH2 + DH3 + 2DH1, Or, , = 737.7 + 1451 + 2 (-328), , Or, , = 1532.7 KJ/mol, , 29. Which of the following order is incorrect?, (a) Ionic character : MCl < MCl2 < MCl3, (b) Polarisibility : F-< Cl– < Br– < I–, (c) Polarising power : Na+< Ca+2 < Mg+2< Al+3, (d) Covalent character : LiF < LiCl < LiBr < LiI, 1, Sol.(a) Ionic character µ, Polarising power of cation, , As positive oxidation state increases or, size of, cation decreases, polarising power of cation, increases hence, the correct order of ionic, character is: MCl > MCl2 > MCl3, 30. Select wrong statement:, (a) A transition metal ion has more polarising, power than S-block ions of comparable size, and charge., (b) Order of solubility in water is AgF > AgCl >, AgBr > AgI, (c) LiCl is soluble in organic solvents, (d) The hydration of ions involves absorption of, heat., Sol.(d) Out of cations having comparable size and charge, the one having, Noble gas configuration has less polarizing, power., LiCl is a covalent compound hence, it is soluble, in organic solvents., Order of solubility of heavy metal halides depends, order on hydration energy., (AgF > AgCl > AgBr > AgI), The hydration of ions involves evolution of heat., M+ (g) + H2O, , M+ (aq); DH = -Ve
Page 56 :
2.15, , Exercise, Covalent and ionic, 1. The phosphate of a metal has the formula M3PO4., The formula of its chloride would be:, MCl, (c) MCl3, , , (b) MCl2, (d) M2Cl3, , 8. The fluorine molecules is formed by:, p-p orbital’s (sideways overlap), p-p orbital’s (end-to-end overlap), sp-sp orbital’s, s-s orbital’s, , 2. Solid NaCl is a bad conductor of electricity, because:, In solid NaCl there are no ions, Solid NaCl is covalent, In solid NaCl there is no mobility of ions, In solid NaCl there are no electrons, , 9. Among given species identify the isostructural, pairs:, [NF3 and BF3], [BF4-and NH4+], [BCl3 and BrCl3], [NH3 and NO3-], , 3. Knowing that Na+ > Mg2+ and S2- > Cl- (Order, of size), predict which compound will be more, covalent?, , 10. The molecule exhibiting maximum number of, non-bonding electron pairs (lp) around the central, atom is:, , MgS, (c) MgCl2, , (b) Na2S, , XeOF4, , XeO2F2, , (d) NaCl, , XeF3-, , XeO3, , 4. Which of the following compound possesses the, largest lattice energy?, LiF, (c) KH, , (b) NaCl, (d) CsI, , 5. Select the incorrect statement:, Lithium is least reactive but the strongest, reducing agent among all the metals., Lithium hydrogen carbonate is not obtained, in the solid form while all other alkali metals, forms solid hydrogen carbonates., Lithium nitrate when heated gives lithium, oxide whereas other alkali metal nitrates, decompose to give the corresponding nitrite., Solubility of alkali metal hydroxides decreases, down the group. It is due to decrement in, hydration energy from Li+ to Cs+., 6. Which pair of element can form multiple bond, with itself and oxygen?, F, N, , N, Cl, , N, P, , N, C, , 7. Bonds present in N2O5 (nitrogen pentaoxide) are:, Only ionic, Only covalent, Covalent and co-ordinate, , 11. The shapes of XeF4, XeF-5 and SnCl2 are:, Octahedral, trigonalbipyramidal and bent, Square pyramidal, pentagonal planar and, linear, Square planar, pentagonal planar and angular, See-saw, T-shaped and linear, 12. Which is not correctly matched?, XeO3 – Trigonalbipyramidal, ClF3 – bent T-shape, XeOF4 – Square pyramidal, XeF2 – Linear shape, 13. The geometry of ammonia molecule can be best, described as:, Nitrogen at one vertex of a regular tetrahedron,, the other three vertices being occupied by, three hydrogens, Nitrogen at the centre of the tetrahedron,, three of the vertices being occupied by three, hydrogens, Nitrogen at the centre of an equilateral, triangle, three corners being occupied by, three hydrogens, Nitrogen at the junction of a T, three open, ends being occupied by three hydrogens
Page 59 :
2.18, , 43. The statement true for azide ion (N3-) is:, (a) It has a non–linear structure, (b) It is called pseudo halogens, (c) The oxidation state of N in this anion is- 1, (d) It is isoelectronic with NO2, 44. The pair of strongest hydrogen bond is:, (a) SiH4 and SiCl4, (b) CH3COOH and CH3OCH3, (c) CH3COOH and CH3COCH3, (d) H2O and H2O2, 45. The strongest force among the following is:, (a) London force, (b) Ion-dipole interaction, (c) Dipole-induced dipole interaction, (d) Dipole-dipole interaction, , 1. In which of the following species maximum, atoms can lie in same plane?, , XeF2O2, AsH+4, , PCl5, XeF4, , 2. The correct order of Cl - O bond order is:, (a) ClO3- < ClO4- < ClO2- < ClO (b) ClO- < ClO4-< ClO3- < ClO2 (c) ClO- < ClO2-< ClO3- < ClO4 (d) ClO4- < ClO3-< ClO2- < ClO3. Resonance structures can be written for:, O3, NH3, CH4, H2O, 4. The number of sp2 – s sigma bonds in benzene, are:, 3, 6, 12, None of these, 5. Which is the following pair of species have, identical shapes?, (a) NO2+ and NO2- (b) PCl5 and BrF5, (c) XeF4 and ICI4- (d) TeCl4 and XeO4, 6. The hybridisation of the central atom will change, when:, (a) NH3 combines with H+, (b) H3BO3 combines with OH (c) NH3 forms NH-2, (d) H2O combines with H+, , 7. Give the correct order of initials T or F for, following statements. Use T if statement is true, and F if it is false:, (I) The order of repulsion between different pair, of electrons is:, lp – lp > lp– bp > bp– bp, (II) In general, as the number of lone pair of, electrons on central atom increases, value, of bond angle from normal bond angle also, increases, (III) The number of lone pair on O in H2O is 2, while on N in NH3 is 1, (IV) The structures of xenon fluorides and xenon, oxofluorides could not be explained on the, basis of VSEPR theory, (a) TTTF, , (b) TFTF, , (c) TFTT, , (d) TFFF, , 8. Among the following species, the least angle, around the central atom is in:, O3, , I3-, , NO2-, , PH3, , 9. BF3 and NF3 both are covalent compounds but, NF3 is polar whereas BF3 is non–polar. This is, because:, Nitrogen atom is smaller than boron atom, N – F bond is more polar than B – F bond, NF3 is pyramidal whereas BF3 is planar, triangular, BF3 is electron deficient whereas NF3 is not, 10. Which statement is incorrect?, MP of H2O, NH3, HF are maximum in, their respective group due to intermolecular, H-bonding, BP of CH4 out of CH4, SiH4, GeH4, and SnH4, is least due to weak intermolecular force of, attraction, Acetic acid forms dimer by H-bonding, NH3 has lower BP than SbH3, 11. The molecular size of ICl and Br2 is approximately, same, but BP of ICl is about 40°C higher than of, Br2. It is because:, ICl bond is stronger than Br-Br bond, IE of iodine < IE of bromine, ICl is polar while Br2 is nonpolar, I has larger size than Br
Page 62 :
2.21, , 39. Which of these is not true for metallic bond ?, (a) Metallic bond is non-directional in nature, (b) Metallic bonds are weaker than covalent, bond., (c) Energy required to vapourise a mole of, Cu metal is high to the energy required to, vapourise a mole of a covalent substance like, diamond., (d) The valency electrons in a metallic bond are, mobile., 40. Which substance has the strongest London, dispersion forces?, SiH4, , (b) CH4, , (c) SnH4, , (d) GeH4, , 41. The type of molecular forces of attraction present, in the following compound is:, OH, NO2, , HO, , (a), (b), (c), (d), , Intermolecular H-bonding, Intramolecular H-bonding, van der Waals’ force, All of these, , 42. Which among the following attraction is, strongest?, (a) HF ……… H2O, (b) Na+ ……… HCl, (c) H2O ……… Cl2, (d) Cl – Cl …… Cl – Cl, 43. In which of the following species back- p bonding, exists?, (a) NF3, , (b) NH3, , (c) BF3, , (d) BF-4, , 44. Which statement about hybridisation is correct?, It involves the mixing of atomic orbitals of, the atom at the time of their participation in, bonding., (b) sp3d3 hybridisation orbital point out towards, the corners of regular hexagon., (c) Hybrid orbitals form weaker bonds than pure, atomic orbitals., (d) For hybridisationto occur, the atom must, have vacant orbitals in the valence shell., , 45. Which of the following has highest melting point?, SF6, (c) SiC, , (b) NaCl, (d) Xe, , ONE OR MORE THAN ONE CORRECT, 1. Which species have same bond order?, CO3-2, (c) NO2-, , (b) NO3(d) NO, , 2. In Which of the following molecule bonding is, taking place in excited state?, CH4, (b) BF3, (c) ICl3, (d) PCl3, 3. Which is/are in linear shape?, NO2+, -, , (c) I3, , (b) XeF2, (d) I3+, , 4. The species which are paramagnetic is/are:, NO, (b) NO2, (c) ClO2, (d) N2O4, 5. Which of the following statements are correct?, O–hydroxybenzaldehyde is a liquid at, room temperature due to intramolecular H–, bonding., Order of boiling point is H2O > H2Te > H2Se, > H2S, Order of boiling point is HF > HI > HBr >, HCl, Order of boiling point is SbH3 > NH3 > AsH3, > PH3, 6. The molecule that is/are having N – N bond:, N2O, (b) N2O3, (c) N2O5, (d) N2O4, 7. There is change in the type of hybridisation when:, NH3 combines with H+, AlH3 combines with HNH3 forms NH2SiF4 forms SiF628. Select correct statement(s) regarding s and, p-bonds:, s-bond lies on the line joining the nuclei of, bonded atoms, p-electron cloud lies on either side to the line, joining the nuclei of bonded atoms
Page 63 :
2.22, , (2pp-3dp) pi-bond is stronger than (2pp-3pp), pi-bond., s-bond has primary effect to decide direction, of covalent bond, while p-bond has no, primary effect in direction of bond, 9. In which of the following there is intermolecular, hydrogen bonding?, Water, , Ethanol, , Acetic acid, , H-F, , 10. Which of the following statements are correct, about sulphur hexafluoride?, All S-F bonds are equivalent, SF6 is a planar molecule, Oxidation number of sulphur is the same as, number of electrons of sulphur involved in, bonding, Sulphur has acquired the electronic structure, of the gas argon, 11. Correct order of decreasing boiling points is:, (a) HF > HI > HBr > HCl, (b) H2O > H2Te > H2Se > H2S, (c) Br2 > Cl2 > F2, (d) CH4 > GeH4 > SiH4, 12. Which of the following species does/do not exist?, OF4, , NH2-, , NCl5, , ICl32-, , 13. Ionic compounds in general do not possess:, high melting points and non-directional, bonds, high melting points and low-boiling points, directional bond and low-boiling points, high solubilities in polar and non-polar, solvents, PASSAGE BASED QUESTIONS, Passage # 1 (Q. 14 and 15 ), The distribution of electrons among various, molecular orbital’s is called the electronic, configuration of the molecule which provides, us the following very important information’s, about the molecule., , (a) Stability of molecule: The molecule is, stable if number of bonding molecular orbital, electrons (Nb) is greater than the number of, anti bonding molecular orbital electrons (Na), and vice – versa., , 1, , (b) Bond order: Bond order =, (Nb - Na), 2, A positive bond order means a stable, molecule while a negative or zero bond order, means an unstable molecule., , (c) Nature of the bond: Bond order 1, 2, or 3, corresponds to single, double or triple bonds, respectively., , (d) Bond length: Bond length decreases as bond, order increases., , (e) Magnetic nature: Molecular orbital’s, in a molecule are double occupied, the, substance is diamagnetic and if one or more, molecular orbital’s are singly occupied, it is, paramagnetic., 14. Select correct statement(s):, (a) Among O2+, O2 and O2- the stability decreases, as O2+ > O2 > O2 (b) He2 molecule does not exist as the effect of, bonding and anti-bonding molecular orbital’s, cancel each other, (c) C2, O22- and Li2 are diamagnetic, (d) In F2 molecule, the energy of s 2pz is more, than p2px and p2py, 15. N2 has greater bond dissociation energy than N2+,, where as O2 has a lower bond dissociation energy, than O2+ because :, Bond order is reduced when O2 is ionised, to O2+ and bond order increased when N2 is, ionised to N2+, Bond order is increased when O2 is ionised to, O2+ and bond order is decreased when N2 is, ionised to N2+, Bond order is deceased when O2 is ionised to, O2+ and bond order is decreased when N2 is, ionised to N2+, None of these., Passage # 2 (Q. 16 and 17), Molecular geometry is the general shape, of a molecule as determined by the relative, positions of the atomic nuclei. VSEPR model, predicts the shape of the molecules and ions, in which valence shell electron pairs are, arranged about the atom as far away from one, another as possible. Thus minimising electron, pair repulsion gives information about the, geometry of a molecule. Information about, the geometry of a molecule can sometimes, be obtained from an experimental quantity, called dipole moment.
Page 66 :
2.25, , 14. The structure of XeO3 is:, Linear, (c) Pyramidal, , (b) Planar, (d) T-shaped, [IIT-2007], , 15. Statement-1: p-hdroxybenzoic acid has a lower, boiling point then o-hdroxybenzoic acid., , Statement-2:, o-hdroxybenzoic, intramolecular hydrogen bonding., , acid, , has, , Statement-1 is True, Statement-2 is True,, Statement-2 is a correct explanation for, Statement-1., Statement-1 is True, Statement-2 is True,, Statement-2 is not a correct explanation for, Statement-1., Statement-1 is True, Statement-2 is False., Statement-1 is False, Statement-2 is True., [IIT-2007], 16. Statement-1: In water, orthoboric acid behaves as, a weak monobasic acid., Statement-2: In water, orthoboric acid acts as a, proton donor., Statement-1 is True, Statement-2 is True,, Statement-2 is a correct explanation for, Statement-1., Statement-1 is True, Statement-2 is True,, Statement-2 is Not a correct explanation for, Statement-1., Statement-1 is True, Statement-2 is False., Statement-1 is False, Statement-2 is True., [IIT-2007], 17. Statement-1: Pb4+ compounds are stronger, oxidizing agents than Sn4+ compounds., Statement-2 : The higher oxidation states for the, group 14 elements are more stable for the heavier, members of the group due to inert pair effect., Statement-1 is True, Statement-2 is True,, Statement-2 is a correct explanation for, statement-1., Statement-1 is True, Statement-2 is True,, Statement-2 is Not a correct explanation for, statement-1., Statement-1 is True, Statement-2 is False., Statement-1 is False, Statement-2 is True., [IIT-2008], , 18. The nitrogen oxide(s) that contain(s) N-N bond(s), is(are):, N2O, (b) N2O3, (c) N2O4, (d) N2O5, [IIT-2009], 19. The bond energy (in kcal mol-1) of a C-C single, bond is approximately:, 1, (b) 10, (c) 100, (d) 1000, [IIT-2010], 20. Assuming that hund’s rule is violated, the bond, order and magnetic nature of the diatomic, molecule B2 is:, 1 and diamagnetic, 0 and diamagnetic, 1 and paramagnetic, 0 and paramagnetic, [IIT-2010], 21. The species having pyramidal shape is:, , SO3, (c), , SiO32-, , (b) BrF3, (d) OSF2, , [IIT-2010], , 22. In allene (C3H4), the type(s) of hybridisation of, the carbon atoms is (are):, sp and sp3, (b) sp and sp2, , (c) Only sp2, (d) sp2 and sp3, [IIT-2012], 23. The shape of XeO2F2 molecule is:, Trigonalbipyramidal, (b) Square planar, (c) Tetrahedral, (d) See-saw, [IIT-2012], 24. Hybridisation of the underlined atom changes, when,, AlH3 changes to AlH4H2O changes to H3O+, NH3 changes to NH4+, All of these, [AlEEE-2002], 25. The maximum number of 90° angles between, bond pair- bond pair of electrons is observed in,, dsp3 hybridisation, (b) sp3d hybridisation, (c) dsp2 hybridisation, (d) sp3d2 hybridisation, [AlEEE-2003]
Page 67 :
2.26, , 26. Based on lattice energy and other considerations,, which one of the following alkali metal chlorides, is expected to have the highest melting point?, RbCl, , (b) KCl, , (c) NaCl, , (d) LiCl, [AlEEE-2004], , 27. The number and types of bonds between two, carbon atoms in calcium carbide are:, One sigma, One pi, (b) One sigma, Two pi, (c) Two sigma, One pi, (d) Two sigma, Two pi, [AlEEE-2005], 28. In silicon dioxide:, Each silicon atom is surrounded by four, oxygen atoms and each oxygen atom is, bonded to two silicon atoms., Each silicon atom is surrounded by two, oxygen atoms and each oxygen atom is, bonded to two silicon atoms., Silicon atom is bonded to two oxygen atoms., There are double bonds between silicon and, oxygen atoms., [AlEEE-2005], 29. The molecular shapes of SF4, CF4 and XeF4 are:, Different with 1, 0 and 2 lone pairs of, electrons on the central atoms, respectively, Different with 0, 1 and 2 lone pairs of, electrons on the central atoms, respectively, Different with 1, 1 and 1 lone pairs of, electrons on the central atoms, respectively, Different with 2, 0 and 1 lone pairs of, electrons on the central atoms, respectively, [AlEEE-2005], 30. The number of hydrogen atom(s) attached to, phosphorus atom hypophosphorus acid is:, Zero, (b) Two, (c) One, (d) Three, [AlEEE-2005], 31. The correct order of the thermal stability of, hydrogen halides (H-X) is:, HI > HBr > HCl > HF, HF > HCl > HBr > HI, HCl < HF < HBr < HI, Hl < HCl < HF < HBr, [AlEEE-2005], , 32. The decreasing values of bond angles from, NH3(106°) to SbH3 (101°) down group-15 of the, periodic table is due to:, Increasing bp-bp repulsion, Increasing p-orbital character in sp3, Decreasing lp-bp repulsion, Decreasing electronegativity, [AlEEE-2006], 33. In which the following ionisation processes, the, bond order has increased and magnetic behaviour, has changed?, C2, , C2+, , (b) NO, , , (c) O2, , O2+, , (d) N2, , NO+, N2+, [AlEEE-2007], , 34. The charge/size of a cation determines its, polarising power. Which one of the following, sequences represents the increasing order, polarizing power of the cationic species, K+,, Ca2+, Mg2+, Be2+?, Mg2+ < Be2+ < K+ < Ca2+, Be2+ < K+ < Ca2+ < Mg2+, K+ < Ca2+ < Mg2+ < Be2+, Ca2+ < Mg2+ < Be2+< K+, [AlEEE-2007], 35. The bond dissociation energy of B–F in BF3 is, 646kJ mol-1 whereas that of C–F bond in CF4, is 515kJ mol-1. The correct for higher B-F bond, dissociation energy as compared to that of C – F, is:, Smaller size of B-atom as compared to that of, C-atom, Stronger s bond between B and F in BF3 as, compared to that between C and F is CF4, Significant pp–pp interaction between B and, F in BF3 whereas there is no possibility of, such interaction between C and F in CF4, Lower degree of pp–pp interaction between, B and F in BF3 than that between C and F in, CF4, [AlEEE-2009], 36. The hybridisation of orbitals of N atom in NO3-,, NO2+ and NH4+ are respectively:, sp, sp2, sp3, , (b) sp2, sp, sp3, , (c) sp, sp3, sp2, , (d) sp2, sp3, sp, [AlEEE-2011]
Page 69 :
2.28, , Answer Key, 1. (a), 11. (c), 21. (b), 31. (d), 41. (a), , 2. (c), 12. (a), 22. (c), 32. (b), 42. (b), , 3. (a), 13. (b), 23. (d), 33. (c), 43. (b), , 4. (a), 14. (b), 24. (d), 34. (c), 44. (d), , 5. (d), 15. (b), 25. (d), 35. (c), 45. (b), , 6. (d), 16. (c), 26. (d), 36. (a), , 7. (c), 17. (b), 27. (a), 37. (d), , 8. (b), 18. (a), 28. (b), 38. (a), , 9. (b), 19. (c), 29. (b), 39. (b), , 10. (c), 20. (c), 30. (c), 40. (a), , 1. (d), 11. (c), 21. (a), 31. (c), 41. (d), , 2. (c), 12. (b), 22. (d), 32. (b), 42. (b), , 3. (a), 13. (a), 23. (d), 33. (b), 43. (c), , 4. (b), 14. (d), 24. (b), 34. (b), 44. (a), , 5. (c), 15. (c), 25. (b), 35. (b), 45. (c), , 6. (b), 16. (d), 26. (d), 36. (a), , 7. (b), 17. (c), 27. (b), 37. (b), , 8. (d), 18. (a), 28. (b), 38. (d), , 9. (c), 19. (c), 29. (d), 39. (c), , 10. (a), 20. (b), 30. (a), 40. (c), , 1. (a, b), 2. (a, b, c), 9. (a, b, c, d) 10. (a, c), 17. (c), 18. (a), 25. (3), 26. (6), 28. A b; B c; C, 29. A c; B a; C, 30. A c,d; B a,b,d; C, , 1. (a), 11. (d), 21. (d), 31. (b), 41. (c), , 2. (b), 12. (d), 22. (b), 32. (d), 42. (b, c), , 3. (b), 13. (a), 23. (d), 33. (b), 43. (c), , 3. (a, b, c), 4. (a, b, c), 11. (a, b, c) 12. (a, c, d), 19. (d), 20. (5), 27. (5), a,d; D a,c, b;d; D b,c, b; D b,c, , 4. (a), 14. (c), 24. (a), 34. (c), 44. (d), , 5. (a), 15. (d), 25. (d), 35. (c), 45. (c), , 5. (b, c, d) 6. (a, b, d) 7. (b, d), 13. (b, c, d) 14. (a, b, c) 15. (b), 21. (9), 22. (4), 23. (4), , 6. (a), 16. (c), 26. (c), 36. (b), 46. (c), , 7. (c), 17. (c), 27. (b), 37. (c), 47. (a), , 8. (a), 9. (b), 18. (a, b, c) 19. (c), 28. (a), 29. (a), 38. (a), 39. (c), 48. (d), 49. (d), , 8. (a, b, c, d), 16. (c), 24. (6), , 10. (d), 20. (a), 30. (b), 40. (b), , Hints and Solutions, | Z+ | . | Z− |, r0, +, –, |Z | and |Z | are magnitude of charge of cation, and anion., r0 r + + r –, , In LiF, both cation and anion are very small., 5., (d) Most reactive metal is Cs but Li is the strongest, reducting agent among all the metals., Only bicarbonates of Na+, K+, Rb+ and Cs+, 4., 1, , 1., (a) M 3 PO 4, Formula of chloride of M is MCl, 2., (c) Due to presence of lettice, there is no free ions, in NaCl hence, there is no mobility of ions., 3., (a) According to Fazan’s rule,, smaller cation and larger anion leads more, covalent character., , (a) Lattic energy (U0) ∝
Page 70 :
2.29, , exist in solid state., 2LiNO3 ∆, → Li2O + 2NO2- + 1/2O2∆, 2NaNO3 , → 2NaNO2 + O2-, (K+, Rb+ or Cs+), Order of solubility in water :, LiOH < NaOH < KOH < RbOH < CsOH, 6., (d) F can not form multiple bond. Cl and P can, not form multiple bond with itself because, 3pp – 3pp bond is not stable., O, 7., (c) O, O, N, N, O, O, 8., (b) In F2 molecule,, , F σ, → F (p – p end-to-end overlapping), 9., (b) Species, Shape, NH3, NF3, Pyramidal, BF3, BCl3, NO3–, Trigonal planar, BF4–, NH4+, Tetrahedral, BrCl3, ‘T’-shape, 10., (c) Species, lp on central atom, XeOF4, 1, XeO2F2, 1, –, XeF3, 3, XeO3, 1, 11., (c) Species, Shape, XeF4, Square planar, –, XeF5, Pentagonal planar, SnCl2, Angular or ‘v’-shape, 12., (a) XeO3 (Pyramidal shape), , O, , 13., , Xe, , H, , N, , Cl, Cl, , 0, , Cl, , Cl, , Cl, , Cl, Cl, , Cl, , , , =0, , Cl, , Cl, , Cl, , µ=x, , µ = 2x, , 18., (a) Species, lp + bp (s), Hybridization, , IF5 , 6 , sp3d2, , –, I 3 , 5 , sp3d, , +, I3 , 4 , sp3, 19., (c) In ICl4–, hybridization of central atom is, sp3d2. In sp3d2, axial d-orbitals dx2–y2 and dz2, participates., 20., (c) General order of melting point:, , Covalent network solid > Ionic solid > Metallic, solic > molecular solid., 21., (b) OF4 does not exist becuase, due to absence of, d-orbitals ‘O’ can form only 2 covalent bonds., Q, 22., (c), OQ, O, O, N, , N, O, , O, , 23., , Q, , O, , N, O, , O, , O, , (d), , C2 H 6, ( Molecular with van der Waal ’s, force of attraction ), , <, , CH3OH, < KCl <, Si, ( Molecular with H − bond ) ( Ionic ) ( Covalent network ), , (d) Hybridisation of ‘O’ in H3O+ is sp3., (d) Bond length directly depends on size of atoms., I3+, I3−, (d), ’V ’−shape, ( Planar ), , Linear, ( Planar ), , 27., H, , H, , , , Geometry is tetrahedral, shape is pyramidal, , 14., , (b), , H2 O, ( ≈ 104.5°), , 15., , (b), , NH 4+, ( ≈ 109.5°), , 16., , (c) If CF4, all C–F bonds are polar but due to, regular geometry it has zero dipole moment., , H 2 S NH3, SO2, ( ≈ 92°) ( ≈ 107°.48′) ( ≈ 109.5°), >, , (b), Cl, , 24., 25., 26., , O, , O, , (b), , 17., , NH3, ( ≈ 107° 48′), , > NH 2−, , ( <<109°⋅5°), , (a) In I2 solid, I2 molecules are attached by London, forces (because I2 is a non-polar molecule), 28., (b) In ClO3, all Cl–O bonds are double bond., , O, , Cl, , O, 29., (b) Species, XeF5–, , , O, , Shape, Pentagonal planar (6 atoms are, in XY-plane)
Page 73 :
2.32, , 26., (d) S + px, no bond, py + pz, no bond, +2, –, 27., (b) SrC2 (Sr – C C–), 28., (b) Due to regular geometry, BF3 has zero dipole, moment., NH3 has higher dipole moment than NF3, because in NH3 lp moment supports dipole, moment while in NF3 lp moment opposes, dipole moment., 29., (d) In sp3d2 hybridisation (octahedral geometry),, 12, 90° angles are observed between bp - bp of, electrons., 30., (a) Order of bond angle:, SO3, >, SO2, >, SO32–, (120°), ( 109.5°), (< 109.5°), 31., (c) N2, N2+, 1e– is removed from s2px or s2py or s2pz, O2, O2+, , , 1e– is removed from *2px or *2py or *2pz, 32., (b) Order of bond length:, , H2O2 > O3 > O2, (Bond order =, 1, 1.5, 2), 33., (b) MgO has high lattice energy, 34., (b) Fe+3 has greater polarising power than Fe+2., 35., (b) K2HPO3 (2K+ HPO3–2), O, P, H, , O, , O, O, , O, , 36., , (a) HF forms KHF2 because HF can form H-bond., , 37., , (b) ‘O’ forms covalent bond with 2 H-atoms and, H-bond with 2 H-atoms., 1, 38., (d) E, (This relation is valid for dipole-dipole, r3, interaction), H-bond is a type dipole - dipole interaction, 39., , (c) Cu is a metallic substance., , , , 42., 43., 44., , 45., , (b) Ion-dipole interaction is stronger than H-bond., (c) In BF3, pp – pp back bonding is present., (a) Hybridization involves the mixing of atomic, orbitals of the atom at the time of their, participation in bonding., (c), , 1., , (a, b), Species, , (c) Strength of London dispersion forces depends, on molecular mass., , 41., , (d) In this compound, intermolecular H-bond,, intramolecular H-bond and van der Waal’s, force, all are present., , Bond order, , O, , CO3–2, , 1.33, , C, , Θ, , O, , Θ, , O, O, , –, NO3, , O, , –, NO2, O, , O, , N, , (a, b, c), In CH4,, C (I excitation), In BF3,, B (I excitation), In ICl3,, I (I excitation), , 5s, 5p, , 1.5, , N, , Θ, , NO, , 1.33, , N, , Θ, , O, , 1.5, , O, , 2., , In PCl3,, , 2s, , 2p, , 2s, , 2p, , 5d, , 3s, , 3p, , P (Ground state), 3., , For metallic substance heat of vapourisation is, lower than for covalent network substance like, diamond., , 40., , Structure, , (a, b, c), Species, , Linear, , XeF2, , ., , Linear, , I3– , , Linear, , I3+ , , ‘V’-shape, , NO2, , 4., , Molecular shape, , +, , (a, b, c), NO, NO2 and ClO2 are odd e– molecules.
Page 74 :
2.33, , 5., , (b, c, d), , 12., , Cause of a particular physical state of a molecular, compound is strength of intermolecular bonding, not intramolecular bonding, 6., (a, b, d), N2O, , N≡N→ O, , N2O3, , O, , N2O5, , O, , O, , O2+ > O2 > O2–, , O, N–N, , Bond order = 2.5, , O, , AlH3 + H, sp2, , In F2, the energy of, , → Al H 4−, sp3, , N2, →, 15., (b), Bond order =3, , , NH3 → N H 2− + H +, sp3, , Bond order = 2, , SiF4 + 2F − → SiF6−2, 8., , sp3 d 2, , s, p, , , X, , 9., , p, , p, p, , p, , O2+, , A, X, , p, , (a, b, c, d), , (a, c), , SF6 has octahedral geometry (Non-planar), In SF6, ‘S’ has 12e– in outermost shell after sharing, hence, it does not acquire e– configuration of Ar gas., 11., , and, , Bond order = 2.5, , In all these compounds H-atom is attached with, highly electronegative atom., 10., , 2px, , Bond order = 2.5, , x 2 + x 2 + 2 x 2 (cos120°) = x, , s, p, , is less than, , 16., (c) Let the bond moment of A–X bond = x, , The dipole moment of AX2 =, , (a, b, c, d), , p, , 2pz, , N 2+, , →, , O2, , sp3, , sp3, , 1.5, , In C2,O22– and Li2, all molecular orbitals are paired, hence, they are diamagnetic., , sp3, , −, , 2, , He2 does not exist because bonding and antibonding, electrons are equal., , (b, d), NH3 + H + → NH 4+, sp3, , 14., , Ionic compounds have high melting point, boiling, point, solubility in polar solvents and have nondirectional bonds., (a, b, c), , Order of stability :, , O, , 7., , NCl5 does not exist., , O, N, , N, , O, , N2O4, , ‘N’ can form only 3 covalent bonds hence,, , O, , O, , O, , ‘O’ can form only 2 covalent bonds hence, OF4 does, not exist., , In octahedral geometry 3lp are not possible hence, ICl32– does not exist., 13., (b, c, d), , O, N–N, , (a, c, d), , (a, b, c), The correct order of boiling point is:, CH4 < SiH4 < GeH4, , s, p, p, p, p, p, d, , 2py.
Page 76 :
2.35, , 16., , (c) In water, orthoboric acid behaves as OH–, acceptor, H3BO3 + H2O $ [B(OH)4]– + H+, , Or, B(OH)3, (c) For heavier members of group 14, due to inert, pair effect, lower oxidation state is more stable., 18., (a, b, c), (N2O), N = N →O, , 28., , O, N–N, , (N2O3), , O, O, , O, N–N, , O, , N, , N, , O, , O, , (a) If Hund’s rule is not followed then Bond order, of B2 is 1 and it is diamagnetic, , 21., , (d), F, , F, , (b) H C = C = C H, H 2, H, sp sp, sp2, , 23., , (d), , :, , O, , Xe, F, , 24., , 100 kCal mol–1., , (Pyramidal shape), , F, , (see-saw shape), O, , (a) AlH3, , AlH4–, sp3, , H2O, H3O+, , sp3, sp3, , NH3, NH4+, , 3, sp, sp3, 25., (d) After sp3d2 hybridisation, geometry, octahedral. In octahedral geometry., 12, 90° angles are present., , sp2, , 26., (c) Order of melting point :, NaCl > KCl > RbCl > LiCl, 27., , 29., (a) Molecule lp on central atom, Shape, , SF4, 1, see-saw, , CF4, 0, Tetrahedral, , XeF4, 2, Square planar, , H, , 20., , 22., , (b) CaC2 (Ca+2 C, , C ), , O, , O, , (N2O5), , (c) Bond energy of C–C bond, , O, , ~ O – Si – O – Si – O ~, , 30., (b) Hypophosphorus acid (H3PO2), O, , 19., , S, , O, , O, , O, , O, , O, , (N2O4), , O, , O, , O, , ~ O – Si – O – Si – O ~, , 17., , O, , (a), , is, , 31., , P, , OH, , H, , (b) As bond length increases, bond breaking, becomes easier., 32., (d) Order of bond angles :, NH3 > PH3 > AsH3 > SbH3, 33., (b) , NO, NO+, Bond order :, 2.5, 3, Magnetic character : paramagnetic Diamagnetic, 34., (c) Order of polarizing power :, K+ < Ca+2 < Mg+2 < Be+2,, 1, Polarising power, size of ion, 35., (c) Due to p –p back bonding, B – F bond has, partial -character hence, its bond energy, becomes more than expected., 36., (b) , lp + bp( ), Hybridisation, –, NO3, 3, sp2, , NO2+, 2, sp, , NH4+, 4, sp3, 37., (c) Order of bond angle:, NCl3 > PCl3 > AsCl3 > SbCl3 (Order depends, on electronegativity of central atom), 38., , (a) Due to absence of d-orbitals, ‘B’ can maximum, form 4 bonds (3 covalent and 1 co-ordinate, bond), , (c) Covalent character µ polarising power of, cation, , µ charge density of cation, 39.
Page 78 :
Chapter, , Key Concepts, A metal atom or ion may combine with neutral molecules or, anions to form a new identifiable species called a complex, or coordination compound. For example, [Co(NH3)6]3+, is a complex species which can be identified as a whole., The groups that surround the metal ion in a coordination, compounds are called ligands. The total number of the, ligands bound around a metal ion is called the coordination, number of the metal ion. Ligands have been classified, depending upon the number of donor atoms it has. A few, examples are given below :Mono or Unidentate Ligand: Examples are F–, Cl–, Br–,, H2O, NH3, CN– and, etc., Bidentate: Examples are ethylenediamine (en), glycinate, ion (gly) etc., Tridadentate: Examples are diethylenetriamine and 2, 2’,, 2”- tripyridine etc., Tetradentate: Examples are triethylenetetrammine and, ethylenebis (salicyladimine) ion., Pentadentate: Examples is ethylenediaminetriacetate ion., Hexadentate: Example is ethylenediamine tetracetate ion., , The International Union of Pure and Applied Chemistry, (IUPAC) have suggested the following basic rules for, naming a coordination compound., , (a) The name of negative ligands end in ‘–o’, e.g., fluoro (F–), chloro (Cl–), hydrido (H–), thio (S2–),, nitro (NO2–), (b) Neutral groups have no special endings, e.g., ammine (NH3), aqua (H2O), carbonyl (CO) and, nitrosyl, (NO)., (c) Positive groups en ‘-ium’, e.g. hydrazinium, (H2NNH3+).
Page 79 :
3.2, , A few example of naming the complex compounds, are given in the following:, [Co(NH3)6]Cl3., 2+, , [CoCl(NH3)5], , Hexaamminecobalt (III) chloride, Pentaamminechlorcoblat (III) ion, , [CoSO4(NH3)4]NO3. Tetraamminesulphatocobalt (III), nitrate, [Co(NO2)3(NH3)3], , Triamminetrinitrocobalt (III), , Na2[ZnCl4], , Sodium tetrachlorozincate (III), , K3[Fe(CN)5(NO)], , Potassium pentacyanonitrosylferrate (II), , Fe(C5H5)2., , Bis(cyclopentadienyl) iron (II), , 2. The cation makes available a number of orbitals, equal to its coordination number, for the formation of, covalent bonds with the ligands., 3. The cation orbitals hybridize to form a new set of, equivalent hybrid orbitals with definite directional, characteristics., 4. The nonbonding metal electrons occupy the inner d, orbitals and do not participate in the hybridization., 5. Each ligand contains a lone pair of electrons. A covalent, bond is formed by the overlap of vacant hybrid metallic, orbitals and a filled orbital of the ligand., The above rules are illustrated with the following, typical examples., [Cr(NH3)6]3+, The outer electronic configuration of Cr is (3d)5, (4s)1. Chromium in the above complex is in +3, oxidation state, hence, Cr(III) has the configuration, of (3d)3. There are six ligands, so six empty orbitals, of chromium are required. These include two 3d, orbitals, one 4s orbital and three 4p orbitals. These, orbitals hybridize to give d2sp3 hybrid orbitals, directed towards the corners of an octahedron., The find configuration of the complex will contain, three unpaired electrons in three 3d orbitals of, chromium. Hence, the complex will be paramagnetic., The above description is diagrametically represented, as follows:, 3d, , On of the earliest theories to explain the formation of, coordination compounds has given by Alfred Werner., According to him, each element exhibits two types of, valencies. There is primary valency (which corresponds, to the oxidation state of the central metal) and secondary, valency (which represents the coordination number of, the central metal). The primary valency is satisfied by, anions whereas the secondary valency is satisfied by either, negative ion or neutral molecules. The primary valencies, are shown by dotted lines while secondary valencies by, solid lines. The secondary valencies are always directed, towards fixed positions in space giving a definite geometry, to the complex., Modern theories to explain the formation of complex, compounds are valence-bond theory area, crystal field, theory. The salient features of the valence bond theory are, as follows:1. The central metal looses a requisite number of, electrons to form the ion. The number of electrons lost, is the valency of the resulting cation., , 4s, , 4p, , Cr atom, 3+, , Cr ion, Cr3+ in, [Cr(NH3)6], 2, , 3, , ds p hybridization six pairs of electrons, from six NH3 molecules, [CoF6], , 3–, , 3d, , 4s, , 4p, , Co atom, 4p, 3+, , Co in, 3+, , Co in, 3–, [CoF6], , 4d, , sp3d2 hybridization six pairs of electrons, from six F– ligands, , The complex [CoF6]3– is octahedral and is strongly, paramagnetic.
Page 81 :
3.4, A, , A, , A, , M, , The isomers involving the exchange of H2O molecules are, known as hydrate isomers., For example : [Co(H2O)6]Cl3 and [Co(H2O)5]Cl2.H2O, , The isomers involving the different geometrical, arrangement of ligands around the central metal atom are, known as geometrical isomers. The two identical ligands, occupying he adjacent position is known as cis isomer, while those occupying the opposite by the lower case, alphabets (such as a, b, c,…) and bidentate by the upper, case alphabets (such as AA and AB), the geometrical, isomerism in complex compounds are as follows. AA is, symmetrical and AB is unsymmetrical bidentate ligand., , The complexes [Ma4] and [Ma3b] do not exhibit, geometrical isomerism., The complexes [Ma2b2] and [Ma2bc] exhibit cis-trans, isomerism., a, , b, , a, , b, , b, , b, , a, , M, , c, , c, , Cis, , a, , a, , M, d, , b, , a, , M, b, , d, , a, , a, , c, , a, , a, , a, , b, , Cis-isomer, , Trans-isomer, , a, , a, , a, , d, , The complex [M(AB)2] also exists in cis-and trans-forms., , b, , M, , a, , a, b, , M, c, , a, , M, , M, , The complex [Mabcd] exists in three isomeric forms, The have different pairs of ligands at the trans positions., c, , b, , a, , a, , Ma2bc, , a, , b, , b, , Trans, , A, , In cis-form, the two ‘b’ ligands have cis positions to, each other. In trans-form, the two ‘b’ ligands have trans, positions to each other., [Ma4bc], , b, , M, a, , b, , a, , Ma2b2, a, , Trans, , In an octahedral complex, if the two similar ligands, occupy positions at the two ends of the twelve edges of the, octahedron, the complex is the cis isomer., If the two similar ligands occupy positions at the end of a, straight line passing through the centre of the octahedron, (which is occupied by the central atom M), the complex is, the trans isomer., A brief account of geometrical isomers of a few complex, compound is given below., The complexes [Ma6], [Ma5b] and [M(AA)3] do not show, geometrical isomerism., The complexes [Ma4b2], [Ma3b3] and [Ma4bc] exhibit two, isomers each., [Ma4b2], , a, , Trans, , B, , M, , M, , Cis, , M, B, , Cis, , b, , M, a, , B, , B, , c, , a, , c, , a, , Cis-isomer, , Trans-isomer, , [Ma3b3], In cis-form, the three ligands ‘a’ occupy positions at, the corners of a triangular face and the three ‘b’ ligands
Page 82 :
3.5, a, , occupy positions at the corners of the opposite face. This, isomer is also known as facial (abbreviation-face) isomer, The trans-form is known as meri-donal (abbreviation-mer), isomer., a, , a, , b, , a, , a, , b, , b, , b, , a, , face-isomer, , mer-isomer, , The complex [Ma2b2c2] exists in five geometrical isomers,, (three are five ways of distributing pairs of ligands in trans, positons), one of which also has its enantionmer, thus, there exists a total of six isomers., a, , a, , b, , c, , c, , b, , c, , a, Trans positions,, aa; bb; cc, a, , c, , c, , a, , A, , b, , A, , M, , A, , b, , A, , b, , b, Cis-form, (and its enantiomer), , a, (Trans-form), , A, , a, , A, , A, , c, , A, , M, , A, , M, , A, , c, , c, ac; bb; ac, , Co-ordination compounds having no plane of symmetry, or centre of symmetry exists in two optically active, configuration which are related to each other through the, mirror image of each other and are not super-imposable, on each other., The complex [Ma2b2cd] exhibits six geometrical isomers, there are six ways of distributing pairs of ligands in trans, positions, two of which have enantiomers, thus making a, total of eight isomers., a, , a, , b, , c, , a, , b, , M, , c, , a, , M, b, , a, , b, , a, , b, (enantiomer), ab; ac; bc, , a, , b, (and its enantiomer), , M, , b, , d, , c, , b, , b, (and its enantiomer), , a, , b, ab; ab; cc, , b, , M, , d, , b, , a, , a, , a, , M, , The complex [Mabcdef] exhibit fifteen isomers (= 6C2, = 6!/(4! 2!)) all of them have their enantiomers, making a, total of 30 isomers., The complexes [M(AA)a2b2], [M(AA)2a2] and [M, (AA)2ab], where AA is a symmetrical bidentate, exists in, two forms, namely, cis and trans forms, of which cis form, has its enantiomer., , b, , a, aa; bc; bc, , d, , c, , c, , c, , a, , M, , d, , M, , b, , c, , M, , a, , M, , a, , a, , M, , a, , b, , M, , b, , a, , M, , b, , a, , b, , b, , M, , a, , M, d, , d, a, , c, , b, , d, b, , A, , A, , A, , a, , Cis-form, (and its enantiomer), , (Trans-form), , A, , a, , A, , A, , a, , M, , M, , b, , A, A, Cis-form, (and its enantiomer), , A, , A, , A, A, , (Trans-form), , The complex [M(AB)3], where AB is unsymmetrical, bidentate ligand in which A and B are two different donor, atoms, exists in cis-and trans-forms. Each of the two also, has its enantiomer.
Page 83 :
3.6, A, , A, , B, , B, , B, , A, , M, , M, A, , B, , 2, 2, , B, , A, , A, , B, , 2, , Trans-form, (and its enantiomer), , Cis-form, (and its enantiomer), , 2, , Example of Geometrical isomers in octahedral complexes, The cis isomers involve the two identical ligands on any, of the twelve edges of the octahrdron and trans isomers, involve the ligands on either end of a straight line passing, through the metal., +, , 2, , Cl, , 2, , Cl, , Cl, , H3N, , +, , NH3, , H3N, , Co, Cl, , 2, 2, 2, 2, 8, , H3N, , NH3, Cis-isomer, , 2, , 6, , Co, , H3N, , 3, , NH3, Cl, trans-isomer, , 15, 30, 2, 3, , A complex having no plane of symmetry or centre of, inversion exists in two optically active isomers. Such a, complex is said to be asymmetric. The two isomers are, mirror image of each other and the are not superimposable, on each other. The two have identical physical and chemical, properties but differ in their action on the polarized light., Examples are, Cl, , Py, , Py, , 2, , +, , Py, , Pt, , Cl, , 2, , H3N, NH3, , NH3, , 2, 4, 6, 12, 4, 8, 11, 4, 6, , Pt, NH3, , Cl, , Py, , +, , 3, , 12, , Cl, , 24, , Mirror, N, , Cl, , N, , +, , Cl, , Co, , N, , Co, Cl, , N, , N, , Cl, N, , N, Mirror, , N, , +, :(N–N is a, bidentate, ligand), , The stability of a complex compound depends on the, nature of the metal and that of the ligand. In general, the, higher the oxidation state of the metal, the more stable the, complex. The cyano complexes are far more stable than, those formed by halide ions., The increasing order of the influence of ligands on the, electronic configuration of central metal atom or ion, (known as spectrochemical series) is:I– < Br– < S2– < SCN– < Cl– < F– < OH– < Ox < O2– < H2O, < NH3 < NO2– < CN– < CO
Page 84 :
3.7, , Thus, halogens are said to be weak ligands while NO2–,, CN– are said to be strong ligands., Thus stability of complexes also decreases with increases, in the ionic size of the central metallic ion having the same, charge number. For example, the stability of complexes, formed from the same ligands increases from Mn2+ to Cu2+, and then decreases at Zn2+, that is the order of stability is, Mn 2 + < Fe2 + < Co 2 + < Ni 2 + < Cu 2 + > Zn 2 +, 91 pm, , 83 pm, , 82 pm, , 78 pm, , 91 pm, , 74 pm, , This sequence is known as Iriving-Willam order of stability, of complexes of M2+ ions., , z2, , eg, , t2, , 3, �0, 5, , 5, , 2�, , �0, , t, , �t, , 2, �0, 5, , 3�, t, 5, eg, , t2g, Octahedron arrangement of ligands, , Many ionic and covalent compounds of transition elements, are coloured. When light passes through a material,, it absorbs some of the wavelengths due to electronic, excitation. If absorption occurs in the visible region of, the spectrum, the transmitted light is coloured with the, complimentary colour to the light absorbed. The material, looks coloured corresponding to the transmitted light., In transition elements, the electronic excitation is due, to d-d electronic excitation. In an isolated metal ions,, d-orbitals are degenerate. This degeneracy is lost when, the ligands approach the metal ion. The energy of some, of d-orbitals is lowered while of the remaining orbitals the, energy is raised. Electrons occupy the orbitals of lower, energy and ca be excited to higher ones with the absorption, of wavelength which lies in the visible region., In some complexes, cause of colour is charge transfer, spectra. It is of three types :-, , Tetrahedral arrangement of ligands, , MnO 4 , MnO 24 , CrO 24 , Cr2 O72, , Solved Examples, 1. Select the incorrect statement:, (a) Hydrazine can act as bidentate ligand., (b) Oxalate is a chelating ligand., (c) All bidentate ligands are chelating ligands., (d) Number of chelate rings = (denticity – 1), Sol.(a) Hydrazine (H2N – NH2) is a monodentate ligand., All bidentate ligands are chelating ligands,, oxalate is a bidentate ligand., , 2. Aqueous solution of Mohr’s salt gives a positive, test for:, (a) Ferrous ions only, (b) Sulphate ions only, (c) Ammonium and sulphate ions only, (d) Ferrous, ammonium and sulphate ions
Page 85 :
3.8, , (a) Diamminechlorido, dipyridinenitrito–N–, cobaltate (III) nitrate, (b) Diammine, chloridonitrito–O–bipyridine, cobalt (III) nitrate, (c) Diamminechloridonitrito–O–dipyridine, cobalt (III) nitrate, (d) Chloridodiamminenitrito–O– bis (pyridine), cobalt (III) nitrate, , , , Sol.(c) Correct IUPAC name is:, , O O, , Q, || || Q, 2–, C2O4, , O, || Q, , , , N, .., , N, .., , N, .., , (b) [V(CO)6] can act as oxidising agent., (c) [Fe(CO)5] can act as both oxidising agent and, reducing agent., (d) [Mn(CO)5] show dimerisation to gain, stability., Sol. (c) [Mn(CO)6], EAN of Mn = 25 – 0 + 12 = 37, To gain stability it has to loose an e– hence, it can, act as reducing agent., [V(CO)6], EAN of V = 23 – 0 + 12 = 35, To gain stability it has to gain an e– hence, it can, act as oxidising agent., [Fe(CO)5], EAN of Fe = 26 – 0 + 10 = 36, It follows EAN rule. It does not behave as, oxidising agent and reducing agent., [Mn(CO)5] can dimerise [Mn2(CO)10] to gain, stability., 5. The IUPAC name for the complex, [Co(NH3)2Cl, (ONO) (Py)2] NO3 is:, , Diamminechloridonitrito–O–dipyridinecobalt, (III) nitrate., 6. Which of the following pairs of name and formula, of complexes is not correct?, (a) Tetraamminecopper (II) sulphate, [Cu(NH3)4]SO4, (b) Diamminesilver (I) chloride [Ag(NH3)2]Cl, (c) Potassium hexacyanidoferrate (III), , K4[Fe(CN)6], (d) Potassium amminepentachloridoplatinate (IV), , K[Pt (NH3)Cl5], c) The correct formula of Potassium hexacyanido, ferrate (III) is K3[Fe(CN)6], 7. Different hydrated isomer of CrCl3.6H2O can not, be differentiated by:, (a) Conductivity measurements, (b) Precipitation by AgNO3, (c) Dipole moment, (d) Magnetic moment, (i) [Cr(H2O)6] Cl3, (ii) [Cr(H2O)5Cl] Cl2. H2O, (iii) [Cr(H2O)4Cl2] Cl. 2H2O, , (a), (b), (c), (d), , participation of d-orbital in hybridization., polarity and magnetic nature., presence of synergic bonding., shifting of ns electron into (n–1) d subshell., , Sol.(a) Hybridization of Fe in [Fe(CO)5] is dsp3 while, hybridization of Ni in [Ni(CO)4] is sp3.
Page 88 :
3.11, , Sol.(c) In aqueous solution (0.001 M), the complexes, will dissociate to give the ions :, ��, �, ��, �, , coordination number is attained by other Mn atom, of (Mn–Mn) bond. Thus it can have structure :, CO, , ��, �, ��, �, , OC, , ��, �, ��, �, , OC, , ��, �, ��, �, 19. The EAN of each Mn (Z = 25) in its carbonyl, is 36. What is the structure of the carbonyl with, molecular formula : Mn2 (CO)10 ?, Sol. Electrons from each Mn = 25, , Thus is the complex five CO (ligands) are, coordinated to each Mn atom and sixth, , CO, , CO, , CO, Mn, , CO, , Mn, , CO, , CO, CO, , 20. Identify the complexes which are expected to be, coloured and explain., (a) Ti(NO3)4, (b) [Cu(NCCH3)4]+BF4–, (c) [Cr(NH3)6]3+ 3Cl–, (d) K3[VF6], Sol.(c & d) (c) and (d) are coloured because Cr3+ in, [Cr(NH3)6]3+ and V3+ in [VF6]3– has unpaired, electron in d subshell., , Exercise, , 1. Incorrect statement for addition compound is:, (a) Simple salts do not lose their identify in, double salt., (b) Complex compounds retain their identity in, aqueous solution., (c) Simple salts lose their identity in complex, compound., (d) Double salts retain their identity in aqueous, solution., 2. Neutral and symmetrical bidentate ligand is:, (a) Oxalate, (b) dien, (c) gly, (d) dipyridyl, 3. Which of the following pair contains only, ambidenate ligand?, , 4. EDTA is a:, (a) Polydenate ligand, (b) Chelating ligand, (c) Flexidenate ligand, (d) All of these, , 5. Chelating ligand is:, (a) thiocyanate, (b) cyanide, (c) Oxalate, (d) Ammonia, 6. Which can’t form chelates?, (a) Didentate ligand, (b) Ambidentate ligand, (c) Tetradentate lignad, (d) Flexidenate ligand, 7. An example for a double salt is:, (a) Cuprammonium sulphate, (b) Mohr’s salt, (c) Potassium ferricyanide, (d) Cobalthexammine chloride, 8. Which of the following complexes are, heteroleptic?, (a) [Cr(NH3)6]3+, (b) [Fe(NH3)4Cl2]+, (c) [Mn(CN)6]4–, (d) [Co(NH3)6]+2, 9. Which of the following can not act as ligand?, (a) H2N—CH2—COO–, (b) H2N–NH2, ∞, , N H3, 10. The oxidizing agent is:, (a) Fe(CO)5., (b) Mn(CO)5, (c) Mn2(CO)10., (d) Fe2(CO)9
Page 90 :
3.13, , (a) 2 and 2., , (b) 2 and 3, , (c) 3 and 2., , (d) 3 and 3, , 36. The type of isomerism present in pentaamminenitro, chromium (III) ion is:, (a) Optical, (b) Linkage, (c) Ionization, (d) Polymerisation, , 29. Which of the following is correct order of, stability?, (a) [NiCl4]–2 < [PdCl4]–2 < [PtCl4]–2, (b) [Co(H2O)6]+3 < [Co(NH3)6]+3 < [Co(CN)6]–3, (c) [Co(H2O)6]+3 < [Rh(H2O)6]+3 < [Ir(H2O)6]+3, (d) All of these, 30. The colour of a compound may be due to :, (a) polarisation, (b) d-d transition, (c) charge transfer spectra, (d) All of these, 31. Which is coloured but not due to d-d transition?, (a) Cr2O72–, , (b) KMnO4, , (c) AgBr, , (d) All of these, , 32. The colour of light absorbed by an aqueous, solution of CuSO4 is:, (a) orange-red, , (b) blue-green, , (c) yellow, , (d) violet, , 33. Which of the following isomerism is not present, in complex [Co(NH3)4 (SCN)2] Br?, (a) Geometrical isomerism, (b) Linkage isomerism, (c) Ionisation isomerism, (d) Optical isomerism, 34. Which is correct statement?, (a) [Co(en)3] [Cr(CN)6] will display coordination, isomerism, (b) [Mn(CO)5(SCN)] will display linkage, isomerism, (c) [Co(NH3)5(NO2)]SO4 will display ionization, isomerism, (d) All are correct, 35. How many isomers exist for [Co(NH3)4Cl2]+ and, [Co(en)2Cl2]+ complex ions, respectively?, , 37. Na2S forms violet colour complex when reacts, with:, (a) Brown ring complex, (b) Sodium nitroprussible, (c) K4[Fe(CN)6], (d) Hypo solution, 38. The incorrect statement is:, (a) Fe+2 salt gives blue colloidal solution with, K3[Fe(CN)6], (b) FeCl3 gives red colour with K SCN, (c) Cu+2 salt gives blue colloidal solution with, K4[Fe(CN)6], (d) Light blue solution of CuSO4 turn into dark, blue in presence of ammonia., 39. Select correct statement regarding [Ni(DMG)2], complex compound:, (a) It acts as oxidizing agent because Ni2+ cation, is having EAN 35., (b) It is extra stabilized by hydrogen bonding, (c) It’s IUPAC name is Bis (dimethylglyoximato), nickelate (II), (d) It’s ligand contains two different donar sites, 40. Sodium thiosulphate is used in photography to, (a) Reduce AgBr to metallic Ag, (b) Convert metallic Ag to Ag salt, (c) Remove undecomposed AgBr as a soluble, silver thiosulphate complex, (d) Remove unreduced silver, , 1. A six coordinate complex of formula CrCl3.6H2O, has green colour. A 0.1 M solution of the complex, when treated with excess of AgNO3 gave 28.7 g, of white precipitate. The formula of the complex, would be:, (a) [Cr(H2O)6)]Cl3, (b) [CrCl(H2O)5]Cl2.H2O, (c) [CrCl2(H2O)4]Cl.2H2O, (d) [Cr(H2O)3Cl3]
Page 91 :
3.14, , 2. Which is not true about metal carbonyls?, (a) Here CO acts as a Lewis base as well as, Lewis acid, (b) Here metal acts as Lewis base as well as, Lewis acid, , 3. Which of the following pair the EAN of central, metal atom is not same?, (a) [Fe(CN)6]3– and [Fe(NH3)6]3+, (b) [Cr(NH3)6]3+ and [Cr(CN)6]3–, (c) [FeF6]3– and [Fe(CN)6]3–, , (d) [Ni(CO)4] and [Ni(CN)4]2–, 4. The IUPAC name for K2[Cr(CN)2O2(O2)NH3] is:, (a) Potassium amminedicyanotetraoxo chromium (III), (b) Potassium, amminedicyanodioxygendioxo, chromate (IV), (c) Potassium amminedicyanosuperoxoperoxo, chromate (III), (d) Potassium, amminedicyanodioxoperoxo, chromate (VI), 5. The magnetic moment of [NiX4]2– ion is found to, be zero. Then the ion is:, , (X = monodentate anionic ligand), (a) sp3 hydridised (b) spd2 hydridised, (c) dsp2 hydridised (d) d2sp hydridised, 6. The magnetic moment of a complex ion is 2.83, BM. The complex ion is :, (a) [V(H2O)6]3+, (b) [Cr(H2O)6]3+, , (c) [Cu(CN)4]2–, (d) [MnCl4]2–, 7. What is the magnetic moment (spin only) and, hybridisation of the brown ring complex, [Fe(H2O)5NO]SO4?, 3 BM, sp3 d2, , 3 BM, d2 sp3, , 15 BM, sp3 d2, , 15 BM, d2sp3, , 8. Select the correct code about complex [Cr(NO2), (NH3)5] [ZnCl4]:, , (I) IUPAC name of compound is pentaamminenitrito-N-chromium (III) tetrachlorozincate, (II), (II) It shows geometrical isomerism, (III) It shows linkage isomerism, (IV) It shows coordination isomerism, (a) III, IV, (b) I, III and IV, (c) II, III and IV, (d) I, II, III and IV, , 9. Cis-trans isomerism is exhibited by:, (a) [PtCl(NH3)3]3+ (b) [Pt(NH3)4]2+, , , (c) [PtCl4]2–, , (d) [PtCl2(NH3)2], , 10. Which one of the following platinum complexes, is used in cancer chemotherapy?, (a) cis-[PtCl2(NH3)2], (b) trans-[PtCl2(NH3)], (c) [Pt(NH3)4]2+, (d) [Pt(Cl4)]2–, 11. The cyanide complex of silver formed in the, silver extraction in Mac-Arthur’s Forrest cyanide, process is:, (a) [Ag(CN)2]–, , (b) K2[Ag(CN)3], , 2–, , (c) [Ag(CN)4], , (d) Na3[Ag(CN)4], , (a) 0.01, 0.02., , (b) 0.02, 0.01, , (c) 0.01, 0.01., , (d) 0.02, 0.02, , 13. In Na2[Fe(CN)5NO], sodium nitroprusside:, (a) oxidation state of Fe is +2, (b) this has NO+ as ligand, (c) both are correct, (d) none is correct, 14. Complexes formed in the following methods are:, , (I) Mond’s process for purification of nickel, , (II) Removal of unreacted AgBr from, photographic plate, (III) Removal of lead poisoning from the body, I, , II, , III, –, , [Pb(EDTA)]2–, , (a) Ni(CO)4., , [Ag(CN)2], , (b) Ni(CO)4., , [Ag(S2O3)2]3– [Pb(EDTA)]2–, , (c) Ni(CO)6., , [Ag(S2O3)2]3– [Pb(EDTA)]4–, , (d) Ni(CO)6., , [Ag(S2O3)2]–, , [Pb(EDTA)]2–, , Ø, C, , (Soluble Complex), , , , D, , (Soluble Complex)
Page 93 :
3.16, , Choose the correct code., , Θ, H 2 O < NH3 < NOΘ, 2 < CN, , (a) D, E are coloured; F is colourless, , H 2 O < NH3 < CN Θ < NOΘ, 2, , (b) B is optically active; A, C are optically, inactive, (c) A, B are optically active; C optically inactive, (d) D is coloured; E, F are colourless, 29. One mole of complex compound Cr(NH3)5Cl3, give 3 moles of ions on dissolution in water. One, mole of the same, complex reacts with two moles, of AgNO3 to yield two moles of AgCl(s). The, complex is, (a) [Cr(NH3)4Cl]Cl2.NH3, (b) [Cr(NH3)4Cl2]Cl.NH3, (c) [Cr(NH3)5Cl]Cl2, (d) [Cr(NH3)3Cl3].2NH3, 30. In which of the following pairs both the complexes, show optical isomerism ?, (a) cis-[Cr(C2O4)2Cl2]3–, cis-[Co(NH3)4Cl2], (b) [Co(en)3]Cl3 , cis-[Co(en)2Cl2]Cl, (c) [Co(NO3)3(NH3)3], cis-[Pt(en)2Cl2], , 35. Which of the following complex is inner orbital, as well as low spin complex?, (a) [Cr(H2O)6]3+, , (b) [Fe(CN)6]3–, , (c) [Cu(CN)4]3–, , (d) [Ni(NH3)6]2+, , 36. Which of the following is incorrect about, Wilkinson’s catalyst?, (a) It is a diamagnetic complex., (b) It is a non-ionic complex., (c) It is tetrahedral complex., (d) It is very effective for selective hydrogenation, of organic molecule at room temperature and, pressure., 37. [Cr(H2O)6]Cl3 (atomic number of Cr = 24) has, a magnetic moment of 3.83 BM. The correct, distribution of 3d electron in the chromium of the, complex is:, 3d1xy ,3d1yz ,3d1z2, , (d) [PtCl(en)Cl], [NiCl2Br2]2–, 31. The correct order of magnetic moment (spin, values in BM) is, , (Atomic number Mn = 25, Fe = 26, Co = 27), (I) [MnBr4], , 2–, , (II) [Fe(CN)6]4–, (III) [CoBr4]2–, (a) II > III > I, , (b) I > II > III, , (c) II > I > III, , (d) I > III > II, , 32. Which of the following compound is not, coloured?, (a) Na2[CuCl4], (b) Na2[CdCl4], (c) K4[Fe(CN)6], (d) K3[Fe(CN)6], , (a) [Co(CN)6]3–, , (b) [CoF6]3–, , (c) [Co(NO2)6]3–, , (d) [Co(NH3)6]3+, , 34. The increasing order of the crystal field splitting, power of some common ligands is, Θ, NH3 < NOΘ, 2 < CN < H 2 O, , 38. What will be the correct order of absorption, of wavelength of light in the visible region,, for the complex, [Co(NH3)6]3+, [Co(CN)6]3–,, [Co(H2O)6]3+?, (a) [Co(CN)6]3– > [Co(NH3)6]3+ > [Co(H2O)6]3+, (b) [Co(NH3)6]3+ > [Co(H2O)6]3+ > [Co(CN)6]3–, (c) [Co(H2O)6]3+ > [Co(NH3)6]3+ > [Co(CN)6]3–, (d) [Co(CN)6]3– > [Co(NH3)6]3+ < [Co(H2O)6]3+, 39. An ion M2+, form the complexes, , [M(H2O)6]2+, [M(en)3]2+ and [M Br6]4– . Colour, of these complexes may be:, (a) Green, blue & Red, , (b) Blue, Red & Green, , (c) Green, Red & Blue, , (d) Red, Blue & Green, , 40. Cu2+ ions will be reduced to Cu+ ions by the, addition of an aqueous solution of:
Page 94 :
3.17, , (b) In [Fe(C2O4)3]2+, geometrical isomerism, does not exist, while optical isomerism exists., ONE OR MORE THAN ONE TYPE QUESTIONS, , (a), (b), (c), (d), , Ionization isomerism, Geometrical isomerism, Optical isomerism, Linkage isomerism, , 2. Find out correct IUPAC name of complex, compound., (a) Pentaaminecyanidocyanidochromium (II), hexanitrito-N-irridate (III), (b) Triamminetricyanidochromium (III), hexanitrito-N-irridate (III), (c) Hexanitrito-N-irridium (III), pentaamminecyanidochromate (II), (d) Pentaamminecyanidchromium (III), hexanitrito-N-irridate (III), 3. Complex ions [NiCl6]4–, [Ni(CN)6]4– similar in, their given properties:, (a) oxidation state, geometry, (b) co-ordination number, EAN, (c) magnetic moment, geometry, (d) stability, colour, 4. Which of the following compound has/have, effective atomic number equal to the atomic, number of noble gas?, (a) K[Co(CO)4], , (b) K2[Fe(CO)4], , (c) [Co(NH3)6]Cl2. (d) [CoCl3(H2O)3], 5. A d-block element forms octahedral complex, but its magnetic moment remains same either in, strong field or in weak field ligand. Which of the, following is/are correct?, , 7. The d-orbitals involved in sp3d2 or d2sp3, hybridisation of the central metal ion are:, , 8. Which of the following pairs of name and formula, of complexes, is correct?, (a) Tetramminecopper (II) sulphate …………., [Cu(NH3)4]SO4, (b) Diamminesliver (I) chloride ………………, [Ag(NH3)2]Cl, , (c) Potassium, hexacyanidoferrate, ………………….. K4[Fe(CN)6], , (d) Potassium, amminepentachloridoplatinate, (IV) ………………. K[Pt(NH3)Cl5], 9. Which of the following is/are correctly matched?, (a) [Ni(CO)4]Cl2-dsp2 and diamagnetic., (b) [Ni(en)3] (NO2)2- sp3d2 and two unpaired, electrons., (c) [V (NH3)6]Cl3-sp3d2 and two unpaired, electrons., (d) [Mn (NO+)3(CO)]-sp3 and diamagnetic., , S1: Generally square planar complexes show, geometrical isomerism but do not exhibit optical, isomerism because they do not posses plane of, symmetry., 4, 9, , (a) Element always forms colourless compound., (b) Number of electrons in t2g orbitals are higher, than in eg orbitals., (c) It can have either d3 or d8 configuration., (d) It can have either d7 or d8 configuration., 6. Which of the following statement (s) is/are false?, (a) In [PtCl2(NH3)4]2+ complex ion, the cisform is optically active, while trans-form is, optically inactive., , (III), , (a) S1 and S3 are correct, (b) S2 and S3 are correct, (c) S1 is incorrect, (d) S2 and S3 are incorrect
Page 95 :
3.18, , 11. Three arrangements are shown for the complex, [Co(en)(NH3)2Cl2]+. Pick up the wrong statement., Cl, , Cl, Co, , H2N, , NH3, , Cl, , NH 3, Co, , en, , Cl, , en, , en, , Co, , NH 3, , NH 3, , NH3, , Cl, , Cl, , (I), , (II), , (III), , (a), (b), (c), (d), , or neutral molecules which have a lone pair of, electrons, if the ligand is a neutral molecule such, as NH3, the negative end of the dipole in the, molecule is directed towards the metal cation., The electrons on the central metal ion are under, repulsive forces from those on the ligands. Thus, the electrons occupy the d-orbitals remain away, from the direction of approach of ligands., , I and II are geometrical isomers, II and III are optical isomers, I and III are optical isomers, II and III are geometrical isomers, , 12. Consider the following complexies [V(CO)6]–,, [Cr(CO)6] and [Mn(CO)6]+. Then incorrect, statement (s) about metal carbonyls is/are., (a) ‘C–O’ bond is strongest in the cation and, weakest in the anion, (b) ‘C–O’ bond order is less in the cation than in, anion., (c) ‘C–O’ bond longer in the cation than in, anionic or neutral carbonyl., (d) ‘M–C’ bond order is higher in the cation than, in anionic or neural carbonyl., BASED ON PASSAGE TYPE QUESTIONS, Passage # 1 (Q. 13 and 14), An isomer of the complex CoBrCl2(en)2(H2O), on, reaction with concentrated H2SO4 (dehydrating, agent), suffers no loss in weight and on reaction, with AgNO3 solution it gives only white, precipitate, which is soluble in NH3 solution., 13. The correct formula of the complex is:, (a) [CoBr(H2O)(en)2]Cl2, (b) [CoCl(en)2(H2O)]BrCl, (c) [CoBrCl(en)2]Cl. H2O, (d) [CoCl2(en)2]Br. H2O, 14. The incorrect statement about complex is:, (a) It can show geometrical isomerism, (b) cis isomer is optically active, (c) Trans isomer is optically active, (d) It can exhibit solvate isomerism, Passage #2: (Q. 15 and 16), According to CFT, attraction between the central, metal ion and ligands in a complex is purely, electrostatic. The transition metal which forms the, central atom cation in the complex is regarded as, positive ion. It is surrounding by negative ligands, , 16. The crystal field-spliting order for Cr3+ cation in, octahedral field for ligands CH3COO–, NH3,H2O, CN– is:, (a) CH3COO– < H2O < NH3 < CN–, (b) CH3COO– < NH3 < H2O < CN–, (c) H2O < CH3COO– NH3 < CN–, (d) NH3 < CH3COO– < H2O < CN–, Passage #3: (Q. 17 and 18), Double salts are addition compounds which, lose their identity in aqueous solution whereas, complexes which are also addition compounds, do not lose their identify in aqueous solution., The coordination compounds show isomerism, and find applications in photography, qualitative, analysis, metallurgy, water purification and in the, treatment of various diseases., 17. Which of the following statements is incorrect?, (a) Alum is a double salt., (b) EDTA salt of calcium is used in the treatment, of lead poisoning., (c) Effective atomic number of the metals in, complexes [Ni(CO)4] and [Fe(CN)6]4– is, same., (d) Chloridotris-(triphenylphosphine) rhodium, (I) is effective heterogenous catalyst for, hydrogenation of alkenes., 18. Which of the following statements is true for the, complex [Co(NH3)4Br2] NO2?, (a) It shows ionisation, linkage and geometrical, isomerism., (b) It does not show optical isomerism because, its cis and trans forms each have at least one, plane of symmetry., (c) Its ionisation isomers cannot be differentiated, by silver nitrate solution., (d) (a) and (b) both.
Page 96 :
3.19, , 27., , INTEGER TYPE QUESTIONS, 19. Brown colour of the complex [Fe(H2O)5(NO)], SO4 is due to charge transfer spectrum which, causes momentary change in oxidation state. Find, out oxidation state of Fe in this complex., 20. Sum of denticity of following ligands are, , Glycinate ion, Oxalate ion, o-phenathroline,, 2,2'-bipyridyl,, diethylenetriamine,, ethylenediamine, 21. Find the sum of number of geometrical isomers, for following complexes., , (1) [CoCl2Br2]2–, , (2) [Rh(en)3]3+, , (3) [Cr(en)2Br2]+, , (4) [Pt(en)Cl2], , 28., , (5) [Co(NH3)3(NO2)3], 22. In the complex Fe(CO)x, the value of x is:, 23. Count the number of ions which can form both, low spin and high spin complexes when coordination number 6, , Co+3, Ni+2, Cr+3, Fe+2, Fe+3, Cu+2, Ti+3, Co+2, 24. The number of unpaired electrons present in, [NiF6]2– is …………………, MATCH THE COLUMN TYPE QUESTIONS, 25., (A) [MnCl4]2–, , (P) sp3 hybridisation, , (B) [Ni(CN)4]–2, , (Q) Diamagnetic, , (C) [Ni(CO)4], , (R) Paramagnetic, , (D) [Cu(NH3)4]2+, , (S) dsp2 hybridisation, , 3, 24, , 15, 8, , 26., (c) Geometrical and optical, (d) Geometrical only, (A) [Ni(CO)4] and K2 (P) Magnetic moment, [Ni(CN)4], (B) [Cu(NH3)4] SO4 (Q) Oxidaton no. of, and K3[Cu(CN)4], central metal, (C) K2[NiCl4] and K4 (R) Geometry, [Ni(CN)4], (D) K2[NiCl4], K2[PtCl4], , and, , 4. If the bond length of CO bond in carbon monoxide, is 1.128 Å, then what is the value of CO bond, length in Fe(CO)5?, (a) 1.15 Å, (b) 1.128 Å, (c) 1.72 Å, (d) 1.118 Å, [IIT-2006], 5. Among the following metal carbonyls, the C—O, bond order is lowest in, (a) [Mn(CO)6]+, (b) [Fe(CO)5], (c) [Cr(CO)6], (d) [V(CO)6]–, [IIT-2007]
Page 98 :
3.21, , (c) diaminetetraquacobalt (III) chloride, (d) diamminetetraaquacobalt (III) chloride, [IIT-2012], 18. Consider the following complex ions, P, Q and R., P = [FeF6]3–, Q = [V(H2O)6]2+ and, R = [Fe(H2O)6]2+., The correct order of the complex ions, according, to their spin-only magnetic moment values (in, BM) is, (a) R < Q < P, , (b) Q < R < P, , (c) R < P < Q, , (d) Q < P < R, [JEE-Advanced-2013], , 19. Match each coordination compound in Column I, with an appropriate pair of characteristics from, Column II and select and correct answer using the, codes given below the Columns, , (en = H2NCH2CH2NH2 ; atomic numbers :, Ti = 22; Cr = 24; Co = 27; Pt = 78), , 22. How many EDTA (ethylenediaminetetraacetate, ion) molecules are required to make an octahedral, complex with a Ca2+ ion?, (a) One, (b) Two, (c) Six, (d) Three, [AIEEE-2006], 23. The “spin only” magnetic moment [in units of, Bohr magneton] of Ni2+ in aqueous solution, would be. (At. No. Ni = 28) (a) 0, (b) 1.73, (c) 2.84, (d) 4.90, [AIEEE-2006], 24. The only of the ‘spin only’ magnetic moment for, one of the following configuration is 2.84 BM., The correct one is:, (a) d 4 (in strong ligand field), (b) d 4 (in weak ligand field), (c) d 3 (in weak as well as in strong field), (d) d5 (in strong ligand field), [AIEEE-2006], 25. Which one of the following has a square planar, geometry–, , (C), , (Co = 27, Ni = 28, Fe = 26, Pt = 78) (a) [CoCl4]2–, (b) [FeCl4]2–, 2–, (c) [NiCl4], (d) [PtCl4]2–, [AIEEE-2007], , Codes:, A B, (a) d b, (c) b a, , 26. The coordination number and the oxidation state, of the element ‘E’ in the complex [E(en)2(C2O4)], NO2 (where (en) is ethylene diamine) are,, respectively–, (a) 6 and +2, (b) 4 and +2, (c) 4 and +3, (d) 6 and +3, [AIEEE-2008], , C D A, c a, (b) c, c d, (d) a, , B, a, c, , C, d, d, , D, b, b, , [JEE-Advanced-2014], 20. The IUPAC name for the complex [Co(NO2), (NH3)5] Cl2 is:, (a) pentaammine nitro-N-cobalt (II) chloride, , (a) [Co(CN)6]3–, , (b) [Co(C2O4)3]3–, , (c) [Co(H2O)6]3+, , (d) [Co(NH3)6]3+, , (b) pentaammine nitro-N-cobalt (III) chloride, (c) nitrito-N-pentaammineocobalt (III) chloride, (d) nitrito-N-pentaammineocobalt (II) chloride, [AIEEE-2006], 29. Which of the following pairs represents linkage, isomers?, (a) [Pd (P Ph3)2(NCS)2] and [Pd (P Ph3)2(SCN)2], (b) [Co(NH3)5(NO3)] SO4 and [Co(NH3)5SO4], NO3
Page 100 :
3.23, , 43. Consider, the, coordination, compound,, [Co (NH3)6]Cl3. In the formation of this complex,, the species which acts as the Lewis acid is (a) [Co(NH)6]3+, (b) Cl–, (c) Co3+, (d) NH3, [JEE-Main Online-2014], 44. Which one of the following complexes will most, likely absorb visible light?, (At number Sc = 21, Ti = 22, V = 23, Zn = 30), (a) [Sc (H2O)6]3+ (b) [Ti (NH3)6]4+, (c) [V(NH3)6]3+, (d) [Zn(NH3)6]2+, [JEE-Main Online-2014], 45. An octahedral complex with molecular, composition M.5NH3. ClSO4 has two isomers,, A and B. The solution of A gives a white, precipitate with AgNO3 solution and the solution, of B gives white precipitate with BaCl2 solution., The type of isomerism exhibited by the complex, is:, (a) Linkage isomerism, (b) Ionisation isomerism, (c) Coordinate isomerism, (d) Geometrical isomerism, [JEE-Main Online-2014], 46. Nickel (Z = 28) combines with a uninegative, monodentate ligand to form a diamagnetic, complex [NiL4]2–. The hybridisation involved and, the number of unpaired electrons present in the, complex are respectively., (a) sp3, two, (b) dsp2, zero, 2, (c) dsp , one, (d) sp3, zero, [JEE-Main Online-2014], 47. The octahedral complex of a metal ion M3+, with four monodentate ligands L1, L2, L3 and L4, absorb wavelengths in the region of red, green,, yellow and blue, respectively. The increasing, order of ligand strength of the four ligands is, (a) L4 < L3 < L2 < L1., , (b) L1 < L3 < L2 < L4, , (c) L3 < L2 < L4 < L1., , (d) L1 < L2 < L4 < L3, , [JEE-Main-2014], 48. Which molecule /ion among the following cannot, act as a ligand in complex compounds, (a) CH4., (c) Br, , –, , (b) CN, , –, , (d) CO, , [JEE-Main Online-2015], 49. Which of the following complex ions has electrons, that are symmetrically filled in both t2g and eg orbits?, , (a) [FeF6]3–, , (b) [Mn(CN)6]4–, , (c) [CoF6]3–, , (d) [Co(NH3)6]2+, [JEE-Main Online-2015], , 50. When concentrated HCl is added to an aqueous, solution of CoCl2, its colour changes from reddish, pink to deep blue. Which complex ion gives blue, colour in this reaction, (a) [CoCl6]3–, (b) [Co(H2O)6]2+, (c) [CoCl6]4–, (d) [CoCl4]2–, [JEE-Main Online-2015], , 52. The equation which is balanced and represents, the correct product (s) is, , NaOH, Excess, , →, , [JEE-Main-2015], 53. The number of geometric isomers that can exist, for square planar [Pt(Cl)(py)(NH3)(NH2OH))]+ is, (py = pyridine)., (a) 2, (b) 3, (c) 4, (d) 6, [JEE-Main-2015], 54. The pair having the same magnetic moment is, [at number Cr = 24, Mn = 25, Fe = 26 and Co =, 27], (a) [Cr(H2O)6]2+ and [Fe(H2O)6]2+, (b) [Mn(H2O)6]2+ and [Cr(H2O)6]2+, (c) [CoCl4]2– and [Fe(H2O)6]2+, (d) [Cr(H2O)6]2+ and [CoCl4]2–, [JEE-Main-2016], 55. Which one of the following complexes shows, optical isomerism?, (a) cis [Co(en)2 Cl2]Cl, (b) trans [Co(en)2 Cl2]Cl, (c) [Co(NH3)4 Cl2]Cl, (d) [Co(NH3)3 Cl3], [JEE-Main-2016]
Page 101 :
3.24, , Answer Key, , 1. (d), 11. (c), 21. (d), 31. (b), , 2. (d), 12. (d), 22. (a), 32. (a), , 3. (b), 13. (c), 23. (d), 33. (d), , 4. (d), 14. (b), 24. (c), 34. (d), , 5. (c), 15. (c), 25. (d), 35. (b), , 6. (b), 16. (d), 26. (c), 36. (b), , 7. (b), 17. (c), 27. (c), 37. (b), , 8. (b), 18. (a), 28. (c), 38. (c), , 9. (c), 19. (a), 29. (d), 39. (b), , 10. (b), 20. (c), 30. (d), 40. (c), , 1. (b), 11. (a), 21. (b), 31. (d), , 2. (d), 12. (c), 22. (c), 32. (b), , 3. (d), 13. (c), 23. (b), 33. (a), , 4. (d), 14. (b), 24. (c), 34. (c), , 5. (c), 15. (c), 25. (c), 35. (b), , 6. (a), 16. (b), 26. (c), 36. (c), , 7. (c), 17. (b), 27. (b), 37. (d), , 8. (b), 18. (c), 28. (b), 38. (c), , 9. (d), 19. (d), 29. (c), 39. (b), , 10. (a), 20. (d), 30. (b), 40. (d), , 1. (a,b,d), 2. (a,d), 3. (a,b,c), 4. (a,b,d), 5. (b,c), 9. (a,b,d), 10. (b,c), 11. (b,c,d), 12. (b,c,d), 13. (a), 17. (d), 18. (d), 19. (1), 20. (13), 21. (4), 25. A " P, R; B " Q, S; C " P, Q; D " R, S, 26. A " Q, R, S ; B " P, Q, R; S; C " P, R; D " P, R, S, 27. A " R, B " Q; C " P, D " S, 28. P " 4; Q " 1; R " 1, 2, 4; S " 3, , 1. (d), 11. (a), 21. (d), 31. (b), 41. (c), 51. (c), , 2. (b), 12. (c), 22. (a), 32. (a), 42. (b), 52. (b), , 3. (a), 13. (b), 23. (c), 33. (b), 43. (c), 53. (b), , 4. (a), 14. (b), 24. (a), 34. (b), 44. (c), 54. (a), , 5. (d), 15. (c), 25. (d), 35. (d), 45. (b), 55. (a), , 6. (b), 16. (c), 26. (d), 36. (a), 46. (b), , 6. (a,c,d) 7. (a.d), 14. (c), 15. (b), 22. (5), 23. (4), , 7. (c), 17. (d), 27. (a), 37. (a), 47. (b), , 8. (c), 18. (b), 28. (c), 38. (b), 48. (a), , 9. (a), 19. (b), 29. (a), 39. (b), 49. (a), , 8. (a,b,d), 16. (a), 24. (0), , 10. (b), 20. (b), 30. (a), 40. (a), 50. (d)
Page 104 :
3.27, , 5., , (c) [NiX4]–2 has zero unpaired e– hence, pairing of, electrons ocurs. X is a strong field ligand., +2, , ", , 8, , Ni = [Ar] 4s° 3d, 3d, , 4p, , 4s, , +2, , " [Cu(NH 3 )4 ] SO 4, , 2, , dsp, , ∆0 =, , 6., (a) Magnetic moment = 2.83 BM, Number of unpaired e– = 2, , hc, 1, or ∆ 0 ∝, λ, λ, , ", , 7., , (c) In brown ring complex, [Fe(H2O)5NO] SO4, Fe, has three unpaired e– hence, magnetic moment, = 15 BM and, hybridization is sp3d2., 8., (b) IUPAC name is., Pentaaminenitrito-N-chromium (III) tetrachlorozincate (II), This compound shows linkage and coordination isomerism but not geometrical, isomerism., 9., (d) Square planar complex Ma2b2 shows, geometrical isomerism., , 1, 3, , ", ", ", ", ", +2, , +1, , Na 2 [ Fe(CN)5 NO], ", ", ", ", , ., , "
Page 106 :
3.29, , 5. (b,c) Electronic , Hybridization, configuration, of central metal, 0, 3 , d – d, d2sp3, , 8, 10 , d –d, sp3d2, , In these cases, magnetic moment remains, same either in strong field or in weak field, ligand., 6. (a,c,d) In Ma4b2 complex both cis and trans-form are, optically inactive., M(AA)3 complex shows optical isomerism, but not geometrical isomerism., Mabcd tetrahedral complex shows optical, isomerism but Mabcd square planar complex, does not show optical isomerism., 7. (a,d) In sp3d2 or d2sp3 hybridization, d-orbitals, involved are dx2– y2 and dz2, 8. (a,b,d) Potassium hexacyanidoferrate (III) is, K3[Fe(CN)6], 9. (a,b,d) , 3d, , 4s, , Complex, Glycinate, , 2, 2, , 4p, , +2, , [Ni(CO)4]Cl2 Ni = [Ar], 4s° 3d8, , 2, , diamagnetic, dsp2, 3d, , [Ni(en)3], (NO2)2, , 4s, , 4p, , 2, , 4d, , +2, , paramagnetic, , Ni = [Ar], 4s° 3d8, 3, , 3, 2, , 2, , sp d, 3d, , [V(NH3)6] Cl3, , 4s, , 4p, , V+3 = [Ar], 4s° 3d2, , paramagnetic, 2, , Complex, , 3, , d sp, 3d, +1, , 4s, , 4p, , –3, , [Mn(NO)3(CO)] Mn 2 = [Ar], 4s 3d8, , diamagnetic, 3, , sp, , 10. (b, c), S1 is incorrect but S2 and S3 are correct., 11. (b, c, d), I and II are geometrical isomers., I and III are geometrical isomers., II and III are identical., 12. (b, c, d), Order of C – O bond order:, [V(CO)6]– < [Cr(CO)6] < [Mn(CO)6]+, , Order of M – C bond order:, [V(CO)6]– > [Cr(CO)6] > [Mn(CO)6]+, 13. (a), If, there is no loss in weight with concentrated, H2SO4 then complex does not have water, of crystalisation. complex gives white, precipitate of AgCl., , [CoCl2Br2]2–, , Zero (tetrahedral complex), , [Rh(en)3]3+, , Zero, +, , [Cr(en)2Br2], , 2, , [Pt(en)Cl2], , Zero, , [Co(NH3)3(NO2)3]
Page 110 :
Chapter, , Key Concepts, Introduction: The process of extraction of metal from its, ore in profitable manner is called metallurgy., (i) Mineral is a substance in which metal is, present in either native state or combined, state., (ii) ‘Ore’ is the mineral from which the metal can, be economically and conveniently extracted., (iii) ‘Gangue or matrix’ is the non metallic, impurities present in the ore., , (iii) Lighter impurity particles washed off with, water and heavier ore particles settle down at, the bottom, (iv) Usually employed for oxide and carbonate, ores., (b) Magnetic separation:, (i) Ore and gangue are separated, if only one of, them having magnetic property, (ii) Mixture of two minerals can also be separated, if one of them is non magnetic and the other, is magnetic., (c) Froth floatation process:, (i) Employed for sulphide ores, , Operation in which size reduction of large lumps to small, pieces followed by finely ground material by the use of, crushers and grinders., , Operation in which the removal of impurities (gangue), from ore by the following methods:, (a) Levigation or gravity separation:, (i) This method is based on the difference in, densities of the ore particles and gangue, particles., (ii) The powdered ore with gangue particles, introduced in the running steam of water., , (ii) It is based on the different wetting, characteristics of the ore and gangue particles, with water and oil., (iii) Usually ore particles are making as aerofillic, and gangue particles as aerophobic by using, different reagents., (iv) Ore particles raised to the surface along with, air bubbles and collected at the surface where, as gangue particles are wetted and settled, down at the bottom of the tank., (v) Reagents acts as frothing agents (pine oil),, collectors (ethyl xanthate and potassium, ethyl xanthate), activators (copper sulphate), and depressors (sodium cyanide, alkali).
Page 111 :
4.2, , (d) Leaching:, (i) Chemical method of concentration, (ii) Selective dissolution of ore in strong reagents, where as gangue particles are undissolved, and gets separated., , (iii) Employed for concentrating ores of, aluminium, silver gold etc., Working of the concentrated ore:, (i) It depends upon the nature of the ore as well as, the nature of impurities., (ii) Processes involved:, (a) Conversion of the concentrated ore to its, oxide form., (b) Conversion of the oxide to the metallic form, (c) Hydrometallurgy, Conversion of the concentrated ore into its oxide form:, (i) Calcination:, (a) Ore is heated in absence of air to remove, water or CO2 from hydrated oxides or, carbonates respectively., (b) Process temperature is below the melting, points of treated ores., , (c) During calcination moisture, volatile, impurities are removed there by one becomes, porous., Example:, , Al2O3. 2H2O, , Al2O3 + 2H2O, , 2Fe2O3. 3H2O, , 2Fe2O3 + 3H2O, , CaCO3, , CaO + CO2, , MgCO3, , MgO + CO2, , (ii) Roasting:, (a) Ore is heated strongly with other substances,, usually with oxygen, (b) Employed for sulphide ores, (c) Process temperature is below the melting, points of treated ore, (d) Chemical conversion of ore is taking place., (e) Some of the impurities removed as volatile, substances., , S + O2, 4As + 3O2, P4 + SO2, Examples:, , SO2, 2As2O3, 2P2O5, , (a) Conversion of metal sulphides into oxides, , , 2ZnS + 3O2, , 2ZnO + 2SO2, , 2PbS + 3O2, , 2PbO + 2SO2, , (b) Metal sulphides converted into sulphates, , PbS + 2O2, , PbSO4, , ZnS + 2O2, , ZnSO4, , (c) Metal sulphides converted into chlorides, , Ag2S + 2 NaCl, , 2AgCl + Na2S, , (d) Conversion of amalgams, AgCl + 2Hg, , Ag – Hg + HgCl, , Conversion of the oxide to metallic form:, The roasted or calcined ore is converted into metallic form, through reduction by using different reducing techniques, which will depends upon the nature of the ore, some of the, methods are mentioned below., (i) Reduction by carbon (smelting):, The oxides of less electropositive metals like, Pb, Zn, Fe, Sn, Cu etc, are reduced by strongly, heating with coal or coke., (a) Reduction of the oxide with carbon at high, temperature is known are as smelting, (b) Flux is added smelting, which reduces the, melting point of impurities to form as easily, fusible substance called as ‘slag’ and can be, separated easily because of its lower density., (c) Selection of flux depends upon nature of, impurity present. Its impurity is acidic, basic, flux is employed and vice versa., (d) Smelting is usually carried out in blast, furnaces or reverberatory furnace., (ii) Reduction by aluminium (Alumino-thermite, reduction):, (a) Aluminium acts as reducing agent due to its, high electropositive nature., (b) Oxides such as Cr2O3, Mn3O4 are reduced, by this method because carbon or CO are not, efficiently reduced., (c) The process is also known as “Gold Schmidt, thermite process”., (iii) Reduction by heating in air (Auto-reduction):, (a) Employed for metals of less active such as, Hg, Cu and Pb, (b) Due to unstable nature in the oxide form, at high temperature, no reducing agent is, required for their reduction .
Page 112 :
4.3, , Example :, , of zinc powder., , , HgS + O2 ∆, → Hg + SO2, ∆, , , 2Cu2S + 3O2 , → 2Cu2S + 2O2 (Roasting), , 2Cu2O + Cu2S ∆, → 6C + SO2 (Auto-reduction), (iv) Electrolytic reduction (Electro-metallurgy):, (a) Employed for highly electropositive metals, such as Na, K, Ca, Mg, Al, etc., (b) These metals are extracted by the electrolysis, of their oxides hydroxides or chlorides in, fused state., Example:, , On fusion:, NaCl Na+ + Cl- (ions become mobile), On electrolysis:, At cathode:, Na+ + e-, , Na, , At anode:, Cl-, , Cl + e-, , Cl + Cl, Cl2, (c) Aluminium is obtained by the electrolysis, of electrolyte which consists of mixture, of alumina, cryolite and calcium fluoride., (cryolite and fluorospar are added to reduce, melting point of electrolyte and to increase, conductivity)., (v) Other methods are:, (a) Reduction by carbon monoxide (employed, for iron (III) oxide), (b) Reduction by water gas (employed for nickel, oxide), (c) Amalgamation method (employed for noble, metals), Hydrometallurgy (Reduction by precipitation):, (i) Process in which more electropositive metals, displace less electropositive metals from salt, solution., (ii) First the concentrated ore is dissolved, in strong reagent and removed insoluble, precipitates., (iii) Now the metal is precipitated by addition of, more electropositive metal., Example:, Silver sulphide dissolved in sodium cyanide which forms a, soluble complex, then silver is precipitated by the addition, , Ag2S + 4 NaCN, , 2Na [Zn(CN)2] + Na2S, , , , Sodium dicyanoargentate (I), , 2Na[Ag(CN)2] + Zn, , Na2 [Zn(CN)4] + 2Ag ., , Refining or purification:, (i) The metals after reduction process consists of, number of impurities like Si, P, Slag, oxides,, other metals etc., (ii) Removal of all these impurities to get pure, metal is called as refining, (iii) Methods as refining are:, (a) Liquation:, (i) This is based on the principle of difference in, melting points of metal and impurity., (ii) Employed for purification of low melting, point metals like Pb, Sn, etc., (b) Distillation process:, (i) This is based on difference in boiling points, of metals and impurities., (ii) Employed for low boiling point metals like, Zn, Hg etc., (c) Oxidation process:, (i) This is a selective oxidation method, (ii) Used for refining those metals in which, the impurities have greater tendency to get, oxidized than the metals it self, (iii) The impurities converted into oxide and, skimmed off from the metal, (iv) Various oxidation processes used for different, metals bear different names, eg, poling,, pudding, bessemerisation and cupellation, (for Ag), (d) Electrorefining:, (i) Employed for refining of highly electro, positive metals like Al, Cu, Ag, Zn, Sn, Cr, and Ni, (ii) Impure metal is made as anode, thin pure, metal sheet is kept as cathode and the, electrolyte is comprising with soluble salt, solution of the metal., (iii) On passing the electric current, pure metal, from the anode dissolved and is deposited on, the cathode.
Page 113 :
4.4, , (iv) The soluble impurities go into the solution, (remains in the solution after the completion, of refining) while the insoluble impurities, settle down below the anode as ‘anode mud’., , , , (e) Van-Arkel process:, (i) Employed to get metal in very pure form of, small quantities., (ii) In this method, the metal is converted into, volatile unstable compound (eg iodide), and, impurities are not affected during compound, formation., (iii) The compound thus obtained is decomposed, to get the metal., (iv) Employed for purification of metals like, titanium and zirconium, , Til4 (g), , Ti (s) + 2I2 (g), , , , 1700K, , 523K, , TiI4 (g), , Ti (s) + 2I2 (g), , (f) Zone refining:, (i) Employed for metals which require very high, purity like semi conductors., (ii) The method is based on the principle that an, impure metal on solidification will deposit, crystals of pure metal and the impurities will, remain behind in the molten part of the metal., (iii) Used to purify the elements such as silicon,, germanium, etc., , Solved Examples, 1. Which of the following is not a concentration, technique?, Levigation, Froth floatation, Leaching, Calcination, Sol.(d) Concentration is the method employed to remove, gangue materials by mechanical separation. In, calcination, volatile impurities are removed by, heating ore below its melting point in absence or, limited supply of air., 2. The ores that are concentrated by froth floatation, method are:Carbonates, Sulphides, Oxides, Phosphates, Sol.(b) In floatation process, the ore particles should, have aerofillic in preference to gangue particles., Sulphide ores having this character., 3. Calcination is the process in which:, Heating the ore in presence of air, Heating the ore in presence of sulphur, Heating the ore in absence of air, Heating the ore in presence of chlorine, Sol.(c) Calcination is the process in which moisture and, , volatile impurities are removed and process is, carried in absence of air or in limited supply of, air., 4. The purpose of adding Na3AlF6 to Al2O3 during, electrolysis is:To decrease melting point of Al2O3, To increase conductivity of electrolyte, To provide reducing conditions in the bath, Both (a) and (b), Sol.(d) Al2O3 is a poor conductor of electricity and, having very high melting point. So, to increase, the conductivity Na3AlF6 is added and to decrease, the melting point Na3AlF6 and CaF2, AlF3 are, added so that melting point of electrolyte comes, to around 930°C., 5. During the process of electro refining of copper,, some metals present as impurity settle down as, anode mud. These are:, Sn and Ag, Pb and Zn, Ag and Au, Fe and Ni, Sol.(c) In anode mud, less electropositive elements are, present. Ag and Au are less electropositive than, Cu., 6. The method of Zone refining of metals is based, on the principle of:
Page 114 :
4.5, , Greater solubility of the impurities in the, molten state than in the solid., Greater solubility of pure metal than that of, impurity., Higher melting point of the impurity than, that of pure metal., Greater noble character of the solid metal, than that of the impurity., Sol.(a) Zone refining is used when pure metal has greater, melting point than impurities and impurities have, greater solubility in molten state than in the solid., 7. Which of the following is not correctly matched?, ZnCO3 " Zn (Calcination followed by, smelting), Al(OH)3 " Al (Calcination followed by, smelting), PbS " Pb (Partial roasting followed by self, reduction), Cu2S " Cu (Complete roasting following by, carbon reduction), Sol.(b) ZnCO3 " Zn, ZnCO3 ∆, → ZnO + CO2- (Calcination), ZnO + C ∆, → Zn + CO- (Smelting), Al(OH)3 " Al, , Poling, Electrolytic process, Sol.(a) In cupellation process, impure metal is heated, with borax and silica. Impurities are removed as, their oxide., 9. What is the appropriate process for the extraction, of lead from galena?, (A) Froth floatation (B) Calcination (C) Roasting, (D) Self reduction (E) Electrolytic reduction, (F) smelting (G) Leaching, G " B" D " E, A"C"D, A"C"E, A"C"F, Sol.(b) Galena is a sulphide ore (PbS). It is concentrated, by froth floatation. After partial roasting, self, reduction process is used., 10. In purification of bauxite ore, it is mixed with coke, and heated at 1800°C in presence of nitrogen., This is:, Hall’s process, Serpeck’s process, Baeyer’s process, Electrolytic reduction, Sol.(b) Serpeck’s process:-, , ∆, , Al(OH)3 , → Al2O3 + H2O- (Calcination), Al2O3 ∆, → Al (electrolytic reduction), , Al2O3. 2H2O + 3C + N2 ∆, → 2AlN+3CO+2H2O, AlN + 3H2O " Al(OH)3. + NH3 2Al(OH)3 ∆, → Al2O3 + 3H2O-, , PbS " Pb, ∆, , PbS + O2 , → PbO + PbSO4 + SO2- (Partial, , roasting), PbO + PbS ∆, → Pb + SO2-, , , , Or PbS + PbSO4 ∆, → Pb + SO2-, , (Self, reduction), , 11. Select the reaction which does not occur in, Bessemer’s convertor:, FeS + O2 " FeO + SO2, 2Cu2S + 3O2 " 2Cu2O + 2SO2, FeO + SiO2 " FeSiO3, 2CuFeS2 + O2 " Cu2S + 2FeS + SO2, Sol.(d) The reaction,, , Cu2S " Cu, , 2CuFeS2 + O2 ∆, → Cu2S + 2FeS + SO2, , Cu2S + O2 ∆, → Cu2O + SO2- (Complete, , roasting), , represents partial roasting of ore. It is carried out, in reverberatory furnace., , Cu2O + C ∆, → Cu + CO- (Carbon reduction), 8. When an impurity in metal has greater affinity for, oxygen and is more easily oxidized than metal, itself then the metal is refined by:, Cupellation, Zone refining, , 12. Which of the following process is not involved in, the extraction of Ag from argentite?, Hydrometallurgy, Formation of cyanide, Levigation, Reduction by Zn metal
Page 115 :
4.6, , Sol.(c) Levigation is a concentration process, used, for oxide ores. Concentration process used for, argentite ore is cyanide leaching., , Removal of Pb impurity from impure, Ag " Cupellation, Obtaining wrought iron from cast, iron " Bessemerisation, Refining of Nickel " Mond’s process, , Ag2S + CN- + H2O + O2 " [Ag(CN)2] Reduction process used is Hydrometallurgy, (reduction by Zn)., -, , -2, , [Ag(CN)2] + Zn " Ag + [Zn(CN)4], , 13. Select the incorrect statement:, Based on reactivity series, occurrence of, certain elements takes place in native state., Due to the basic nature of oxides of alkaline, earth metals, they combine with atmospheric, acidic oxides giving salts., Chalcopyrite ore contains both Cu and Fe., Both anglesite and cassiterite contain Pb., Sol.(d) Due to less reactivity, Au occurs in native state., Due to basic nature of alkaline earth metals, oxides, they occur in the form of their salts., Anglesite is PbSO4 and cassiterite is SnO2., 14. Which of the following is not correctly matched?, Removal of oxide impurity from impure, Cu " Poling process, , Sol.(c) Wrought iron is obtained from cast iron in, reverberatory furnace., 15. Which of the following statement is not correct?, Tin stone is separated from non-magnetic, impurity of wolframite by electrolytic, separation., CO acts as reducing agent is most parts of, blast furnace during extraction of iron., A silicate slag is obtained during the, extraction of Cu from CuFeS2 and iron from, haematite., In Puddling process, impurities present in, cast iron are oxidized by haematite., Sol.(a) Tin stone (SnO2) is non-magnetic while, wolframite (FeWO4) is magnetic Tin stone is, separated from wolframite by electromagnetic, separation., , Exercise, 5. Which one of the following is not an ore of, aluminum?, 1. Three most occurring elements into the earth, crust are:, (a) O, Si, Al, , (b) Si, O, Fe, , (c) Fe, Ca, Al, , (d) Si, O, N, , 2. An example of an oxide ore is, (a) Bauxite, , (b) Malachite, , (c) Zinc blende, , (d) Feldspar, , 3. Which of the following set of elements mostly, occur as sulphide ores?, (a) Zn, Cu, Na, (b) Zn, Cu, Pb, (c) Fe, Al, Ti, (d) Cu, Ag, Au, 4. Which of the following is not an ore of, Magnesium?, , (a) Bauxite, , (b) Corundum, , (c) Epsomite, , (d) Cryolite, , 6. Which one contains both Ca and Mg?, (a) Limestone, , (b) Dolomite, , (c) Chalk, , (d) Feldspar, , 7. Which of the following minerals does not contain, iron?, (a) Magnetite, (b) Magnesite, (c) Haematite, (d) Limonite, 8. Metals which exist as native ore?, (a) Ni, Pt, (b) Zn, Cd, (c) Pt, Au, (d) Sn, Pb, 9. Which of the following contain Fe as well as Cr?, , (a) Carnallite, , (b) Magnesite, , (a) Wolframite, , (b) Chromite, , (c) Dolomite, , (d) Gypsum, , (c) Pyrolusite, , (d) Chalcopyrite
Page 116 :
4.7, , 10. Which ore is concentrated by ‘wetting by oil’?, (a) Oxide ore, , (b) Sulphate ore, , (c) Carbonate ore, , (d) Sulphide ore, , 11. Haematite ore is concentrated by:, (a) Gravity separation method, (b) Froth floatation process, (c) Amalgamation, , (d) Oxide ores are concentrated by froth, floatation process., 19. Purification of silicon element, semiconductors is done by:, , used, , in, , (a) Zone refining, (b) Heating, (c) Froth floatation, (d) Heating in vacuum, , (d) Hand picking, 12. In the extraction of copper from its sulphide ore,, the metal is formed by reduction of Cu2O with:, (a) FeS, , (b) CO, , (c) Cu2S, , (d) SO2, , 13. The element which is recovered from electrolytic, process is:, , 20. Which one of following beneficiation processes is, used for the mineral Al2O3.2H2O?, (a) Froth floatation (b) Leaching, (c) Liquation, , (d) Magnetic separation, , 21. In blast furnace, maximum temperature is in:, (a) Zone of fusion, , (a) Iron, , (b) Lead, , (b) Zone of combustion, , (c) Aluminium, , (d) Zinc, , (c) Zone of slag formation, , 14. Refining of silver can be done by:, (a) Cupellation, (b) Electrorefining, , (d) Zone of reduction, 22. When a metal is to be extracted from its ore and, if the gangue associated with the ore is silica,, then:, , (c) Both (a) and (b), , (a) An acidic flux is needed, , (d) None of these, , (b) A basic flux is needed, , 15. Impure aluminium is purified by:, (a) Baeyer’s process, (b) Hall’s process, (c) Hoop’s Process, , (c) Both acidic and basic fluxes are needed, (d) Neither of them is needed, 23 Silver containing lead as an impurity is removed, by:, , (d) Serpeck’s process, , (a) Poling, , (b) Cupellation, , 16., , , , , , (c) Lavigation, , (d) Distillation, , In the alumino-thermite process, Al metal acts as:, (a) Oxidizing agent, (b) Reducing agent, (c) Catalyst, (d) Flux, , 17. Slag is formed by reaction between:, (a) Impurities and coke, (b) Impurities and ore, (c) Impurities and flux, (d) Flux and coke, m18. Which of the following statement is correct?, (a) All ores are minerals, (b) All minerals are ores, (c) Calcination is generally carried out in blast, furnace, , 24. Cassiterite is concentrated by:, (a) Levigation, (b) Electromagnetic separation, (c) Froth-floatation, (d) Liquification, 25. For which ore of the metal, froth floatation, method is used for concentration?, (a) Horn silver, , (b) Bauxite, , (c) Cinnabar, , (d) Haematite, , 26. In which of the following minerals, aluminium is, not present?, (a) Cryolite, , (b) Mica, , (c) Feldspar, , (d) Fluorspar
Page 117 :
4.8, , 27. Gravity separation process may be used for the, concentration of:, , 5. Which one of the following reactions is an, example for calcination process?, , (a) Chalcopyrite, , (b) Bauxite, , (a) 2Ag + 2HCl + (O) " 2AgCl + H2O, , (c) Haematite, , (d) Calamine, , (b) 2Zn + O2 "2ZnO, , 28. Electrolytic reduction method is used in the, extraction of:, (a) Highly electropositive elements, (b) Highly electronegative elements, (c) Transition metals, (d) Noble metals, 29. Bauxite is leached with:, (a) KCN, , (b) NaCN, , (c) NaOH, , (d) Na2CO3, , 30. In the equation:, 4M + 8CN- + 2H2O + O2 " 4[M(CN)2]- + 4OH Identify the metal M:, (a) Cu, , (b) Fe, , (c) Au, , (d) Zn, , 1. Which of the following process is not a physical, process of separation?, (a) Levigation, (b) Magnetic separation, (c) Leaching, (d) Froth floatation, 2. In the extraction of copper from copper pyrites,, iron is removed as:, (a) FeSO4, , (b) FeSiO3, , (c) Fe3O4, , (d) Fe2O3, , 3. In zone refining method, the molten zone:, (a) Consists of impurities only, (b) Consists more impurity than the original, metal, (c) Contains the purified metal only, (d) Moves to either side, 4. Bauxite, Siderite and argentite are respectively:, (a) Sulphide, oxide and carbonate ore, (b) Oxide, carbonate and sulphide ore, (c) Oxide, oxide and sulphide ore, (d) Oxide, sulphide and oxide ore, , (c) 2ZnS + 3O2 " 2ZnO + 2SO2, (d) MgCO3 " MgO + CO2, 6. Cupellation process is used in the metallurgy of:, (a) Cu, , (b)Ag, , (c) Zn, , (d)Al, , 7. In electrorefining of metal the impure metal, is made the anode and a strip of pure metal the, cathode during the electrolysis of an aqueous, solution of a complex metal salt. This method, cannot be used for refining of:, (a) Silver, (b) Copper, (c) Aluminium, (d) Gold, 8. Among the following groups of oxides, the group, that cannot be reduced by carbon to give the, respective metals is:, (a) Cu2O, SnO2, , (b) Fe2O3, ZnO, , (c) CaO, K2O, , (d) PbO, Fe3O4, , 9. The reason for floating of ore particles in, concentration by froth floatation process is that:, (a) They are light, (b) They are insoluble, (c) They are charged, (d) They are hydrophobic, 10. Which of the following statements is correct, regarding the slag obtained during the extraction, of a metal like copper or iron?, (a) The slag is lighter and has lower melting, point than the metal, (b) The slag is heavier and has lower melting, point than the metal, (c) The slag is lighter and has higher melting, point than the metal, (d) The slag is heavier and has higher melting, point than the metal, 11. Consider the following reactions at 1000°C., 1, (i) Zn(s) + O2 (g) "ZnO(g); DG°= -360 kJ, 2, mol-1, 1, (ii) C(s) + O2 (g) " CO(g); DG°= -460 kJ, 2, mol-1
Page 118 :
4.9, , (a), (b), (c), (d), , ZnO is more stable than CO, ZnO can be reduced to Zn by C, ZnO and CO are formed at equal rate, ZnO can not be reduced to Zn by C, , 12. DG° vs T plot in the Ellingham’s diagram slopes, downward for the reaction:, 1, (a) Mg + O2" MgO, 2, (b) 2Ag +, , 1, O2" Ag2O, 2, , 1, O2" CO, 2, 1, (d) CO + O2 " CO2, 2, 13. The incorrect match in the following is:, (c) C +, , (a) Purification of Al metal : Baeyer’s method, (b) Polling : Reduction of Cu2O, (c) FeCr2O4 (Chromite ore) : NaOH/ Na2CO3, (d) Ag : Mac Arthur cyanide process, 14. An ore after levigation is found to have acidic, impurities. Which of the following can be used as, flux during smelting operation?, (a) H2SO4, , (b) CaCO3, , (a) SiO2, , (d) Both CaCO3 and SiO2, , (a) In froth floatation process, pine oil decreases, the surface tension of the solution., (b) In Poling refining, non volatile oxides are, removed as sucm., (c) Dolomite ore can be considered as ore of, both Ca and Mg., (d) Aqueous Al2(SO4)3 is used for electrorefining, of Al., 19. Which is incorrectly matched for refining of, elements?, (a) Si, Ge from impurities, zone refining, method, (b) Sn refined from impurities with high boiling, point poling method, (c) Zn, Cd from impurities with high boiling, point distillation, (d) Al from impurities Cu, Fe hoop method, 20. A mixture of alumina and coke is heated in a, current of nitrogen to about 1800°C. And the, product obtained is treated with water. A gas is, evolved. The gas is:, (a) N2, , (b) N2O, , (c) NH3, , (d) NO, , 21. The slag obtained during the extraction of copper, pyrites is composed mainly of:, (a) MgSiO3, , (b) CuSiO3, , 15. Which of the following metals never occurs in, free sate in nature?, (a) Gold, (b) Copper, (c) Silver, (d) Sodium, , (c) FeSiO3, , (d) CuFeS2, , 16. Select the incorrect statement in the following:, (a) Silica present in bauxite is removed by, addition of NaOH(aq.), (b) Silica present in haemetite is removed by the, addition of CaCO3 during smelting, (c) Fe2O3 present in bauxite is removed by, Bayer’s process, (d) Magnetic separation is used for cassitarite, , (B) 2C (gr) + O2 (g) " 2CO(g); DG° = –500kJ, mol-1, , 17. In which of the following process, roasting and, self reduction is required?, (a) Cu2S " Cu2O, (b) CaCO3" CaO, (c) ZnS " Zn, (d) PbS " Pb, 18. Which of the following statement is not correct?, , 22. Consider the following at 1000°C, (A) 2Zn (s) + O2 (g) " 2ZnO(g); DG° = –360kJ, mol-1, , Choose the correct statement at 1000°C:, (a) Zinc can be oxidized by carbon monoxide, (b) Zinc oxide can be reduced by graphite, (c) Both statements (a) and (b) are true, (d) Both statements (a) and (b) are False, 23. When ZnS and PbS minerals are present together,, them NaCN is added to separate them in the froth, floatation process as a depressant because:, (a) Pb(CN)2 is precipitated while no effect on, ZnS., (b) ZnS forms soluble complex Na2[Zn(CN)4]., (c) PbS forms soluble complex Na2[Pb(CN)4]., (d) It decreases the floatation property of PbS by, making it hydrophilic
Page 119 :
4.10, , 24 The materials mixed, before haemetite ore is, subjected to smelting in the extraction of iron,, are:, (a) Coke and silica, (b) Coke and limestone, (c) Limestone and silica, (d) Cock, silica and limestone, 25. Which of the following reaction forms the basis, of Goldschmidt alumino-thermite process?, (a) 2Al + N2 " 2AlN, (b) 2Al + 3Cl2 " 2AlCl3, , (c) Zinc blende and iron pyrites are sulphides., (d) Malachite and azurite are ores of copper., , ONE OR MORE THAN ONE CORRECT TYPE, 1. Which of the following is (are) manufactured by, the electrolysis of their fused salts?, (a) Copper , , (b) Sodium, , (c) Aluminium , , (d) Platinum, , 2. Complexes formed in the cynide process are:, , (c) 2Al + 6HCl " 2AlCl3 + 3H2, , (a) [Au(CN)2]- , , (b) [Ag(CN)2]-, , (d) 2Al + Fe2O3 " Al2O3 + 2Fe, , (c) [Cu(CN)4]2- , , (d) [Zn(CN)4]2-, , 26. In the extraction of copper, metal is formed in the, Bassemer converter due to reaction:, , 3. Which of the following process (es) occur(s) during, the extraction of copper from chalcopyrites?, , (a) Cu2S + 2Cu2O "6Cu + SO2, , (a) Froth floatation , , (b) Roasting, , (b) Cu2S " 2Cu + S, , (c) Bessemerisation , , (d) Calcination, , (c) Fe + Cu2O " 2Cu + FeO, , 4. Calcium silicate (slag) formed in the slag, formation zone in extraction of iron from, Haematite ore:, (a) Does not dissolve in molten iron., (b) Being lighter floats on the molten iron., (c) Is used in cement industry and as building, material., (d) Prevents the re-oxidation of molten iron., , (d) 2Cu2O " 4Cu + O2, 27. The extraction of zinc from zinc blende is, achieved by:, (a) Electrolytic reduction, (b) Roasting followed by reduction with carbon, (c) Roasting followed by reduction with another, metal, (d) Roasting followed by self-reduction, 28. Which method is not correct given for refining of, crude metals?, (a) Distillation : Zinc and mercury, (b) Liquation : Tin, (c) Van Arkel : Zirconium, (d) Mond process : Lead, 29. Poling process is used for:, (a) The removal of Cu2O from Cu, (b) The removal of Al2O3 from Al, (c) The removal of Fe2O3 from Fe, (d) All of these, 30. Among the following statements, the incorrect, one is, (a) Calamine and siderite are carbonates., (b) Argentite and cuprite are oxides., , 5. Liquation process may be applied for the, purification of:, (a) Copper , , (b) Tin, , (c) Iron , , (d) Zinc, , 6. Roasting of copper pyrites is done:, (a) To remove moisture., (b) To oxidize free sulphur and antimony., (c) To convert pyrites completely into Cu2O and, FeO., (d) To remove volatile organic impurities., 7. Select the correct statement:, (a) Dolomite contains both magnesium and, calcium., (b) Extraction of lead from galena involves, roasting in limited supply of air at moderate, temperature followed by self reduction at, higher temperature (to melt the charge).
Page 120 :
4.11, , (c) Extraction of zinc from Zinc blende involves, roasting followed by reduction with carbon., (d) The chemical composition of ‘slag’ formed, during the extraction of iron and copper is, FeSiO3., 8. Which of the following is a correct statement?, (a) Calamine is the ore of zinc., (b) Proustite is the ore of silver., (c) Cassiterite is the ore of tin., (d) Diaspore is the ore of aluminium., 9. Froth floatation:, (a) Is a physical method of separating mineral, from the gangue, (b) Is a method of concentration of ore depending, on the difference in wetability of gangue and, the ore particles., (c) Is used for the concentration of sulphide ores, (d) Is a method in which impurities sink to, the bottom and ore particles pass on to the, surface with froth., 10. Which of the following are correct processes?, (a) Fe + Al2O3 " 2Al + Fe2O3, (b) ZnO + C " Zn + CO, (c) Cr2O3 + 2Al " 2Cr + Al2O3, (d) 2[Ag(CN)2]- + Zn " 2Ag + [Zn(CN)4]211. Which of the following reactions occur during, calcination?, (a) CaCO3 " CaO + CO2, (b) 2Al(OH)3 " Al2O3 + 3H2O, (c) 4FeS2 + 11O2 " 2Fe2O3 + 8SO2, (d) Cu2S + 2CuO " 4Cu + SO2, 12. Silver containing lead as an impurity is not, purified by:, (a) Poling, , (b) Cupellation, , (c) Levigation, , (d) Distillation, , 13. Select the correct statements for Ellingham, diagram:, (a) Any metal will reduce the oxide of other, metals which lie above it in the Ellingham, diagram., (b) According to Ellingham diagram, Al will not, reduce MgO at temperature below 1350°C, (c) According to Ellingham diagram, Al will, reduce MgO at temperature below 1350°C, , (d) Any metal will not reduce the oxide of other, metals which lie above it in the Ellingham, diagram., PARAGRAPH BASED QUESTIONS, Paragraph # 1 (Q. 14 to 15), , Metallic gold frequently is found in, aluminosilicate rocks and it is finely dispersed, among other minerals. It may be extracted by, treating the crushed rock with aerated Sodium, cyanide solution. During this process metallic, gold is slowly converted to [Au(CN)2]-, which, is soluble in water. After equilibrium has been, reached, the aqueous phase is pumped off and, the metallic gold is recovered from it by reacting, the gold complex with zinc which is converted to, [Zn(CN)4]2-gold in nature is frequently alloyed, with silver which is also oxidized by aerated, sodium cyanide solution., 14. The correct ionic reaction for the process is:, (a) 4Au + 8CN- + 2H2O + O2(air) " 4[Au(CN)2](soluble) + 4OH (b) Au + 2CN- " [Au(CN)2] (c) Zn + 2CN- " [Zn(CN)2] (d) Zn + 4CN- " [Zn(CN)4]215. The process described above in the paragraph, represents:, (a) Ore concentration, (b) Pyrometallurgical extraction, (c) Hydrometallurgical extraction, (d) Purification of metal, Paragraph # 2 (Q. 16 to 17), Extraction of copper is done using copper pyrites., After roasting, the ore is mixed with silica and, coke and then smelted in a blast furnace. The, matte obtained from the blast furnace is charged, into a silica-lined converter. Some silica is also, assed, and a hot air blast is blown into the mixture, to obtain blister copper, which is purified by, electrorefining., 16. The chemical formula of copper pyrites is:, (a) CuFeS2, , (b) Cu2O, , (c) Cu2S, , (d) CuCO3. Cu(OH)2, , 17. The chemical composition of the slag formed, during smelting is:, (a) CuSiO3, , (b) FeSiO3, , (c) CaSiO3, , (d) Cu2O. SiO2
Page 121 :
4.12, , Paragraph # 3 (Q. 18 to 19), , (a) Bauxite, , (b) Corundum, , Extraction of aluminium can be understood by:, , (c) Dolomite, , (d) Malachite, , (e) Magnetite, , (f) Pyrolusite, , (g) Argentite, , (h) Horn silver, , (i) Quartz, , (j) Cryolite, , (k) Siderite, , (l) Zincite, , (m) Calamine, , (n) Sylvine, , Pure alumina, (Al2O3), Reduction, of alumina, By electrolysis, , Concentration of ore by, Chemical method, , Impure Al, , Electrolysis, , Bauxite, (Al2O3.2H2O), , Pure Al, , Using suspended graphite rods an anode and, C-lining inside the Fe container., 18. The purpose of adding cryolite is:, (a) To remove the impurities as slag, (b) To lower the melting point of Al2O3, (c) To decrease the electrical conductivity of, pure aluminium, (d) To increase the Al percentage in the yield, 19. The molten electrolytes contain Na+, Al3+ and, Ca2+ but only Al gets deposited at Cathode, because,, (a) Standard reduction potential of Al is more, than that of Na and Ca, (b) Standard oxidation potential of Al is more, than that Na and Ca, (c) Graphite reacts only with Al3+ and not with, Na+ and Ca2+, (d) Discharge potential of Al3+ is higher than Na+, and Ca2+, INTERGER TYPE QUESTIONS, 20. Number of metals among following which are, obtained by electrometallurgy in molten state are:, Li, Ba, Na, Al, Fe, Cu, Pb, Sn, Ag, Au, Zn, Ca,, Mg, 21. How many of the following process of refining is/, are chemical methods:, (a) Liquation process, (b) Fractional distillation process, (c) Zone refining method, (d) Chromatographic method, (e) Cupellation, (f) Poling process, (g) Hoop’s process, (h) Kroll’s process, 22. How many of the following minerals are oxides, of metals/ metalloids., , (o) Carnellite, 23. Poling process is applied when impurity is a, compound of a metal and a non-metal. Atomic, number of non-metal is…………………, MATCH THE COLUMN TYPE QUESTIONS, 24., , 25., Column I, , Column II, , A, , Copper pyrites, , (a), , Fluoride ore, , B, , Cryolite, , (b) Sulphate ore, , C, , Rock salt, , (c), , D, , Alumina, , (d) Sulphide ore, , E, , Dolomite, , (e), , Chloride ore, , F, , Gypsum, , (f), , Carbonate ore, , Oxide ore, , 1. Electrolytic reduction of alumina to aluminium, by Hall – Heroult process is carried out:, (a) In the presence of NaCl, (b) In the presence of fluorite, (c) In the presence of cryolite which forms a, melt with lower melting temperature, (d) In the presence of cryolite which forms a, melt with higher melting temperature, [IIT-2000]
Page 122 :
4.13, , 2. The chemical composition of ‘slag’ formed during, the smelting process in the extraction of copper is, (a) Cu2O + FeS, , (b) FeSiO3, , (c) CuFeS2, , (d) Cu2S + FeO, , [IIT-2001], , 3. Which of the following process is used in, extractive metallurgy of magnesium?, (a) Fused salt electrolysis, (b) Self - reduction, (c) Aqueous solution electrolysis, (d) Thermite reduction, [IIT-2002], 4. In the process of extraction of gold,, , O, Roasted gold ore + CN + H2O, , -, , [X] + HO, , [X] + Zn, , [IIT-2007], 9. Native silver metal forms a water soluble complex, with a dilute aqueous solution of NaCN in the, presence of, (a) Nitrogen, , [Y] + Au, , [IIT-2008], 10. Match the conversions in Column I with the, type(s) of reaction(s) given in Column II., , Identify the complexes [X] and [Y], , Column I, , -, , 2-, , (a) X = [Au(CN)2] , Y = [Zn(CN)4], 3-, , 2-, , (b) X = [Au(CN)4] , Y = [Zn(CN)4], -, , 4-, , -, , 2-, , (c) X = [Au(CN)2] , Y = [Zn(CN)6], , (d) X = [Au(CN)4] , Y = [Zn(CN)4], , [IIT-2003], , 5. The methods chiefly used for the extraction of, lead and tin from their ores are respectively:, (a) Self-reduction and carbon reduction, (b) Self-reduction and electrolytic reduction, (c) Carbon reduction and self-reduction, (d) Cyanide process and carbon reduction, [IIT-2004], 6. Which ore contains both iron and copper:, (a) Cuprite, , (b) Chalcocite, , (c) Chalcopyrite, , (d) Malachite, [IIT-2005], , 7. Match entry in Column (I) is in some way related, to the entries in columns (II), Column I, , (b) Oxygen, , (c) Carbon dioxide (d) Argon, , 2, , -, , 8. Extraction of zinc from Zinc blende is achieved, by:, (a) Electrolytic reduction, (b) Roasting followed by reduction with carbon, (c) Roasting followed by reduction with another, metal, (d) Roasting followed by self-reduction, , Column II, , A Self - reduction, , (a), , Lead, , B Carbon reduction, , (b), , Silver, , C Complex formation and, displacement by metal, , (c), , Copper, , D Decomposition of iodide, , (d), , Boron, [IIT-2006], , Column II, , A, , PbS " PbO, , (a) Roasting, , B, , CaCO3 " CaO (b) Calcination, , C, , ZnS " Zn, , (c) Carbon reduction, , D, , Cu2S " Cu, , (d) Self-reduction, , Passage (Q. 11 to 13), Copper is the most noble of the first row transition, metals and occurs in small deposits in several, countries. Ores of copper include chalcanthite, (CuSO4.5H2O),, atacamite, (Cu2Cl(OH)3),, cuprite (Cu2O), copper glance (Cu2S) and, malachite (Cu2(OH)2CO3). However, 80% of, the world copper production comes from the ore, chalcopyrite (CuFeS2). The extraction of copper, from chalcopyrite involves partial roasting,, removal of iron and self–reduction. [IIT-2010], 11. Partial roasting of chalcopyrite produces:, (a) Cu2S and FeO, (b) Cu2O and FeO, (c) CuS and Fe2O3, (d) Cu2O and Fe2O3, 12. Iron is removed from chalcopyrite as:, (a) FeO, (b) FeS, (c) Fe2O3, (d) FeSiO3, 13. In self-reduction, the reducing species is:, (a) S, (b) O2 (c) S2(d) SO2
Page 123 :
4.14, , 14. Oxidation states of the metal in the minerals, haematite and magnetite, respectively, are:, (a) II, III in haematite and III in magnetite, (b) II, III in haematite and II in magnetite, (c) II in haematite and II, III magnetite, (d) III in haematite and II, III in magnetite, [IIT-2011], 15. In the cyanide extraction process of silver from, argentite ore, the oxidizing and reducing agents, used are, (a) O2 and CO respectively, (b) O2 and Zn dust respectively, (c) HNO3 and Zn dust respectively, (d) HNO3 and CO respectively, [IIT-2012], 16. Sulphide ores are common for the metals, (a) Ag, Cu and Pb, (b) Ag, Cu and Sn, (c) Ag, Mg and Pb, (d) Al, Cu and Pb, , 20 Calcination is the process in which –, (a) Ore is heated in absence of air, (b) Used for sulphides ores, (c) Ore is heated in presence of air, (d) None of these, [JEE Main Online – 2013], 21. In Goldschmidt alumino thermite process which, of the following reducing agents is used –, (a) Calcium, , (b) Coke, , (c) Al - powder, , (d) Sodium, [JEE Main Online – 2013], , 22. The metal that cannot be obtained by electrolysis, of an aqueous solution of its salt is:, (a) Ag, , (b) Ca, , (c) Cu, , (d) Cr, [JEE Main 2014], , 23. The form of iron obtained from blast furnace is:, (a) Steel, , (b) Cast Iron, , (c) Pig iron, , (d) Wrought Iron, , [JEE Advanced 2016], 17. During the process of electrolytic refining of, copper, some metals present as impurity settle as, ‘anode mud’ these are –, (a) Pb and Zn, , (b) Sn and Ag, , (c) Fe and Ni, , (d) Ag and Au, [AIEEE- 2005], , 18. Heating mixture of Cu2O and Cu2S will give, [AIEEE- 2005], , , (a) Cu + SO3, , (b) Cu + SO2, , (c) Cu2SO3, , (d) CuO + CuS, , 19. Which method of purification is represented by, the following equation:, , Ti (s) + 2I2 (g), , , , 523K, , ", , TiI4(g), , 1700K, , ", , Ti (s) + 2I2 (g), , (a) Cupellation, , (b) Poling, , (c) Van Arkel, , (d) Zone refining, [AIEEE- 2012], , [JEE Main Online – 2014], 24. Calamine is an ore of:, (a) Copper, , (b) Aluminium, , (c) Iron, , (d) Zinc, [JEE Main Online – 2015], , 25. In the context of the Hall – Heroult process for, the extraction of Al, which of the following, statements is false?, (a) CO and CO2 are produced in this process, (b) Al2O3 is mixed with CaF2 which lowers, the melting point of the mixture and brings, conductivity, (c) Al3+ is reduced at the cathode to form Al, (d) Na3AlF6 serves as the electrolyte, [JEE Main-2015], 26. Which one of the following ores is best, concentrated by froth floatation method?, (a) Siderite, , (b) Galena, , (c) Malachite, , (d) Magnetite, [JEE Main-2016]
Page 124 :
4.15, , Answer Key, , 1. (a), 11. (a), 21. (b), , 2. (a), 12. (c), 22. (b), , 3. (b), 13. (c), 23. (b), , 4. (d), 14. (c), 24. (a), , 5. (c), 15. (c), 25. (c), , 6. (b), 16. (b), 26. (d), , 7. (b), 17. (c), 27. (a), , 8. (c), 18. (a), 28. (a), , 9. (b), 19. (a), 29. (c), , 10. (d), 20. (b), 30. (c), , 1. (c), 11. (b), 21. (c), , 2. (b), 12. (c), 22. (b), , 3. (b), 13. (a), 23. (b), , 4. (b), 14. (b), 24. (b), , 5. (d), 15. (d), 25. (d), , 6. (b), 16. (a), 26. (a), , 7. (c), 17. (d), 27. (b), , 8. (c), 18. (d), 28. (d), , 9. (d), 19. (b), 29. (a), , 10. (a), 20. (c), 30. (b), , 1. (b,c), 2. (a,b,d), 9. (a,b,c,d) 10. (b,c,d), ", ", , 1. (c), 8. (b), 14. (d), 24. (d), , ", ", , 3. (a,b,c), 11. (a,b), ", ", , ", ", , 4. (a,b, c,d), 12. (a,c,d), ", ", , 5. (b,d), 13. (a,b), , 6. (a,b,c,d), 14. (a), , 7. (a,b,c), 15. (c), , 8. (a,b,c,d), , ", ", , 2. (b), 3. (a), 4. (a), 5. (a), 6. (c) 7. A " (a,c) ; B " (a,c) ; C " (b) ; D " (d), 9.(b) 10. A " (a) ; B " (b) ; C " (a,c) ; D " (a,c,d) 11. (a) 12. (d), 13. (c), 15. (b), 16. (a), 17. (d), 18. (b), 19. (c), 20. (a), 21. (c), 22. (b), 23. (c), 25. (d), 26. (b), , Hints and Solutions, 8., 1., , (a) Three most occurring elements into the earth, crust are O, Si, Al, 2., (a) Bauxite (Al2O3.2H2O) is an oxide ore., 3., (b) Zinc blende, ZnS, chalcopyrites, CuFeS2, Galena, PbS, , 4., (d) Gypsum is CaSO4.2H2O, 5., (c) Epsomite is MgSO4.7H2O, 6., (b) Dolomite is CaCO3.MgCO3, 7., (b) Magnesite is MgCO3, , 9., 10., 11., 12., 13., 14., , (c) Less reactive metals like Au, Pt exist as native, ore., (b) Chromite ore is FeCr2O4, (d) Sulphide ore is concentrated wetting by oil., (a) Oxide ore (Haematite, Fe2O3) is concentrated, by gravity separation method., (c) Cu2S + 2Cu2O $ 6Cu + SO2 (c) Highly electropositive elements are recovered, from electrolytic process., (c) Refining of silver can be done by cupellation, and electrorefining.
Page 125 :
4.16, , 15., , (c) Impure aluminium is purified by Hoop’s, process., (b) In the alumino-thermite process, Al acts as, reducing agent., (c) Slag is formed by reaction between impurities, and flux., (a) All ores are minerals but all minerals are not, ores., (a) Purification of Si is done by zone refining., (b) Concetration method used for bauxite ore is, leaching (chemical method)., (b) In blast furnane, maximum temperature is in, combustion zone where combustion of coke, (C) takes place., (b) Silica (SiO2) is an acidic impurity hence, a, basic flux is needed., (b) In cupellation method, Pb and Zn impurity, present in silver are removed as their oxides., (a) Cassiterite (SnO2) is an oxide ore. It, is concentrated by gravity sepatation, (Levigation)., (c) Froth floatation method is used for sulphide, ore (cinnabar, HgS)., , 4., (b) Bauxite (Al2O3 . 2H2O), Siderite (FeCO3), Argentite (Ag2S), , 26., , (d) Fluorspar is CaF2., , 27., , (a) Gravity separation is used for oxide ore, (Haematite, Fe2O3)., , 28., , (a) Electrolytic reduction method is used in the, extraction of high electropositive elements like, , DG° = – 460 + 360 = –100 kJ/mol., For a feasible reaction, DG° must be negative., It means, ZnO can be reduced by C into Zn., 12., (c) DG° Vs T plot in the Ellingham’s diagram, slopes downward for the reaction,, 1, C + O2 $ CO, 2, 13., (a) Purification of Al metal is done by Hoop’s, process. (Baeyer’s process is used for, purification of bauxite ore)., 14., (b) To remove acidic impurities, basic flux is used., 15., (d) Because Na is highly reactive., 16., (a) Silica impurity present in bauxite is removed, by sepeck’s process (by addition of coke and, N2), , 16., 17., 18., 19., 20., 21., , 22., 23., 24., , 25., , , 29., , Na, Ca, Mg, K, Al etc., , (c) Bauxite is leached with NaOH, , Al2O3.2H2O + NaOH $ NaAlO2 + H2O, 30., , (c) Cyanide process is used for Ag and Au., , 1., , (c) Leaching is a chemical process in which a, suitable reagent is used which forms a soluble, complex only with desired metal leaving, behind impurities., , (b) FeO + SiO2 $ FeSiO3, Slag, , , 5., , (d) MgCO3 $ MgO + CO2, Calcination is heating of ore in absence or, , limited supply of air., 6., (b) Cupellation is refining process for Ag., 7., (c) This method can not be used for highly, electropositive matals., 8., (c) Highly electropositive metals are extrached by, electrolysis of their fused salt., 9., (d) Ore particles are hydrophobic and they are, wetted by oil., 10., (a) The slag is lighter and has lower melting point, than the metal hence it floats over the molten, metal., 1, 11., (b) (i) Zn(s) +, O (g) $ ZnO(g) ;, 2 2, DG° = – 360 kJ/mol., 1, , (ii) C(s) +, O (g) $ CO(g) ;, 2 2, DG° = – 460 kJ/mol., , (ii–i) ZnO(g) + C(s) $ CO(g) + Zn(s) ;, , 17., , (d) PbS $ PbO + PbSO4 (Roasting), PbS + PbO → Pb + SO2 , Self Re duction, PbS + PbSO 4 → Pb + SO2 , , 18., 19., , (d) Electrorefining process is not used for Al., (b) Poling process is used if oxide of metal is, present as impurity in metal., (c) Al2O3.2H2O+3C+N2 ∆, → 2AlN+3CO+2H2O, , 2., 3., , (b) In zone refining method, the molten zone, consists more impurity than the original metal, becuase impurities are moving in the direction, of movement of furnace., , 20., , , , AlN + 3H2O $ Al(OH)3. + NH3-
Page 126 :
4.17, , 21., , (c) FeO + SiO2 $ FeSiO3, (slag), , 22., (b) A reaction is feasible only when its G° is, negative., , (i): 2Zn(s) + O2(g) $ 2ZnO(g) ;, DG° = –360 kJ/mol, , (ii): 2C(gr) + O2(g) $ 2CO(g) ;, DG° = –500 kJ/mol, , (ii–i):2ZnO(s) + 2C(gr.) $ 2CO(g) + 2Zn(s) ;, DG° = –140 kJ/mol, Hence, ZnO can be reduced by graphite., 23., (b) Impurity ZnS forms a soluble complex, Na2[Zn(CN)4] with depressant NaCN., 24., (b) In extraction of iron, coke (C) and limestone, (CaCO3) are added with haematite ore (Fe2O3)., 25., (d) Fe2O3 + 2Al $ Al2O3 + 2Fe, It is an example of goldschmidt aluminothermite process., 26., (a) In Bassemer converter copper is extracted by,, Cu2S + 2Cu2O $ 6Cu + SO2, 27., (b) ZnS (Zinc blende) $ Zn, ZnS $ ZnO (Roasting), ZnO $ Zn (C-Reduction process), 28., (d) Mond’s process is used for nickel (Ni), 29., (a) Poling process is used for the removal of Cu2O, from Cu., 30., (b) Argentite (Ag2S), Cuprite (Cu2O), , 7. (a,b,c) Slag in the extraction of Fe is CaSiO3 while, slag in the extraction of Cu is FeSiO3., 8. (a,b,c,d), Calamine (ZnCO3), Proustite (Ag3AsS3), Cassiterite (SnO2), Diaspore (Al2O3.H2O), 9. (a,b,c,d) Froth floatation, is a physical method, mainly, used for sulphide ores, depends on the, difference in wetability of gangue and the ore, particles., 10. (b,c,d) Al is more electropositive than Fe hence, Fe, can not be used as reducing agent for Al2O3., 11. (a, b) Calcination is the heating of ore in absence or, limited supply of air., 12. (a,c,d) Lead impurity from silver can be removed by, cupellation., 13. (a, b) Metal present lower in the Ellingham diagram, can reduce metal oxide present higher in this, diagram., 14. (a) , In cyanide process, Au forms soluble complex, with CN– in the presence of O2., 15. (c), This process represents hydrometallurgical, extraction., 16. (a) , Copper pyrites is CuFeS2, 17. (b), 18. (b) , 19. (a) , , 1. (b,c), , Highly electropositive metals like Na, Al etc, are extracted by electrolysis of their fused salt., , 2. (a,b,d) Cynide process is used for metallurgy of Ag, and Au., 3. (a,b,c) Concentration by froth floatation followed by, Roasting. Reduction by Bessemerisation, 4. (a,b,c,d) Slag (CaSiO3) is lighter than molten Fe, 5. (b,d), , Liquation is the process used for metal having, impurities greater melting point than metal., 6. (a,b,c,d) Aim of roasting :, (a) to convert ore into oxide of metal., (b) to remove moisture., (c) to remove volatile organic impurities., (d) to oxidize free sulphur and antimony., , FeO + SiO2 ", , (impurity), , 20. , 21. , , (flux), , FeSiO3, (slag), , Cryolite (Na3AlF6) is added to lower the, melting point of Al2O3., Al has higher SRP (standard reduction, potential) than Na and Ca., Li, Ba, Na, Al, Ca, Mg, Cupellation, Poling process, Hoop’s process,, Kroll’s process., , 22., Bauxite , (Al2O3. 2H2O), Magnetite , (Fe3O4), Quartz , (SiO2), Corundum , (Al2O3), Pyrolusite , (SnO2), Zincite , (ZnO), 23. Poling is applied when metallic oxide is, present as impurity., The atomic number of ‘O’ is 8., 24., (A " e ; B " b ; C " c ; D " a ; E " d ; F " f), 25., (A " d ; B " a ; C " e ; D " c ; E " f ; F " b)
Page 128 :
Chapter, , Key Concepts, , Hydrogen is the first element of the periodic table. Its, electronic configuration is 1s1 and it behaves like an alkali, metal as well as a halogen. There are three isotopes of, hydrogen namely, hydrogen (1H1), deuterium (1H2 or D), and tritium (1H3 or T)., Based on the spinning of two nuclei in dihydrogen, two types, of dihydrogen may be distinguished. Ortho-dihydrogen, involves parallel spinning while para-dihydrogen involves, antiparallel spinning of the two nuclei., Dihydrogen is relatively inactive (because of high enthalpy, 435.kJ Mol-1) at ordinary temperature but quite reactive at, high temperature or in the presence of catalysts., , These are classified as follows:, Ionic Hydrides: When elements of Groups 1 and 2, (except Be and Mg) and lanthanides, (electronegativity, in the range 0.9 to 1.2) from compounds with hydrogen,, they are called ionic hydrides. These hydrides are, crystalline solids with higher melting points. The, stability of hydrides decreases with increase in atomic, number of the element in a group. Examples are NaH,, CaH2 etc., , Covalent Hydrides: When element of p-block from, compound with hydrogen, they are called covalent, hydrides. Examples are NH3, H2O etc. These are, generally gaseous compound. Their stability with, increase in atomic number of elements within group, decreases., Interstitial Hydrides: These are formed by some of the, transition metals with electronegativity ranging from, 1.2 to 1.4. Mostly these are non-stoichiometric solids, and may be considered as interstitial compounds., Varying temperature and pressure may vary the, proportion of hydrogen in the compound. Examples, are TiH1.73, ZrH1.92 , VH0.6 etc., Polymeric Hydrides: Some elements with, electronegativity in the range 1.4 to 2.0 form polymeric, hydrides. These are solids containing molecules, linked by hydrogen-bridged bonds. Examples include, (BeH2)n, (MgH2)n and (AlH3)n, Hydrogen finds many uses. For example, for the, preparation of NH3, CH3OH, synthetic petrol,, acetylene, vegetable ghee, H2 – F2 flame, an oxyhydrogen flame etc., , Water (H2O) is the most abundant liquid i.e., 75% of, the earth’s surface is full oceans, lakes and rivers. It has, bent angular structure with H-O-H bond angle of 104.5°., Because of hydrogen bondings, water unusually has high
Page 129 :
5.2, , melting and boiling points. Because of these bonds, ice is, less dense than liquid water. When ice (density = 0.917, g cm-3) is heated, its density increases to 1 g cm-3 at 4°C, followed by decrease in density as the temperature is, further increased., Water is termed as soft water if it is free from calcium or, magnesium salts. If these salts are present, it is termed as, hard water. The latter is not useful for washing purposes as, soap forms insoluble scum of calcium or magnesium state, in hard water., Temporary hardness in water is due to dissolved, bicarbonates of calcium or magnesium. It can removed, by boiling water when carbonates are precipitated out., Permanent hardness is due to soluble chloride or sulphate, of calcium or magnesium. This hardness can be removed, by adding sodium hydroxide, carbonate or sodium, phosphate. Ion exchange resins are also used to soften the, water., , The two O-H groups in hydrogen peroxide (H2O2) do not, lie in the same plane. The angle between two planes is, 111.5° and it reduces to 90.2° in the crystalline phase., The O-O-H bond on the other hand changes from 94.8°, to 101.9°., Hydrogen peroxide is a strong oxidizing agent. It oxidizes, ferrous to ferric, iodide to iodine, lead sulphide to lead, sulphate, potassium ferrocyanide to potassium ferricyanide, (in acidic medium) and manganese (II) to manganese (IV)., Hydrogen peroxide also acts as a reducing agent. It, reduces permanganate to manganese (II), iron (III) to, iron (II), ferricyanide to ferrocyanide (in alkaline, medium), periodate to iodate, ozone to oxygen and silver, to metallic.
Page 133 :
5.6, , S-Block Elements, Element of group 1 (or I A) and 2(or II A) are known as s-block elements., General electronic configuration are, Group 1 [Inert gas]ns1 Alkali metals, Group 2 [Inert gas]ns2 Alkaline Earth metals, Property, , Alkali metal, (a) All are silvery white., , Physical, state, , Alkaline earth metal, (a) All are grayish white., , (b) Light soft, malleable and ductile metals (b) Relatively harder., with metallic luster., (c) Both are diamagnetic and colourless., (a) Both produce characteristic colours in (a) Be and Mg do not show any colour as their elecBunsen flame due to easy excitation of, trons are more strongly bound., electron to higher energy levels., , Flame, colour, , (b) Characteristic flame colours are, Li – Crimson, Na – Golden Yellow,, K – Pale violet, Rb and Cs – Violet, , (b) Ca – Brick red, Sr - Crimson, Ba – apple green, Ra - Crimson, , (c) Energy released, Li+ < Na+ < K+ < Rb+ < Cs+, , (c) Be and Mg atoms due to their small size, bind their, electrons more strongly because of higher effective, nuclear charge. Hence these posses high excitation, energy and are not excited by the flame energy and, do not show any colour., , (d) The flame energy cause an excitation of, the outermost electron which on reverting, back to its initial position gives out the, absorbed energy as visible light., , lonisation, energy, , (a) Due to unpaired lone electrons in ns sub (a) Due to smaller size, electrons are tightly held as, shell as well as due to their larger size,, compared to alkali metal., the outermost electron is far from the, nucleus, the removal of electron is easier, and these have low values of ionization, potential., (b) IP of these metals decreases from Li to (b) The IP value decreases with increase of atomic, Cs, radii from Be to Ba., (a) Hydration represents for the dissolution, of a substance in water to absorb water, molecule by weak valency forces., Hydration of ions in the process when, ions on dissolution in water get hydrated., , Hydration of ions, , (b) Smaller the cation greater is the degree of (b) Hydration energyhydration. Hydration energyBe+2 > Mg+2 > Ca+2 > Sr+2 > Ba+2, Li+ > Na+ > K+ > Rb+ > Cs+, (c) Li+ being smallest in size has maximum, Degree of hydration and that is why, lithium Salts are mostly hydrated and, moves very slowly under the influence of, electric field.
Page 134 :
5.7, , Property, Oxidation, , Electro negativity, , Standard, oxidation, potentials, are reducing, properties, , Alkali metal, , Alkaline earth metal, , These metals easily form univalent +ve ion, The IP1 of these metals are much lower than IP2 and, 1, by losing solitary ns electron due to low IP thus it appears that these metals should form univalent, ion rather than bivalent ions but in actual practice, all, value., these give bivalent ions., (a) These metals are highly electropositive (a) Their electro negativities are also small but are, thereby posses low values of electrohigher than of alkali metals., negativities, (b) Electro-negativity of alkali, decreases down the group, Li > Na > K > Rb > Cs, , metals (b) Electro-negativity decrease form Be to Ba., , (a) Since alkali metals easily loose ns1 (a) They lose two electrons to give M+2 ion., electron they have high value of oxidation, potential, i.e., M, M+(aq) + e(b) Standard oxidation potential are listed, below, Li Na K Rb Cs, 3.05 2.71 2.93 2.99 2.99, , (b) Standard oxidation potential are, Be Mg Ca Sr, 1.69 2.35 2.87 2.90, , (c) Li have greatest reducing nature due to, maximum hydration energy of Li+ ion., (a) On exposure to moist air, all alkali metals (a) Except beryllium these metals are easily tarnish in, except lithium tarnish quickly., air as a layer of oxide is formed on their surface., Action, with, air, , (b) They generally form oxides, peroxides., O2 O2, M+O2, M2O, M2O2, MO2, , and (b) They give oxides of ionic nature M+2O-2 which are, crystalline in nature., , (a) Alkali metals decompose water with the (a) Ca, Sr, Ba and Ra decompose cold water readily, evolution of hydrogen, with evolution of hydrogen., 2M + 2H2O " 2MOH + H2, 2M + 2H2O " 2M(OH)2 + H2, Action, with, water, , (b) Li decompose water slowly, sodium (b) Magnesium decomposes boiling water beryllium, reacts water quickly. K, Rb and Cs react, is not attacked even at high temperatures., with water vigorously., (c) Alkali metals react with alcohols forming, alkoxides with the evolution of hydrogen:, 2Li + 2C2H5OH $ 2C2H5OLi + H2, Ethy alcohol, Lithium ethoxide, (a) These metals combine with H2 to give (a) Except Be, all alkaline earth on heating directly, white crystalline ionic hydrides of the, with H2., general formula MH., , Hydride, , (b) The metal hydrides react with water to (b) BeH2 is prepared by the action of LiAIH4 on, give MOH and H2:, BeCl2:, MH + H2O $ MOH + H2, BeCl2 + LiAlH4 $ 2BeH2 + LiCl + AlCl3, (c) The ionic hydrides of Ca, Sr, Ba liberate H2 at, anode and metal at cathode.
Page 135 :
5.8, , Property, , Alkali metal, , Alkaline earth metal, , (a) The, carbonates, (M2CO3), and (a) All these metal carbonates MCO3 are insoluble in, bicarbonates (MHCO3) are highly stable, neutral medium but soluble in acids and decompose, to heat, where M stands for alkali metals, on heating., (b) The stability of these salts increases with (b) The stability of carbonates, the increasing electropositive character, electropositive character metal., Carbonates, from Li To Cs. Therefore Li2CO3, And bicarbonates, decompose on heating., , increase, , in, , (c) Bicarbonates are decomposed, relatively high temperature., , at (c) Bicarbonates of alkaline earth metals do not exit, in solid state but are known in solution only on, heating their solution bicarbonate decomposed to, , 300°C, liberate:, 2MHCO3 $ M2CO3 + H2O + CO2, M(HCO3)2 $ MCO3 + CO2 + H2O, (a) Alkali metals combine directly with (a) The alkaline earth metals directly combine with, halogens on heating to give metal halides MX2., halogens to from ionic halide MX., (b) The ease with which the alkali metals (b) The ionic character of halides increases from Be to, form halides increases form Li to Cs due, Ra., to increase in electropositive character., (c) LiX has more covalent character., , Halides, , (c) Beryllium halides have covalent Character due to, size and high effective nuclear charge and thus do, not conduct electricity in molten state., , (d) Halides having ionic nature high melting (d) The solubility of halides in water decreases down, point and are good conductor of current, the group. Except fluorides, all are fairly soluble in, in fused state. These are readily soluble in, water., water., (e) Halides of potassium, rubidium and (e) The decrease is solubility of halide down the group, ceasium have property of combining, is due to decreas in hydration energy because of, with extra halogen atoms forming, increasing size of metal cation. Solubility OH, polyhalides:, increase down the group, KI + I2 – KI3, (f) The halides are hygroscopic and readily form, hydrates, CaCl2.6H2O, BaCl2. 2H2O, Otherwise down the group lattice and hydration, energy incomplete, (a) All these form sulphates of type M2SO4. (a) MSO4, , Sulphates, , (b) Except Li2SO4 rest are soluble in water. (b) The solubility of sulphates decreases on moving, down the group. BeSO4 is soluble in water while, Down the group stability and solubility, BaSO4 is completely insoluble, increases., (a) Nitrates of both are soluble in water and, decompose on heating., , Nitrates, , (b) LiNO3 decompose to give NO2 and O2 (b) On heating they decompose into their corresponding, rest all give nitrites and oxygen., oxides with evolution of a mixture of nitrogen, 2MNO3 2MNO2 + O2 (except Li), dioxide and oxygen, 1, 4LiNO3 2Li2O + 4NO2 + O2, M(NO3)2 MO + 2NO2 + O2, , 2, , Forms deep blue solution with liquid Ammonia Except Be and Mg, all others form a deep blue-black, Solution of Liquid, which is conducting and paramagnetic in solution with liquid ammonia., NH3, nature.
Page 136 :
5.9, , Solved Examples, 1. Incorrect statement for H2O2 is:, (a) Decomposition of H2O2 is a disproportionation reaction., (b) Aqueous solution of H2O2 is weakly acidic., (c) Bleaching action of H2O2 is due to its, reducing nature., (d) H2O2 is used in refreshing old lead paintings, PbS (black) converts into PbSO4 (white) in, presence of H2O2., , Sol. (b) Mg and Cu do not produce hydrogen on treatment, with caustic soda (NaOH), , Sol. (c) 2H2O2 " 2H2O + O2 (disproportionation), , (b) PbS + H2O2 ", , H2O2 is very good oxidizing and poor reducing, agent. Its bleaching action is due to its oxidising, nature., PbS + H2O2 " PbSO4 + H2O, (black), (white), 2. In which property listed below hydrogen does not, resemble alkali metals?, (a) Tendency to form cation, (b) Nature of oxide, (c) Combination with halogens, (d) Reducing character, Sol. (b) H2O is neutral while alkali metal oxides are basic, in nature., 3. Calgon causes the softening of hard water by:, (a) Sequestraction of Ca2+ and Mg2+ ion, (b) Sequestraction of Cl- and SO42- ion, (c) Precipitation the Ca2+ and Mg2+ ions as, phosphates, (d) Precipitation the Ca2+ and Mg2+ ions as, sulphates, Sol. (a) Calgon is sodium hexametaphosphate Na6(PO3)6, or Na2[Na4(PO3)6], 2Ca+2+ Na2[Na4(PO3)6] " Na2[Ca2(PO3)6] + 4Na+, , Soluble complex, It is sequestraction of Ca+2 and Mg+2, 4. Which elements out of the following do not, produce hydrogen on treatment with caustic, soda?, A (Zn); B (Sn); C (Mg); D (Cu); E (Al), (a) A, E (b) C, D (c) D, E (d) B, D, , Zn + 2NaOH " Na2ZnO2 + H2 Sn + 2NaOH + H2O " Na2SnO3 + 2H2 2Al + 2NaOH + 2H2O " 2NaAlO2 + 3H2 5. In which reaction, hydrogen peroxide neither acts, as oxidizing agent nor reducing agent?, (a) Na2CO3 + H2O2 ", (c) Cr2O72- + H+ + H2O2 ", (d) SO32- + H2O2 ", Sol. (a) Na2CO3 + H2O2 " Na2O2 + CO2 + H2O, Here, H2O2 acts as acid., 6. Alkali metals dissolve in liquid ammonia to give a, blue colored solution which is due to the presence, of –, (a) M – atoms, , (b) M + ions, , (c) Solvated anions (d) Solvated electrons, Sol. (d) The blue colored solutions of an alkali metal in, ammonia is explained on the basis of formation, of ammoniated (solvated) metal cations and, ammoniated (solvated) electrons in the metal, ammonia solution in the following way:, M, , M+ + e-, , M+ + xNH3, , [M(NH3)x]+, , e- + yNH3, , [e(NH3)y]-, , M + (x + y)NH3, , [M(NH3)x]+, , , , (Solvated metal cation), , , , , , + [e(NH3)y](solvated electron), , The blue colour of the solution is due to, excitation of free electrons to higher energy, levels. The absorption of photons takes place in, the red region of the spectrum and hence, the, solution appears blue in the transmitted light. As, the concentration of the alkali metal increases,, the metal ion cluster formation takes place and, at very high concentration the solution becomes, colored like that of metallic copper.
Page 137 :
5.10, , 7. Which of the following is an incorrect statement?, (a) Sodium oxide is more basic then magnesium, oxide., (b) Beryllium oxide is amphoteric., (c) The thermal stability of beryllium carbonate, is more than of calcium carbonate., (d) Beryllium is amphoteric., Sol. (c) The thermal stability of calcium carbonate is, more as compared to that of beryllium carbonate., The ionic potential (F) value of Be2+ is more than, that Ca2+. So Be2+ attracts the oxygen of CO32more and on heating beryllium carbonate looses, CO2 more easily., 8. In the Solvay process of manufacture of sodium, carbonate, the raw materials used are:, (a) aqueous NaOH, NH3 and CO2, (b) molten NaOH, NH3 and CO, (c) brine NaCl, NH3 and CO, (d) brine NaCl, NH3 and CO2, Sol. (d) The chemical reactions involved in Solvay, process are as below:, NH3 + CO2 + H2O, NH4HCO3 + NaCl, , , 2NaHCO3, , 250°C, , 30°C, , Ca(HCO3)2, [Z], , Ca(HCO3)2 ∆, → CaCO3 + H2O + CO2, , [Y], [X], 11. Number of crystal water in Gypsum, Plaster of, Paris and Epsom salt respectively are:, (a) 2, 0.5, 7, , (b) 7, 2,1, , (c) 7, 0.5, 2, , (d) 3, 4, 2, , Sol. (a) The formulae of Gypsum, Plaster of Paris and, Epsom salt are CaSO4 .2H2O, CaSO4.0.5H2O and MgSO4. 7H2O, 12. Nitrolim (a nitrogenous fertilizer) is a mixture of:, (a) Calcium carbide and calcium cyanamide, (b) Calcium oxide and calcium carbide, (c) Calcium cyanamide and carbon, (d) Calcium oxide and carbon, 1000°c, , NaHCO3. + NH4Cl, , , Cal. Cynamide, , , Nitrolim, , CaCl2 + 2H2O + 2NH3, (used again), , 9. The ion of which of the following metals has least, ionic conductivity in the aqueous solution?, (a) Lithium, (b) Sodium, (c) Potassium, (d) Rubidium, Sol. (a) Li+ forms [Li(H2O)4]+ in water because of its, smallest size and highest charge to size ratio. The, size of this hydrated ion is biggest and thus ionic, conductivity is least., 10. The compound X on heating gives a colorless, gas. The residue is dissolved in water to obtain, Y. Excess of CO2 is bubbled through aqueous, solutions of Y and Z is formed. Z on gentle, heating gives back X. The compound X is:, (a) CaCO3, (b) Na2CO3, (c) CaSO4. 2H2O (d) K2CO3, Sol. (a) CaCO3 ∆, → CaO + CO2, [X], , Ca(OH)2 + CO2, [Y], (excess), , Sol. (c) CaC2 + N2, , Na2CO3 + H2O + CO2, , Slaked lime, , Ca(OH)2, [Y], , NH4 HCO3, , (used again), , 2NH4Cl + Ca(OH)2, , CaO + H2O, , , CaCN2 + C, , 13. On exposure to air, sodium hydroxide becomes, liquid and after sometimes it changes to whites, powder. Why?, Sol. Sodium hydroxide continuously absorbs carbon, dioxide of atmosphere and is converted into, sodium carbonate. A stage reaches when the, solution becomes saturated and the crystals are, formed. These crystals, with the crystallization, (efflorescence) and crumble to white powder., 14. An aqueous solution of iodine becomes colourless, on adding excess of sodium hydroxide solution., Why?, Sol. Iodine reacts with NaOH forming colourless, compounds. Thus, the color of iodine disappears, on addition of NaOH., 2NaOH + I2, , NaI + NaIO + H2O, , , , , Colourless products, , 15. The addition of NaOH solution to a solution, of ZnCl2 produces a white precipitate which, dissolves on further addition of NaOH. Why?
Page 139 :
5.12, , 20. Calcium burns in nitrogen to produce a white, powder which dissolves in sufficient water, to produce gas A and alkaline solution. The, solution on exposure to air produces a thin solid, layer of on the surface. Identify the compounds, A and B., Sol. Ca + N2 ∆, → white powder, , , , , , , Ca on heating with N2 produces calcium nitride,, Ca3N2, a white powder. Ca3N2 on reacting with, water produces ammonia gas NH3, i.e. A and, alkaline solution, atmospheric CO2 to give, insoluble CaCO3., ∆, → Ca3N2, 3Ca + N2 , , Ca3N2 + 6H2O, , , , , H2O, Gas (A) + alkaline solution, Air, thin solid layer, (B), , Ca(OH)2 + CO2, (air), , 3Ca(OH)2 + 2NH3, , Calcium, hydroxide, (alkaline solution), , CaCO3 + H2O, (B), , Exercise, 1. Which pair of species can undergo chemical, reaction with each other?, (a) CO and NO, (b) LiH and H2O, (c) CO2 and HCl, (d) CaH2 and SiH4, 2. Which type of element forms ionic hydrides?, (a) Transition elements, (b) Metalloids, (c) Elements with high electronegativity, (d) Elements with high electropositivity, 3. The three isotopes of hydrogen differ from one, another in:, (a) Atomic number, (b) Number of protons, (c) Nuclear charge, (d) Nuclear mass, 4. Electrolysis of which of the following liberates, hydrogen gas at anode?, (a) Aq. H2SO4, (b) Aq. CuSO4, (c) Molten calcium hydride, (d) Aq. barium hydroxide, 5. Which of the following operation would cause, removal of temporary hardness of water?, , (a), (b), (c), , (d), , passing CO2 gas through it, passing SO2 gas through it, adding calculated amount of Ca(OH)2, adding calculated amount of sodium, hypophosphate., , 6. When temporary hard water containing, Mg(HCO3)2 is boiled the percipitate formed is of:, (a) MgCO3, (b) MgO, (c) Mg(OH)2, (d) None of these, 7. In which of the following reactions hydrogen act, as oxidizing agent?, (a) Ca + H2 ", (b) H2 + O2 ", (c) H2 + F2 ", (d) CuO + H2 ", 8. Which forces of attraction are responsible for, liquefication of H2?, (a) Dispersion forces, (b) Hydrogen bonding, (c) Dipole force, (d) All of these, 9. Adsorbed hydrogen by Palladium is known as:, (a) Atomic, (b) Nascent, (c) Occuluded, (d) Heavy
Page 140 :
5.13, , 10. Which of the following is not a peroxide?, (a) Na2O2, , (b) BaO2, , (c) PbO2, , (d) H2O2, , 11. The ortho and para-hydrogens possess:, (a) Same physical properties but different, chemical properties, (b) Different physical properties but same, chemical properties, (c) Same chemical and physical properties, (d) Different physical and chemical properties, 12. Which is correct about the reaction between H2O2, and O3?, (a) It is a case of mutual reduction, (b) O3 will oxidise H2O2 into O2, (c) It is not a redox reaction, (d) H2O2 being a stronger oxidizing agent will, decompose ozone into oxygen, 13. Alkali metal superoxides contain the (O2-) ion., They are:, (a) Paramagnetic, (b) Coloured compounds, (c) Oxidizing agents, (d) All of these, 14. On heating sodium metal in the current of dry, ammonia leads to the formation of which gas?, (a) NaNH2, , (b) NaN3, , (c) NH3, , (d) H2, , 15. Which of the following s-block elements react, with NaOH to give water soluble complex?, (a) Al, (b) Ca, (c) Be, (d) Li, 16. Which of the following element is common in, microcosmic salt and Glauber’s salt?, (a) N, (b) Na, (c) K, (d) Both (a) and (b), 17. Which of the following elements does not form, hydride by direct heating with dihydrogen?, (a) Be, , (b) Mg, , (c) Sr, , (d) Ba, , 18. A metal chloride, when placed on a platinum wire, in Bunsen flame, does not produce any distinctive, colour. The cation of chloride is:, (a) Li+, , (b) Mg2+, , (c) Na+, , (d) Ca2+, , 19. Which of the following properties of IA group, metals increases as the atomic number rises?, (I) Metallic character, (II) Ionic radius, (III) Melting point, (IV) Density, (V) Ionization potential, (a) I, II, III, , (b) I, II, , (c) III, IV, V, , (d) All, , 20. Which of the following statements is not true, about the dilute solutions of alkali metals in liquid, ammonia?, (a) They are deep blue coloured solutions, (b) They are highly conducting in nature, (c) They are diamagnetic in nature, (d) Ammoniated cation and ammoniated anion, are formed in the solution., 21. Which of the following equations is not involved, in the Solvay process?, (a) CaCO3 " CaO + CO2, (b) NaCl + NH3 + H2O + CO2 " NH4Cl +, NaHCO3, (c) CaO + 2NH4Cl " 2NH3 + H2O + CaCl2, (d) Na2CO3 + CO2 + H2O " 2NaHCO3, 22. Which of the following property of alkaline earth, metals increases with increasing atomic number?, (a) Ionization potential, (b) Solubility of hydroxides, (c) Solubility of sulphates, (d) Density, 23. Among the carbonates of alkali metals which one, has highest thermal stability?, (a) Cs2CO3, , (b) Rb2CO3, , (c) K2CO3, , (d) Na2CO3, , 24. A solution of sodium in liquid ammonia is blue in, colour due to:, (a) The presence of ions Na+, (b) The presence of ammoniated electron, (c) The formation of NaNH2, (d) The formation of sodium hydride, 25. The order of basic strength of the hydroxides of, alkali metals is:, (a) Li > Na > Rb > Cs, (b) Na > Li > Rb > Cs
Page 141 :
5.14, , (c) Cs > Rb > Na > Li, (d) Rb > Cs > Na > Li, 26. Which of the following compounds liberate(s), oxygen on heating?, (a) Li2CO3, , (b) LiOH, , (c) LiNO3, , (d) NaOH, , 27. When MgCl2.6H2O is strongly heated, then it, forms:, (a) MgO, , (b) Mg(OH)2, , (c) Mg(OH)Cl, , (d) MgCl2, , 28. Magnesium liberates H2 on reaction with:, (a) dil. HCl, (b) dil. H2SO4, (c) very dil. HNO3, (d) all of these, 29. Calcium hydride on hydrolysis forms:, (a) CaO + H2, (b) Ca(OH)2 only, (c) Ca(OH)2 + H2, (d) Only CaO, 30. Which one on reaction with NaOH solution gives, inflammable gas?, (a) S, , (b) Zn, , (c) NH4Cl, , (d) I2, , 31. Which of the following is the most important, factor in making lithium metal, the strongest, reducing agent?, (a) Ionization energy, (b) Hydration energy, (c) Heat of sublimation, (d) None of these, 32. Compound having highest melting point:, (a) LiCl, (b) CsCl, (c) NaCl, (d) KCl, 33. The solubility of metal halides depends on their, nature, lattice enthalpy and hydration enthalpy of, the individual ions. Amongst fluorides of alkali, metals, the lowest solubility of LiF in water is due, to:, (a) Ionic nature of lithium fluoride, (b) High lattice enthalpy of lithium and fluoride, ion, (c) High hydration enthalpy of lithium ion, (d) Low ionisation enthalpy of lithium atom, , 34. Which of the following compound is consumed, during the preparation of Na2CO3 by Solvay’s, Process?, (a) NH3 + CaCO3 + NaCl, (b) NH4Cl + CaO + NaCl, (c) CaCO3 + NaCl, (d) NaCl + NH4HCO3, 35. Select the correct statement:, (a) Be and Al show diagonal relationship, (b) Be forms tetrahedral complexes [Be(C2O4)2]2 (c) Al forms AlF6-3, an octahedral complex, (d) All are correct statements, , 1. When a mixture of ammonium sulphate and 50%, H2SO4 is electrolysed the products formed at, anode and cathode are:, (a) H2 and H2O2, (b) (NH4)2S2O8 and H2, (c) H2 and NH4HSO4, (d) H2O2 and H2, 2. When H2O2 is added to ice cold solution of, acidified potassium dichromate containing ether., The contents are shaken and allowed to stand then, (a) a blue colour is obtained in ether due to, formation of Cr2(SO4)3, (b) a blue colour is obtained in ether due to, formation of CrO5, (c) CrO3 is formed which dissolves in ether to, give blue colour, (d) Chromyl chloride is formed., 3. Which of the following species is reduced by, H2O2?, (a) [Fe(CN6)]4 (b) [Fe(CN6)]3- in alkaline medium, (c) NO2 (d) I-/HCl, 4. Which of the following on oxidation gives H2O2?, (a) 2-Ethylanthraquinol, (b) 2-Ethylanthraquinone, (c) Anthracene, (d) 2-Ethylanthracene, 5. One of the following is an incorrect statement., Point out the incorrect one:
Page 142 :
5.15, , (a) Hardness of water depends upon its soap, consuming power, (b) Temporary hardness is due to bicarbonates of, calcium and magnesium, (c) Permanent hardness is due to soluble, sulphates and chlorides of Ca and Mg, (d) Permanent hardness can be removed by, boiling water., 6. Incorrect statement about ortho and para, hydrogen:, (a) Para hydrogen is present in pure state at low, temperature (zero kelvin), (b) The ratio of ortho : para hydrogen at room, temperature is 3:1, (c) Entropy of ortho hydrogen is more than para, hydrogen at high temperature., (d) 100% pure ortho hydrogen may be obtained, at high temperature, 7. Which of the following is an incorrect statement, for heavy water?, (a) It is used as moderator in nuclear reactor, (b) It gives deuteromathane when react with, Al4C3, (c) Ionic compounds are more soluble in D2O, than in H2O, (d) Bond energy of D2O is higher than that of, H2O, 8. In which of the following reaction hydrogen, peroxide is a reducing agent?, (a) 2FeCl2 + 2HCl + H2O2 " 2FeCl3 + 2H2O, (b) Cl2 + H2O2 " 2HCl + O2, (c) HI + H2O2 " 2H2O + I2, (d) H2SO3 + H2O2 " H2SO4 + H2O, 9. Which one of the following removes temporary, hardness of water?, (a) Slaked lime, (b) Plaster of Paris, (c) CaCO3, (d) Hydrolith, 10. Which physical constant for H2O has higher, magnitude than D2O?, (a) Boiling point, (b) Temperature of maximum density, (c) Dielectric constant, (d) Bond dissociation energy, , 11. Identify incorrect statement regarding H2O2:, (a) It can be prepared by acidifying BaO2 and, hydrolyzing H2S2O8 and H2SO5., (b) It is thermodynamically stable., (c) It has non planar structure., (d) It is oxidizing as well as reducing agent., 12. In which of the following method of the removal, of hardness, Ca+2 and Mg2+ are not separated, from sample of hard water?, (a) By boiling of temporary hard water, (b) Addition of sodium carbonate, (c) Using sodium hexametaphosphate, (d) Synethetic resins and zeolite method., 13. Which of the following statement is not correct, regarding the diagonal relationship between Al, and Be?, (a) BeO and Al2O3 are amphoteric in nature., (b) Al4C3 and Be2C give same gas on hydrolysis., (c) Both can from complexes with same, maximum co-ordination., (d) Both form electron deficient and covalent, hydride., 14. A+H2O " NaOH, B+H2O " NaOH+O2; A and B are respectively:, (a) Na2O2 and Na2O, (b) Na2O and Na2O2, (c) NaO2 and Na2O2, (d) Na2O and NaO2, 15. Which of the following pair of metal form nitride, on reaction with Nitrogen?, (a) Li, Mg, , (b) Mg, Na, , (c) Al, K, , (d) Al, Na, , 16. Which gas responsible for leaving holes in cakes, or pastries and making them light and fluffy?, (a) O2, , (b) CO2, , (c) H2, , (d) CH4, , 17. When sodium is placed in moist air, finally change, into:, (a) NaOH, , (b) Na2O2, , (c) Na2O, , (d) Na2CO3, , 18. Which of the following statement is not correct?, (a) AlCl3 is soluble in excess NaOH and form, soluble complex.
Page 143 :
5.16, , (b) LiHCO3 is not found in solid state., (c) K2O2 is diamagnetic but KO2 is paramagnetic., (d) Hydrated MgCl2 gives anhydrous MgCl2 on, heating in dry air., 19. Which of the following statement is not correct?, (a) BeF2 forms complex ion with NaF in which, Be goes with cation., (b) BeCO3 is kept in the atmosphere of CO2, since it is least thermally stable., -2, , (c) Be dissolves in alkali forming [Be(OH)4] ., (d) BeH2 can exist as planar dimer in vapour, state., 20. CO2 gas along with solid Y is obtained when, sodium salt X is heated, X is again obtained when, CO2 gas is passed into aqueous solution of Y. X, and Y are:, (a) Na2CO3, Na2O, (b) Na2CO3, NaOH, (c) NaHCO3, Na2CO3, (d) Na2CO3,, , , NaHCO3, , 21. Which of the following statement is not correct?, (a) Lithium halide are most covalent among, alkali metal halides., (b) Li2O is more thermal stable than Li2CO3., (c) Except Be halides, all other halides of II A, metals are ionic in nature., (d) Charge and size ratio for Be+2 and Al+3 is, nearly same., 22. NH3 + H2O + CO2 " A;, A + H2O + CO2 " B, B + NaCl " C + NH4Cl;, C " D + H2O + CO2., Which of the following is incorrect statement?, (a) A is (NH4)2CO3, (b) D, , , is Na2CO3, , (c) C is NaHCO3, (d) B, , , is (NH4)2C2O4, , 23. When powered Be is heated with air, it form, A and B. Compound A gives C after reductive, chlorination. C produces white fumes in presence, of moisture and forms D. Then A, B, C and D,, respectively, are:, (a) BeO, Be3N2, BeCl2, Be(OH)2, (b) Be3N2, BeO, BeCl4-2, Be(OH)2, , (c) BeO, Be(OH)2, Be3N2, BeCl2, (d) BeO, Be3N2, Be, Be(OH)2, 24. A solid compound X on heating gives CO2 gas, and a residue. The residue mixed with water, forms Y on passing an excess of CO2 through Y, in water, a clear solution, Z is obtained. On boiling, Z compound X is reformed. The compound X is:, (a) CaCO3, , (b) Na2CO3, , (c) K2CO3, , (d) Ca(HCO3)2, , 25. Select the incorrect choice:, (a) Solubility of alkaline earth metal’s carbonates,, sulphates and chromates decreases from Be, to Ba., (b) Solubility of alkaline earth metal’s hydroxides, is less than alkali metal hydroxides., (c) Solubility of alkaline earth Metal’s oxides, decreases from Be to Ba., (d) SO2 on passing in lime water turns lime water, milky., 26. Which of the following statement is not correct?, (a) All alkali-metal salts impart a characteristic, colour to the Bunsen flame., (b) The correct order of increasing thermal, stability of the carbonates of alkali metals is, Li2CO3 < Na2CO3 < K2CO3 < Rb2CO3 <, Cs2CO3., (c) Among the alkali metal’s cesium is the most, reactive, (d) The reducing character of the alkali metal, hydrides follow the order:, LiH > NaH > KH > RbH > CsH., 27. Identify the product A,B,C,D of reaction sequence, respectively:, X NaCl " A + B + Cl2, . Al, NaAlO2 + B(g), Y, (a), (b), (c), (d), , A+Cl2 " C + D + H2O, NaOH, NaCl, NaClO, H2O, Na2CO3, H2, NaCl, NaClO3, NaOH, H2, NaCl, NaClO3, Na, H2, NaClO3, NaCl, , 28. Which of the following metal, on burning in moist, air, does not give smell of ammonia?, (a) Mg, , (b) Ca, , (c) K, , (d) Li
Page 144 :
5.17, , 29. Mg2C3 reacts with water forming propyne gas., C34- ions has:, (a) Two sigma and two pi bonds, (b) Three sigma and one pi bond, (c) Two sigma and one pi bond, (d) Two sigma and three pi bonds, 30. The fluoride which is most soluble in water is:, (a) CaF2, , (b) BaF2, , (c) SrF2, , (d) BeF2, , 31. Amongst the following hydroxides, the one, which has the highest value of Ksp at ordinary, temperature?, (a) Mg(OH)2, , (b) Ca(OH)2, , (c) Sr(OH)2, , (d) Ba(OH)2, , 32. At high temperature, nitrogen combines with, CaC2 to give:, (a) Calcium cyanide, (b) Calcium cyanamide, (c) Calcium carbonate, (d) Calcium nitride, 33. Which metal bicarbonates does not exist in solid, state?, (i) LiHCO3, , (ii) Ca(HCO3)2, , (iii) Zn(HCO3)2, , (iv) AgHCO3, , (a) i, ii, iii, iv, , (b) i, ii, iii, , (c) i, ii, iv, , (d) ii, iii, iv, , 34. The reaction of sodium highly exothermic with, water. The rate of reaction is lowered by:, (a) Lowering the temperature, (b) Mixing with alcohol, (c) Mixing with acetic acid, (d) Making an amalgam, 35. The alkali metals dissolve in liquid NH3, it is, found that:, (a) The dilute solution are blue but the, colour changes to bronze with increasing, concentration., (b) The blue solutions is due to the presence of, solvated electrons., (c) The blue solutions are paramagnetic but, the bronze coloured solutions are, diamagnetic., (d) All the facts given above are found., , ONE OR MORE THAN ONE CORRECT TYPE, 1. The reagent(s) used for softening the temporary, hardness of water is (are):, (a) Ca3(PO4)2, (b) Ca(OH)2, (c) Na2CO3, (d) NaOCl, 2. The oxidation states of the most electronegative, element in the products of the reaction between, BaO2 with dilute H2SO4 are:, (a) -1, (b) +1, (c) -2, (d) 0, 3. Which of the following reaction(s) is/are correct?, (a) Cl2 + NaOH " NaCl + NaClO3 + H2O, (b) P4 + NaOH + H2O " NaH2PO2 + PH3, , D, (c) S + NaOH, Na2S2O3 + Na2S + H2O, , D, (d) Si + NaOH, Na2SiO3 + H2, 4. Which of the following is/are correct?, (a) Sodium thiosulphate is called hypo., (b) Sodium peroxide is called oxone., (c) Potassium carbonate is called pearl ash., (d) Sodium nitrate is called Indian nitre., 5. Which of the following is/are found in the solid, state?, (a) LiHCO3, , (b) KHCO3, , (c) NaHCO3, , (d) NH4HCO3, , 6. Which of the following compound(s) will impart, a golden yellow colour to the Bunsen flame?, (a) KCl, (c) NaCl, , (b) K2CO3, (d) Na2CO3, , 7. Nitrogen dioxide cannot be obtained by heating:, (a) KNO3, , (b) NaNO3, , (c) AgNO3, , (d) Cu(NO3)2, , 8. Which of the following metals dissolve in liquid, ammonia?, (a) Sr, (b) Ca, (c) Ba, (d) Be, 9. In which of the following, hydration enthalpy is, greater than the lattice enthalpy?, (a) BaSO4, (b) BaCO3, (c) Na2SO4, (d) Na2CO3
Page 145 :
5.18, , PASSAGE BASED QUESTIONS, Passage # 1 (Q. 10 and 11), Hydrogen peroxide is a powerful oxidizing agent,, both in the acidic and alkaline medium., In acidic medium: H2O2 + 2H+ + 2e- " 2H2O, In alkaline medium: H2O2 + 2e- " 2-OH, Hydrogen peroxide acts as a reducing agent, towards powerful oxidizing agents., In acidic medium: H2O2 " 2H+ + O2 + 2e In alkaline medium, however, its reducing nature, is more effective., -, , -, , H2O2 + 2OH " 2H2O + O2 + 2e, , 11. H2O2 behaves as a bleaching agent due to:, (a) Oxidizing nature, (b) Reducing nature, (c) Acidic nature, (d) Unstable nature, , Red hot coke + Steam, , , Steam, , (X), , (Z) + H2., , 500°c catalyst (y), , 12. ‘X’ is:, (a) Water gas, , (b) Producer gas, , (c) Coal gas, , (d) Oil gas, , 13. Catalyst ‘Y’ is:, (a) V2O5, , (b) Cr2O3, , (c) Fe2O3, , (d) Fe2O3 + Cr2O3, , The formation and stability of these metals can be, explained on the basis of size of alkali metal ion and the, anion. Peroxides are colourless, while superoxides are, coloured. The normal oxides are basic while peroxides, and superoxides. Act as oxidizing agents., 15. On heating in excess of oxygen, lithium gives:, (a) Li2O, , (b) LiO, , (c) Li2O2, , (d) LiO2, , 16. On heating excess of oxygen, potassium gives:, , 10. On addition of H2O2 to acidified KMnO4, KMnO4, gets decolourised due to:, (a) Oxidation of KMnO4, (b) Reduction of KMnO4, (c) Both oxidation and reduction, (d) None of the above of KMnO4, , Passage # 2 (Q. 12 to 14), , 1000°c, , formation of oxides, hydroxides and carbonates on their, surface. When heated in air or oxygen they burn vigorously, forming different types of oxides depending upon the, nature of the metal., , 14. ‘Z’ is removed by passing the gaseous mixture, through, (a) acidic solution, (b) alkaline solution, (c) water under high pressure of 25 atm, (d) an organic solvent, Passage # 3 (Q. 15 and 16), On exposure to air, alkali metals get tarnished due to, , (a) K2O, , (b) KO, , (c) K2O2, , (d) KO2, , Passage # 4 (Q. 17 and 18), According to Fajan’s rules, the percentage of covalent, character in an ionic compound increases if the cation is, highly charged or small in size and the anion is large or, cation has pseudo inert gas configuration. As a result of, the increased covalent character, solubility in less polar, solvent increases and the melting point decreases., 17. Which of the following has the lowest melting, point?, (a) KCl, , (b) LiCl, , (c) CsCl, , (d) RbCl, , 18. The correct order of increasing ionic character is:, (a) BeCl2 < MgCl2 < CaCl2 < BaCl2, (b) BeCl2 < MgCl2 < BaCl2 < CaCl2, (c) BeCl2 < BaCl2 < MgCl2 < CaCl2, (d) BaCl2 < CaCl2 < MgCl2 < BeCl2, INTEGER VALUE TYPE QUESTIONS, 19. What is the sum of protons, electrons and neutrons, in the lightest isotope of hydrogen?, 20. How many moles of phosphine are produced, when one mole of the calcium phosphide reacts, with water?, 21. Potassium iodide reacts with acidified K2Cr2O7., How many moles of KI are required for one mole, of K2Cr2O7?, 22. How many water molecules are associated with, washing soda?
Page 146 :
5.19, , MATCH THE COLUMN TYPE QUESTIONS, , statement-1., (c) Statement-1 is True, statement-2 is False, , 23., Column I, , Column II, , (d) Statement-1 is False, statement-2 is True, [IIT-2007], , A, , Calgon, , 1., , More reactive form of hydrogen, as compared to H2, , B, , D2O, , 2., , Open book-type structure, , C, , Nascent hydrogen, , 3., , Sodium polymetaphosphate, , (a) Ca3(PO4)2, , (b) Ca(OH)2, , D, , H2O2, , 4., , Heavy water, , (c) Na2CO3, , (d) NaOCl, , 24., Column I, , Column II, 2+, , A, , Sodium ion in zeolite gets 1., exchanged with, , Ca, , B, , Hardness, , 2., , Mg2+, , C, , Temporary hardness, , 3., , Ca(HCO3)2, , D, , Permanent hardness, , 4., , MgSO4, , 25., Column I, , Column II, , 2. The reagent(s) used for softening the temporary, hardness of water is (are):, [IIT-2010], , 3. Hydrogen peroxide in its reaction with KIO4 and, NH2OH respectively, is acting as a:, (a) Reducing agent, oxidizing agent, (b) Reducing agent, reducing agent, (c) Oxidizing agent, oxidizing agent, (d) Oxidizing agent, reducing agent, [JEE Advanced - 2014], 4. A piece of magnesium ribbon was heated to, redness in an atmosphere of nitrogen and on, cooling water was added, the gas evolved was-, , A, , Gives CO2 on heating, , 1., , Na, , (a) Ammonia, , (b) Hydrogen, , B, , Pink-violet flame colouration, , 2., , Cs, , (c) Nitrogen, , (d) Oxygen, , C, , Forms superoxide on heating, With O2, , 3., , K2CO3, , D, , Used in photoelectric cells, , 4., , NaHCO3, , E, , Form monoxide on heating, with oxygen, , 5., , K, , F, , Forms peroxide on heating, with oxygen, , 6., , Li, , 1. Statement-1: Alkali metals dissolve in liquid, ammonia to give blue solutions., , Statement-2: Alkali metals in liquid ammonia, give solvated species of the type [M(NH3)n]+, (M = alkali metals)., (a) Statement-1: is True, statement-2 is True;, Statement-2 is a correct explanation for, statement-1., (b) Statement-1: is True, statement-2 is True;, Statement-2 is not a correct explanation for, , [AIEEE - 2005], 5. The ionic mobility of alkali metal ions in aqueous, solution is maximum for (a) Rb+, (b) Li+, +, (c) Na, (d) K+, [AIEEE - 2006], 6. In context with the industrial preparation of, hydrogen from water gas (CO+H2), which of the, following is the correct statement?, (a) CO is removed by absorption in aqueous, Cu2Cl2 solution, (b) H2 is removed through occlusion with Pd, (c) CO is oxidized to CO2 with steam in the, presence of a catalyst followed by absorption, of CO2 in alkali, (d) CO and H2 are fractionally separated using, difference in their densities, [AIEEE - 2008], 7. The products obtained on the heating LiNO3 will, be (a) Li2O + NO2 + O2, (b) Li3N + O2, (c) Li2O + NO + O2, (d) LiNO2 + O2, [AIEEE - 2011]
Page 147 :
5.20, , 8. What is the best description of the change that, occurs when Na2O(s) is dissolved in water?, (a) Oxide ion accepts a pair of electrons, (b) Oxide ion donates a pair of electrons, , (d) Reaction of methane with steam, [AIEEE - 2012], 11. The solubility order for alkali metal fluoride in, water is-, , (c) Oxidation number of oxygen increases, , (a) LiF < RbF < KF < NaF, , (d) Oxidation number of sodium decreases, [AIEEE - 2011], , (b) RbF < KF < NaF < LiF, , 9. Which of the following on thermal decomposition, yields a basic as well as an acidic oxide?, , (d) LiF < NaF < KF < RbF, , (a) KClO3, , (b) CaCO3, , (c) NH4NO3, , (d) NaNO3, [AIEEE - 2012], , 10. Pure hydrogen (99.9%) can be made by which of, the following processes?, (a) Mixing natural hydrocarbons of high, molecular weight, (b) Electrolysis of water, (c) Reaction of slat like hydrides with water, , (c) LiF > NaF > KF > RbF, [JEE Main Online - 2013], 12. In which of the following reactions H2O2 acts as a, reducing agent?, (i) H2O2 + 2H+ + 2e-" 2H2O, (ii) H2O2 – 2e- " O2 + 2H+, (iii) H2O2 + 2e-" 2OH (iv) H2O2 + 2OH- - 2e- " O2 + 2H2O, (a) (iii), (iv), (b) (i), (iii), (c) (ii), (iv), (d) (i), (ii), [JEE Main - 2014], , Answer Key, , 1. (b), 11. (b), 21. (d), 31. (b), , 2. (d), 12. (b), 22. (b), 32. (c), , 3. (d), 13. (d), 23. (a), 33. (b), , 4. (c), 14. (d), 24. (b), 34. (c), , 5. (c), 15. (c), 25. (c), 35. (d), , 6. (a), 16. (b), 26. (c), , 7. (a), 17. (a), 27. (a), , 8. (a), 18. (b), 28. (d), , 9. (c), 19. (b), 29. (c), , 10. (c), 20. (c), 30. (b), , 1. (b), 11. (b), 21. (c), 31. (d), , 2. (b), 12. (c), 22. (d), 32. (b), , 3. (b), 13. (c), 23. (a), 33. (a), , 4. (b), 14. (b), 24. (a), 34. (d), , 5. (d), 15. (a), 25. (c), 35. (d), , 6. (d), 16. (b), 26. (d), , 7. (c), 17. (d), 27. (c), , 8. (b), 18. (d), 28. (c), , 9. (a), 19. (a), 29. (a), , 10. (c), 20. (c), 30. (d), , 1. (b, c, d), 2. (a, c), 3. (a, b, c, d), 4. (a, b, c) 5. (b, c, d), 6. (c, d), 9. (c, d), 10. (b), 11. (a), 12. (a), 13. (d), 14. (c), 17. (b), 18. (a), 19. (2), 20. (2), 21. (6) 22. (10), 23. (A, 3; B 4; C 1; D 2), , 24. (A, 1, 2; B 1, 2; C 3; D 4), 25. (A, 4; B 3; C 2, 5; D 2; E 1,2,5, 6; F, 1), , 7. (a, b), 15. (a), , 8. (a, b, c), 16. (d)
Page 148 :
5.21, , 1. (a), 11. (d), , 2. (b, c, d) 3. (a), 12. (c), , 4. (a), , 5. (a), , 6. (c), , 7. (a), , 8. (b), , 9. (b), , 10. (c), , Hints and Solutions, Mg+2 do not give flame test., (b) LiH + H2O, , 19. (b) In alkali metals (IA), metallic character and ionic, radius increases as the atomic number rises., , LiOH + H2, , (d) Highly electropositive elements (s-block metals), can form ionic hydrides., , 20. (c) Solutions of alkali metals in liquid ammonia are, paramagnetic in nature., , (d) Isotopes have different nuclear mass., , 21. (d) Na2CO3 + CO2 + H2O " NaHCO3, (this reaction is not possible), , Ca+2 + 2H-, , (c) CaH2 (Molten), at anode:- 2H-, , The actual reaction is,, , H2 + 2e-, , (c) Temporary hardness of water can be removed by, adding calculated amount of Ca(OH)2., ∆, → MgCO + CO + H O, a) Mg (HCO ) , 3 2, , (a) Ca + H2, , 3, , 2, , 2, , CaH2, , ∆, → Na2CO3 + CO2 + H2O, 2NaHCO3 , , 22. (b) Order of solubility of hydroxides: Be(OH)2 < Mg(OH)2 < Ca(OH)2 < Sr(OH)2 <, Ba(OH)2, , R.A O.A, , 23. (a) Order of thermal stability:-, , 8. (a) Intermolecular interaction present in H2 is, dispersion forces., , Na2CO3 < K2CO3 < Rb2CO3 < Cs2CO3, , 9. (c) Adsorption of H2 by various metals is also known, as occulusion., 10. (c) In PbO2, oxidation state of ‘O’ is ‘-2’., 11. (b) Ortho and para – hydrogen possess different, physical properties but same chemical properties., 12. (b) O3 is a stronger oxidizing agent than H2O2. O3, will oxidize H2O2 into O2, 13. (d) Due to presence of unpaired e- in superoxide ion,, they are paramagnetic, coloured and oxidizing, agents., , 1 H, 14. (d) Na + NH3 , → NaNH2 +, 2, 2, 15. (c) Be + 2NaOH Na2BeO2 + H2, ∆, , , , water soluble, , 16. (b) Microcosmic salt is Na(NH4)HPO4. 4H2O, Glauber’s salt is Na2SO4. 10H2O, 17. (a) Be is less reactive s-block metal. It does not form, hydride by direct heating with H2., 18. (b) Due to high ionisation energy, salts of Be+2 and, , 24. (b) Cause of blue colour is presence of ammoniated, electron., 25. (c) Down the group, basic strength of hydroxides, increases., ∆, → Li2O + 2NO2 + 1 O2, 26. (c) 2LiNO3 , 2, ∆, → MgO + HCl, 27. (a) MgCl2. 6H2O , 28. (d) Mg displaces H2 from acids, 29. (c) CaH2 + 2H2O, 30. (b) Zn + 2NaOH, gas), , Ca(OH)2 + 2H2, Na2ZnO2 + H2 (Inflammable, , 31. (b) Li is the strongest reducing agent because Li+ has, exceptionally high hydration energy., 32. (c) Order of melting point is,, NaCl > KCl > CsCl > LiCl, 33. (b) Both Li+ and F- ions are very small in size. Hence,, lattice energy of LiF is very high and it has lowest, solubility in water amongst alkali metal fluorides., 34. (c) Raw materials used in Solvay process are CaCO3,, NH3 and NaCl but only CaCO3 and NaCl are, consumed during the preparation of Na2CO3.
Page 150 :
5.23, , Ksp µ solubility, , With H2O2, KMnO4 behaves as oxidising agent., , Order of solubility :, , H2O2 behaves as a bleaching agent due to its, oxidizing nature., ∆, Steam, , Mg(OH)2 < Ca(OH)2 < Sr(OH)2 < Ba(OH)2, , , → CO + H, , �2, Red hot coke + steam ∆, → ����, , ∆, → CaCN2, CaC2 + N2 , , , , (Calcium cyanamide), , Only bicarbonates of Na+, K+, Rb+ and Cs+ exist is, solid state., Hg is less reactive metal. Hence, by making an, amalgam the rate of reaction of Na with water is, lowered., 35 (d) The alkali metals dissolve in liquid NH3., This solution is blue coloured due to solvated, electrons. As concentration of metal increases,, colour changes to bronze., , Catalyst, , → CO2 + H 2, , Water gas, (X), , Steam, ∆, → CO + H 2 Catalyst, , → CO2 + H 2, �, ���, �, (Z), Water gas, , , ), Catalyst ‘Y’ is( XFe, O + Cr2O3, 2 3, , CO2 is removed by passing the gaseous mixture, through water under high pressure of 25 atm., CO2 + H2O, , H2CO3, ∆, , Li + O2(excess) , → Li2O, K + O2(excess) ∆, → KO2, Order of melting point:, KCl > RbCl > CsCl > LiCl, Covalent character µ polarising power of cation., , 1.(b, c, d), Ca(OH)2 (slaked lime), Na2CO3 (washing soda), and NaOCl are used for softening the temporary, hardness., , Order of ionic character is reverse of order of, covalent character., Lightest isotope of hydrogen is protium. It has 1, proton, 1 electron and zero neutron., Ca3P2 + 6H2O, , NaOCl + H2O " NaOH + HOCl, OH– + HCO3– "CO32– + H2O, BaO2 + H2SO4 (dilute) "BaSO4 + H2O2, The most electronegative element in products is, , oxygen. In H2O2, oxidation state of ‘O’ is –1 and, in BaSO4, oxidation state of ‘O’ is –2., 3. (a, b, c, d), All reactions are correct., 4. (a, b, c), Indian nitre is potassium nitrate (KNO3), 5. (b, c, d), LiHCO3 exists only in solution., , 3Ca(OH)2 + 2PH3, , 1 mole, , 2 moles, , , , K2Cr2O7 + 7H2SO4 + 6KI " Cr2(SO4)3 + 3I2, + 7H2O + 4K2SO4, 1, mole, 6, moles, , , , Washing soda is Na2CO3.10H2O, (A "3; B "4 ; C "1; D " 2), (A "1, 2; B "1, 2 ; C "3; D "4), (A "4; B "3 ; C "2, 5 ; D "2 ; E "1, 2, 5,, 6; F "1], , 6. (c, d) Na+ will impart a golden yellow colour to the, Bunsen flame., 7. (a, b) Nitrates of Na+, K+, Rb+ Cs+ do not release NO2, gas by heating., 8. (a, b, c), In s-block, Be and Mg do not dissolve in liquid, ammonia., 9.(c, d) For salts, which are soluble in water, hydration, enthalpy is greater than the lattie enthalpy., Na2SO4 and Na2CO3 are soluble in water., , 1. (b) Both statements are correct but blue colour is due, to presence of solvated e–., 2. (b, c, d), Ca(OH)2 + Ca(HCO3)2, , 2CaCO3 + 2H2O, , Na2CO3 + Ca(HCO3)2, , CaCO3 + 2NaHCO3, , NaOCl + H2O, OH– + HCO3–, , NaOH + HOCl, CO3–2 + H2O, , (Z)
Page 152 :
Chapter, , Key Concepts, 5d-series or III transition series: The elements lying in the middle of the periodic table, between group 2 and group 13 are known as d-block, elements., These d-block elements are called transition elements, because they exhibit transitional behaviour between, s-block and p-block elements., Depending upon the subshell (3d, 4d, 5d) involved,, transition elements are mainly classified into three, series., 1. First transition series or 3d series., 2. Second transition series or 4d series., 3. Third transition series or 5d series., Outer electronic configuration of the transition, elements, 3d-series or I transition series:-, , 4d-series or II transition series:-, , 1. Metallic character:- All the transition elements are, metallic in nature and nearly all of them have simple, hcp, ccp or bcc lattices. Due to their greater effective, nuclear charge and the large number of valence, electrons, the metallic bond is quite strong and, hence they are hard, posses high densities and high, enthalpies of atomization., 2. Oxidation states:- Transition elements exhibit variable, oxidation state due to the participation of ns as well as, (n –1) d electrons., Except scadium, the most common oxidation state of, the first row (3d series) elements is +2 which arises, from the loss of two 4s electrons, which means, that after scandium, d-orbital become stable than, s-orbital., In the +2 and +3 oxidation states, bond formed are, generally ionic while in higher oxidation states the, bond formed are essentially convalent. For example in, MnO4–, CrO42–, etc. the bond formed between metal, and oxygen are covalent.
Page 153 :
6.2, , Sc Ti V, Cr Mn Fe, Co Ni Cu, +1 (+1), +2 +2 +2 (+2) (+2) (+2) (+2) (+2), (+3) (+3) (+3) (+3) +3 (+3) (+3) (+3), (+4) +4 +4 +4 +4, +4 +4, , (+5) +5 +5, , (+6) +6 +6, (+7), , Zn, (+2), , Oxidation states of the first row of transition metals (the, most common ones are in circles), 3. Complex formation (complexation):- Transition, metal ions form variety of complex due to the, following reasons:, (i) Small size and high nuclear charge, (ii) Availability of vacant d-orbital of suitable energy,, which can accept lone pair of electrons donated, by the molecule or ion (ligand)., 4. Magnetic Properties:- Two types of magnetic, behaviour are found in substances diamagnetism, and paramagnetism. Paramagnetic substances are, attracted by the magnetic field and weigh more while, the diamagnetic substances are slightly repelled by, the magnetic field and weight less., As the transition metal ions generally contain one, or more unpaired electrons in them and hence their, complexes are generally paramagnetic. Paramagnetic, character increases with increase in number of, unpaired electrons. Paramagnetism is expressed in, terms of magnetic moment., µ =, , n(n 2) BM (Bohr magneton), , n – number of unpaired electrons, More the magnetic moment, more will be the, paramagnetic character., 5. Formation of Alloys:- As the transition elements, have similar atomic sizes hence in the crystal lattice,, one metal can be readily replaced by another metal, giving solid solution and smooth alloys. The alloys so, formed are hard and have often high melting point., 6. Interstitial compound:- Transition metal form, number of interstitial compounds, in which they take, up atoms of small size, e.g., H, C and N in the vacant, spaces in the their lattices. The presence of these, atoms results in decrease in ductility and malleability, of the metals but increases their tensile strength., 7. Catalytic properties:- Transition metals and their, compounds are known to act as a good catalyst due to, the following reasons:, , (i) Due to their variable oxidation state, they form, unstable intermediate compounds and provide, a new path with lower activation energy for the, reaction (intermediate compound formation, theory)., (ii) In some cases the finely divided metal or, their compounds provide a large surface area, for adsorption and the adsorbed reactants react, faster due to the closer contact (Adsorption, theory)., 8. Ionization energy:- The ionization energies of, transition elements are higher than those of S-block, elements but lower than p-block elements., In a particular transition series, ionization, energy increases gradully as we move from left, to right, and it is due to the increase in nuclear, charge., provide an indication of the energy needed to, raise the metal to a particular oxidation state in, a compound. From the knowledge of values of, ionization energies of the metal it is possible, to rationalize the relative stabilities of various, oxidation state., stable than Pt(II) compounds, on the other hand, Pt(IV) compounds are more stable than Ni(IV), compounds. It is due to that sum of first four, ionization energies is less for platinum whereas, sum of the first two ionization energies is less for, nickel., 9. Coloured compounds:- Compounds of transition, elements are usually coloured due to the promotion, of an electron from one d-orbital to another by the, absorption of visible light. It can be clearly explained, as follows:, In the transition elements which have partly filled, d-orbitals, the transition of electron can take from, one of the lower d-orbitals to some higher d-orbital, within the same subshell. The energy required for this, transition falls in the visible region. So when white, light falls on these complexes they absorb a particular, colour from the radiation for the promotion of electron, and the remaining colours are emitted. The colour of, the complex is due to this emitted radiation., A few of the transition metal ions such as Cu+, Ag+,, Sc3+ are colourlesss. In these ions, the d-orbital are, either completely filled or empty.
Page 160 :
6.9, , 9. (a) Of the lanthanides, cerium (Z = 58) forms a, tetrapositive ion, Ce4+ in aqueous solution., Why?, (b) The +3 oxidation states of lanthanum (Z =, 57), gadolinium (Z = 64) and lutetium (Z =, 71) are especially stable. Why?, (c) Why do Zr and Hf or Nb and Ta exhibit, similar properties ?, (d) Which out of the two, La(OH)3 and Lu(OH)3,, is more basic and why?, Sol. (a) Ce3+ having the configuration 4f15d06s0, can easily loose an electron to acquire the, configuration 4f0 and form Ce4+. In fact, this, is the only +4 state lanthanide which exists in, solution., (b) This is because they have empty, halffilled and completely filled 4f subshells, respectively., (c) Due to the consequence of lanthnoid, contraction, Hf (Z = 72) has size similar to, that of Zr (Z = 40). Hence, their properties are, similar. For the same reason. Nb and Ta have, similar size and hence similar properties., (d) La(OH)3 is more basic than Lu(OH)3. As, the size of the lanthanid ions decreases from, La3+ to Lu3+, the covalent chracter of the, hydroxides increases (Fajan’s rules). Hence,, the basic strength decreases from La(OH)3 to, Lu(OH)3., 10. Identify A to F., (a) A powdered substance A on fusion with, (Na2CO3 + KNO3) mixture gives a green, , coloured compound B., (b) The solution of B in boiling water on, acidification with dilute H2SO4 gives a pink, coloured compound C., (c) The aqueous solution of A on treatement with, NaOH and Br2 - water gives a compound D., (d) A solution of D in conc. HNO3 on treatment, with lead peroxide at boiling temperature, produced a compound E which was of the, same colour as that of C., (e) A solution of A in dilute HCl on treatment, with a solution of barium chloride gave a, white precipitate of compound F which was, insoluble in conc. HNO3 and conc. HCl., Sol. A is MnSO4, (a) MnSO4 + Na2CO3 + 2KNO3 " Na 2 MnO 4, (A), , ( B) Green coloured, , , , + 2KNO2, , (b) 3Na2MnO4 + 2H2SO4 " 2NaMnO 4 +, ( C ) Pink coloured, , , , MnO2 + 2Na2SO4 + 2H2O, , (c) MnSO4 + 4NaOH + Br2 " MnO2 + Na2SO4, ( D), , , , + 2NaBr + 2H2O, , (d) 2MnO2 + 10HNO3 + 5PbO2 " 2HMnO 4, , ( E ) Pink coloured, , , , + 5Pb(NO3)2, , (e) MnSO4 + BaCl2 ", , BaSO4 + MnCl2, ( F ) ( Inso lub le in conc. HNO3, and conc. HCl ), , Exercise, 1. Which of the following statements concerning, transition elements, is not true?, (a) They are all metals., (b) They easily form complexes., (c) Compounds containing their ions are, coloured., (d) They show multiple oxidation states always, differing by two units., 2. The stability of particular oxidation state of a, metal in aqueous solution is determined by:, , (a), (b), (c), (d), , Enthalpy of sublimation of the metal, Ionization energy, Enthalpy of hydration of the metal ion, All of these, , 3. Which of the following is likely to form white, salts?, (a) Cu2+, , (b) Sc3+, , (c) Ti3+, , (d) Fe2+, , 4. Brass is an alloy of, (a) Silver and copper, (c) Copper and tin, , (b) Copper and zinc, (d) Copper, zinc and tin
Page 161 :
6.10, , 5. Zr and Hf have almost equal atomic and ionic, radii because:, (a) of diagonal relationship., (b) both are in the same group., (c) of lanthanide contraction., (d) they have same outermost shell., 6. Which of the following compounds is expected to, be coloured?, (a) Ag2SO4, , (b) CuF2, , (c) MgF2, , (d) CuCl, , 7. Stainless steel contains, (a) Fe + Cr + Cu, , (b) Fe + C + Ni, , (c) Fe + Cr + Ni, , (d) Fe + Ni + Cu, , 8. The catalytic activity of the transition metals and, their compounds is ascribed to, (a) Their chemical reactivity, (b) Their magnetic behavior, (c) Their unfilled d-orbitals, (d) Their ability to adopt multiple oxidation, states and their complexing ability., 9. In the reaction Zn + NaOH ∆, → X, the product, X is:, (a) Na2ZnO2, , (b) 2NaZnO2, , (c) Zn (OH)2, , (d) None of these, , 10. Which of the following is not correctly matched?, (a) SiC – Covalent carbide, (b) WC – Interstitial carbide, (c) Al4C3 – Ionic carbide, (d) B4C – Molecular carbide, 11. Which of the following is not a property of, intersitial compounds?, (a) Neither ionic nor covalent, (b) High chemically reactivity, (c) Retain metallic conductivity, (d) Non-stoichiometric compound, 12. K2MnO4 can be converted into KMnO4 by:, (a) Passing CO2 gas, (b) By passing Cl2, (c) Electrolytic oxidation, (d) All of these, 13. Which of the following metals of 3d series do not, show variable oxidation state?, , 14. The metals which are present in insulin and, vitamin B12 respectively are:, (a) Zn, Co, , (b) Fe, Cr, , (c) Co, Fe, , (d) Zn, Fe, , pH = X, 2−, �����, �, 15. CrO24− �, ����, � Cr2 O7, pH = Y, , The pH values of X and Y are respectively:, (a) 4 and 5, , (b) 4 and 8, , (c) 8 and 4, , (d) 8 and 9, , 16. In which of the following oxoanions, the oxidation, state of the central atom is not the same as that of, its group number in the periodic table?, (a) MnO4–, , (b) Cr2O72–, , (c) VO43–, , (d) FeO42–, , 17. Interstitial compounds are not formed by:, (a) Co, , (b) Ni, , (c) Fe, , (d) Ca, , 18. Which compound does not exist?, (a) MnF6, , (b) MnF4, , (c) MnO3F, , (d) MnO4–2, , 19. The incorrect match is:, (a) CrO5 peroxide, (b) Mn2O7 Acidic oxide, (c) CrO3 Amphoteric, (d) FeO, Basic oxide, 20. Solder is an alloy of:, (a) Pb + Sn, , (b) Mg + Al, , (c) Cu + Sn, , (d) Al + Mn + Cu, , 21. Most common oxidation states are matched below, with the elements. Which one is mismatched ?, (a) Iron (+2, +3), (b) Chromium (+1, +2), (c) Manganese (+2, +7), (d) Titanium (+3, +4), 22. Which of the following pair of ions has same, value of “spin-only" magnetic moment:, (a) Cu+, Cu2+, , (b) Co3+, Fe2+, , (c) Ti2+, Cu2+, , (d) Sc2+, Zn+2, , 23. CO2 and SO2 gas can be distinguish by:, , (a) Sc, Ti, , (b) Ti, Cu, , (a) Slaked lime , , (b) Beryta water, , (c) Sc, Zn, , (d) Co, Ni, , (c) Acidified KMnO4, , (d) All of these
Page 162 :
6.11, , 24. Acidified K2Cr2O7 can not oxidise:, (a) Green vitriol, , (b) Mohr’s salt, , (c) Ferric oxalate, , (d) Ferric sulphate, , 25. In which of the following oxo-anion, all M–O, bond length are not identical?, (a) MnO4–, , (b) MnO4–2, , (c) Cr2O7–2, , (d) CrO4–2, , 26. Which of the following is not a similarity between, sulphur and chromium?, (a) Both exhibit hexacovalency, (b) Sulphate and chromate of Ba2+ are water, insoluble, (c) Trioxide (MO3) both are acidic, (d) Sulphate (SO42–) and chromate (CrO42–) have, same colouration, 27. Copper (II) ions gives reddish brown precipitate, with potassium ferrcroyanide. The formula of the, precipitate is:, (a) Cu[Fe(CN)6], , (b) Cu2[Fe(CN)6], , (c) Cu3[Fe(CN)6], , (d) Cu3[Fe(CN)6]2, , 28. CeO2 is:, (a) A good oxidising agent, (b) Diamagnetic in nature, (c) Colourless compound, (d) All of these, 29. Which of the following show highest oxidation, state?, (a) Cl, , (b) Mn, , (c) Np, , (d) All of these, , 30. Which of the following ion has maximum, complex forming tendency?, (a) La+3, , (b) Ce+3, , (c) Eu+3, , (d) Lu+3, , 1. Number of Cr—O bonds in dichromate ion (Cr2O72–), is:, , products obtained from it in the three conditions, are, respectively, (a) MnO42–, Mn3+ and Mn2+, (b) MnO2, MnO42– and Mn2+, (c) MnO2, MnO2+ and Mn3+, (d) MnO2 , MnO2 and Mn3+, 3. Amongst TiF62–, CoF63–, Cu2Cl2 and NiCl42– (Atomic, numbers : Ti = 22, Co = 27, Cu = 29, Ni = 28) the, colourless species are, (a) TiF62– and Cu2Cl2, (b) Cu2Cl2 and NiCl42–, (c) TiF62– and CoF63–, (d) CoF63– and NiCl42–, 4. CrO3 dissolves in aqueous NaOH to give:, (a) CrO42–, , (b) Cr(OH)3, , (c) Cr2O7, , (d) Cr(OH)2, , 2–, , 5. A compound of a metal ion Mx+ (Z = 24) has a spin, only magnetic moment of 15 Bohr Magnetons., The number of unpaired electrons in the compound, are:, (a) 2, , (b) 4, , (c) 5, , (d) 3, , 6. Which one of the following statement is not correct?, (a) La(OH)3 is less basic than Lu(OH)3., (b) In lanthanide series, ionic radius of Ln3+ ions, decreases., (c) La is actually an element of transition series, rather than lanthanide series., (d) Atomic radii of Zr and Hf are same because of, lanthanide contraction., 7. Which of the following compounds has colour but, no unpaired electrons?, (a) KMnO4, , (b) K2MnO4, , (c) MnSO4, , (d) MnCl2, , 8. Zn gives H2 gas with H2SO4 and HCl but not with, HNO3 because:, (a) Zn acts as an oxidising agent when react with, HNO3., , (a) 6, , (b) 7, , (c) 8, , (d) 4, , (c) In electrochemical series Zn is above hydrogen., , 2 Potassium permanganate acts as an oxidant in, neutral, alkaline as well as acidic media. The final, , (d) NO3– ion is reduced in preference to hydronium, ion., , (b) HNO3 is weaker acid than H2SO4 and HCl.
Page 164 :
6.13, , (c) W = Ag; X = NO2;, , Y = AgNO3; Z = Na3, [Ag(S2O3)2], , (d) W = AgO ; X = N2; Y = AgNO3; Z = Na, [Ag(S2O3)2], , , 23. Which of the following electronic configuration, is associated with the highest stable oxidation, state?, (a) [Ar] 3d1 4s2, , (b) [Ar] 3d5 4s1, , (c) [Ar] 3d5 4s2, , (d) [Ar] 3d6 4s2, , 24. A white precipitate of AgCl dissolves in excess of:, (I) NH3(aq), , (II) Na2S2O3, , (III) NaCN, , , , (a) III only, , (b) I, II, III, , (c) I, II, , (d) I only, , 25. Zinc (II) ion on reaction with NaOH first gives, a white precipitate which dissolves in excess of, NaOH due to the formation of:, (a) ZnO, , (b) Zn(OH)2, , (c) [Zn(OH)4], , 2–, , (d) [Zn(H2O)4]2+, , 26. Dilute nitric acid on reaction with silver liberates:, (a) NO gas, , (b) NO2, , (c) N2 gas, , (d) O2 gas, , 27. Acidified permanganate solution does not oxidize:, (a) C2O42– (aq.), , (b) NO2– (aq.), , (c) S2– (aq.), , (d) F–(aq.), , 28. Which of the following characteristic is not the point, of resemblance between lanthanoids and actinoids?, (a) Reducing property, (b) Oxidation state of +3, (c) Trends of ionic radii for M+3 ions, (d) Radioactivity, 29. Which of the following statement is not correct?, (a) Lu+3 has the strongest tendency toward complex, formation among trivalent lanthanoid ions., (b) Ce has maximum composition in misch metal., (c) f-block elements can have electrons from f0 to, f14., (d) Nd, Np and Nb all are f-block elements., 30. Which of the following lanthanoid has one electron, in 6d subshell?, (a) La, , (b) Ce, , (c) Gd, , (d) None of these, , One or more than one correct type, 1. The metal oxide which decomposes on heating is/, are:, (a) ZnO, , (b) Al2O3, , (c) Ag2O, , (d) HgO, , 2. Which of the following acids attack(s) on copper, and silver?, (a) dilute HNO3, , (b) dilute HCl, , (c) conc. H2SO4, , (d) aqua regia, , 3. Which statements are correct regarding copper, sulphate?, , , , , , (a), (b), (c), (d), , It reacts with NaOH and glucose to give Cu2O., It reacts with KCl to give Cu2O., It gives CuO on strong heating in air., It reacts with KI to give brown colouration., , 4. Pick out the correct statements (s):, (a) MnO2 dissolves in conc. HCl, but does not form, Mn4+ ions., (b) Decomposition of acidic KMnO4 is not, catalysed by sunlight., (c) MnO42– is strongly oxidising and stable only, in very strong alkali. In dilute alkali, water or, acidic solutions, it disproportionates., (d) KMnO4 does not act as oxidising agent in, alkaline medium., 5. The species that undergoes disproportionation in an, alkaline medium are:, (a) Cl2, , (b) MnO42–, , (c) NO2, , (d) ClO4–, , 6. Mercuric chloride is converted into mercury by:, (a) Placing copper metal in aqueous solution of, HgCl2., (b) Treating aqueous solution of HgCl2 with excess, of stannous chloride., (c) Treating aqueous solution of HgCl2 with PbCl4, solution., (d) None of these., 7. Choose correct statement (s) regarding the following, reaction:, , Cr O2 − + 3SO2 − + 8H + → 2Cr 3+ +, 2, , 7(aq.), , 3(aq.), , (aq.), , 3SO24(−aq.), , + 4H 2 O
Page 165 :
6.14, , (a) Cr2 O27 is an oxidising agent., (b) SO32 is a reducing agent., (c) The oxidation number of per S-atom in, SO32 is increased by two., (d) The oxidation number of per Cr-atom in is, Cr2 O72(aq.) decreased by three., 8. Transition elements have greater tendency to form, complexes because they have:, , (a), (b), (c), (d), , vacant d-orbitals, small size, higher nuclear charge, variable oxidation states, , 9. Which of the following ions give(s) coloured, aqueous solution?, (a) Ni2+, (b) Fe2+, (c) Cu2+, (d) Cu+, 10. What are the characteristics of products obtained, when green vitriol is strongly heated?, (a) Basic oxide, (b) Neutral oxide, (c) Acidic oxide, (d) Reducing agent, 11. Which of the following statements are correct when, a mixture of NaCl and K2Cr2O7 is gently warmed, with conc. H2SO4?, , (a) Deep red vapours are liberated, (b) Deep red vapours dissolve in NaOH forming, a yellow solution., (c) Greenish yellow gas is liberated, (d) Deep red vapours dissolve in water forming, yellow solution, PASSAGE-BASED QUESTIONS, Passage # 1 (Q. 12 and 13), ∆, →, , High, Temp., , →, , (i) ‘D’ and ‘E’ are two acidic gas., (ii) ‘D’ is passed through HgCl2 solution to give yellow, precipitate., (iii) ‘E’ is passed through water first and then H2S is, passed, white turbidity is obtained., (iv) A is water soluble and addition of HgCl2 in it, white, ppt is obtained but white ppt does not turn into grey, on addition of excess solution of ‘A’., 12. ‘D’ and ‘E’ are respectively., (a) SO2 and SO3, (c) SO2 and CO2, , (b) SO3 and SO2, (d) CO2 and CO, , 13. Yellow ppt in the above observation is :, , (a), (b), (c), (d), , Mercuric oxide, Basic mercury (II) sulphite, Basic mercury (II) sulphate, Mercuric iodide, , Passage # 2 (Q. 14 and 15), MnO2 is the most important oxide of maganese. It occurs, naturally as the black coloured mineral pyrolusite. It is an, oxidising agent, and decomposes to Mn3O4 on heating to, 530°C. It is used in the preparation of potassium permanganate, and in the production of Cl2 gas. Over half a million tonnes, per year of MnO2 is used in dry batteries., 14. When MnO2 is fused with KOH in the presence at, air, the product formed is:, , , , , , (a), (b), (c), (d), , purple colour KMnO4, green colour K2MnO4, colourless MnO4–, purple colour K2MnO4, , 15. In which of the following species, the colour is due, to charge transfer., (I) [Mn(OH)4]2–, (III) MnO2, , (II) MnO42–, , , , (IV) KMnO4, , (a) I, II, III, , (b) II, IV, , (c) I, II, , (d) Only IV, , Passage # 3 (Q. 16 and 17), Iron forms iron halide salts by reacting the metal directly with, halogen. Fel3 does not exist. FeF3 is white solid inspite of five, unpaired electrons with d5 configuration. FeCl3 is soluble in, water and is used as a mordant in dyeing industry., 16. FeI3 does not exist because:, (a) of its large size., (b) Fe3+ oxidises I– to I2., (c) of low lattice energy., (d) iodine is not highly electronegative enough to, oxidise Fe to Fe3+., 17. FeCl3 solution added to K4[Fe(CN)6] gives A while, with KSCN gives B. A and B respectively are:, (a) Fe3[Fe(CN)6]2, Fe(CNS)3, (b) Fe4[Fe(CN)6]3, KFe(CNS)3, (c) Fe4[Fe(CN)6]3, K3[Fe(CNS)6], (d) Fe4[Fe(CN)6]3, K3[Fe(SCN)6], , Passage # 4 (Q. 18 and 19), Pyrolusite ore on oxidation with KClO3/KNO3 in basic, medium produces dark green coloured compounds (A),
Page 166 :
6.15, , which on electrolysis produces a purpule coloured compound, (B). The purple coloured compound can be crystallisd to, deep purple rhombic prisms. It shows different reactions, in different mediums. Excess of compound (B) on heating, with concentrated H2SO4 gives an explosive oil (C), which, on heating decomposes to give another compound (D) along, with oxygen., , Column I, , Column II, , 18. The nature of compound (C) is:, (a) basic, , (b) acidic, , (c) neutral, , (d) amphoteric, , 19. Identify (D), (a) Mn2O7, , (b) MnO2, , (c) MnSO4, , (d) Mn2O3, , INTEGER VALUE TYPE QUESTIONS, 20. Sum of highest stable oxidation states of following, elements is:, Sc, Zn, Ti, Mn, Cr, 21. Determine total number of unpaired electrons in, following ions., , 1. Match the reactions in column-I with nature of, the reaction/type of the products in column-II, Column-I, , Ti3+, V3+, Cr3+, Cr2+, Mn3+, Fe3+, Fe2+, Co2+, Ni2+, Cu2+, ∆, , 22. FeC2O4 , → products, , (A), , Number of dimagnetic products = x, Number of unpaired electrons in paramagnetic, product = y, Report your answer as (x + y)., +, , H, 23. KMnO4 , → Mnx, R . A., , (B), , Column-II, , O2– " O2 + O22–, +, CrO2−, 4 +H ", , (p), , Redox reaction, , (q) One of the products, has trigonal planar, structure, , (C), , MnO4– + NO2– +, H+ ", , (r), , Dimeric bridged, tetrahedral metal ion, , (D), , NO3– + H2SO4 +, Fe2+ ", , (s), , (s) Disproportionation, , OH −, , KMnO4 R, → Mny, . A., , , , [IIT-2007], , 2. Among the following, the coloured compound is:, , −, , K Cr O7 OH, , → Crz, , , 2, 2, , x + y + z is:, , , , (here x, y and z are oxidation states), , COLUMN MATCHING TYPE QUESTIONS, , (a) CuCl, , (b) K3[Cu(CN)4], , (c) CuF2, , (d) [Cu(CH3CN)4]BF4, [IIT-2008], , 3. The oxidation number of Mn in the product of, , alkaline oxidation fusion of MnO2 is:, , 24., Column I, , [IIT-2009], , Column II, , (A), , Kipp’s apparatus waste, , (P), , (NH4)2SO4.FeSO4.6H2O, , (B), , Green coloured, compound, , (Q) Cu(OH)2. CuCO3, , (C), , Leave(s) brown residue, on heating, , (R) FeSO4, , (D), , Leave(s) black residue, on heating, , (S), , CuCl2.2H2O, , 4. Reduction of the metal centre in aqueous, , permanganate ion involves :, (a) 3 electrons in neutral medium, (b) 5 electrons in neutral medium, (c) 3 electrons in alkaline medium, (d) 5 electrons in acidic medium, [IIT-2011]
Page 167 :
6.16, , 5. The colour of light abosrbed by an aqueous, , solution of CuSO4 is:, (a) orange-red, , (b) blue-green, , (c) yellow, , (d) violet, [IIT-2012], , 6. The correct statement(s) about Cr2+ and Mn3+ is/, , are:, [Atomic numbers of Cr = 24 and Mn = 25], (a) Cr2+ is a reducing agent, (b) Mn3+ is an oxdizing agent, (c) Both Cr2+ and Mn3+ exhibit d4 electronic, configuration, (d) When Cr2+ is used as a reducing agent,, the chromium ion attains d5 electronic, configuration., [JEE (Advanced) 2015], 7. Fe3+ is reduced to Fe2+ by using, , (a), (b), (c), (d), , H2O2 in presence of NaOH, Na2O2 in water, H2O2 in presence of H2SO4, Na2O2 in presence of H2SO4, [JEE (Advanced) 2015], , 8. The “spin-only” magnetic moment [in units of, , Bohr magneton, (µs) of Ni2+ in aqueous solution, would be (atomic number of Ni = 28), (a) 2.84, (b) 4.90, (c) 0, (d) 1.73, [AIEEE-2006], , 9. Lanthanoid contraction is caused due to:, (a) the appreciable shielding on outer electrons, by 4f electrons from the nuclear charge., (b) the appreciable shielding on outer electrons, by 5f electrons from the nuclear charge., (c) the same effective nuclear charge from Ce to, Lu., (d) the imperfect shielding on outer electrons by, 4f electrons from the nuclear charge., [AIEEE-2006], 10. Identify the incorrect statements among the, following:, (a) The chemistry of various lanthanoids is very, similar., (b) 4f and 5f orbitals are equally shielded., (c) d-block elements show irregular and erratic, chemical properties among themselves., , (d) La and Lu have partially filled d orbitals and, no other partially filled orbitals., [AIEEE-2007], 11. The actinoids exhibit more number of oxidation, states in general than the lanthanoids. This is, because:, (a) The actinoids are more reactive than the, lanthanoids., (b) The 5f orbitals extend farther from the, nucleus than the 4f orbitals., (c) The 5f orbitals are more buried than the 4f, orbitals, (d) There is a similarity between 4f and 5f orbitals, in their angular part of the wave function., [AIEEE-2007], 12. Larger number of oxidation states are exhibited, by the actinoids than those by the lanthanoids, the, main reason being:, (a) lesser energy difference between 5f and 6d, than between 4f and 5d orbitals, (b) more energy difference between 5f and 6d, than between 4f and 5d orbitals, (c) more reactive nature of the actinoids than the, lanthanoids, (d) 4f orbitals more diffusion than the 5f orbitals., [AIEEE-2008], 13. In context with transition elements, which of the, following statements is incorrect?, (a) In the highest oxidation states, the transition, metal show basic character and forms cationic, complexes., (b) In the highest oxidation states, of the first five, transition elements (Sc to Mn), all the 4s and, 3d electrons are used for bonding., (c) Once the d5 configuration is exceeded, the, tendency to involve all the 3d electrons in, bonding decreases., (d) In addition to the normal oxidation states, the, zero oxidation state is also shown by these, elements in complexes., [AIEEE-2009], 14. Knowing that the chemistry of lanthanoids (Ln) is, dominated by its +3 oxidation state, which of the, following statements is incorrect?, (a) The ionic sizes of Ln(III) decreases in general, with increasing atomic number., (b) Ln(III) compounds are generally colourless.
Page 168 :
6.17, , (c) Ln(III) hydroxides are mainly basic in, character., (d) Because of the large size of the Ln(III) ions the, bonding in its compounds is predominently, ionic character., [AIEEE-2009], 15. In context of the lanthanoids, which of the, , following statement is not correct?, (a) There is a gradual decreases in the radii of the, members with increasing atomic number in, the series., (b) All the member exhibit +3 oxidation state., (c) Because of similar properties the separation, of lanthanoids is not easy., (d) Availability of 4f electrons results in the, formation of compounds in +4 state for all, the members of the series., [AIEEE-2011], 16. The outer electron configuration of Lu (Atomic, , number : 71) is:, (a) 4f3 5d5 6s2, , (b) 4f8 5d10 6s2, , (c) 4f4 5d4 6s2, , (d) 4f14 5d1 6s2, , (d) Ferrous compouds are more easily hydrolysed, than the corresponding ferric compounds., [AIEEE-2012], 18. Which of the following arrangements does not, , represent the correct order of the property stated, against it?, (a) V2+ < Cr2+ < Mn2+ < Fe2+; paramagnetic, behaviour, (b) Ni2+ < Co2+ < Fe2+ < Mn2+: ionic size, (c) Co3+ < Fe3+ < Cr3+ < Sc3+: stability in aqueous, solution, (d) Sc < Ti < Cr < Mn: number of oxidation, states, [JEE-Main - 2014], 19. Which series of reactions correctly represents, chemical relations related to iron and its, compound?, H 2 SO 4, H 2 SO 4 ,O2, (a) Fe dil, , → FeSO4 , →, , Fe2(SO4)3 heat, , → Fe, O2 , heat, dil H 2 SO 4, (b) Fe , → FeO , → FeSO4, heat, , → Fe, , , , [AIEEE-2011], 17. Iron exhibits +2 and +3 oxidation states. Which of, , the following statements about iron is incorrect?, (a) Ferrous oxide is more basic in nature than the, ferric oxide., (b) Ferrous compounds are relatively more ionic, than the corresponding ferric compounds, (c) Ferrous compounds are less volatile than the, corresponding ferric compounds., , heat , air, 2 , heat, (c) Fe Cl, → FeCl2, , → FeCl3 , Zn, Fe, →, 2 , heat, , 600°C, (d) Fe O, → Fe3O4 CO, , → FeO, , 700°C, , CO, , → Fe, , [JEE-Main - 2014], 20. The colour of KMnO4 is due to:, (a) M " L charge transfer transition, (b) d " d transition, (c) L " M charge transfer transition, (d) s " s transition, [JEE-Main - 2015], , Answer Key, , 1. (d), 11. (b), 21. (b), , 2. (d), 12. (d), 22. (b), , 3. (b), 13. (c), 23. (c), , 4. (b), 14. (a), 24. (d), , 5. (c), 15. (b), 25. (c), , 6. (b), 16. (d), 26. (d), , 7. (c), 17. (d), 27. (b), , 8. (d), 18. (a), 28. (d), , 9. (a), 19. (c), 29. (d), , 10. (d), 20. (a), 30. (d), , 1. (c), 11. (d), 21. (a), , 2. (b), 12. (a), 22. (c), , 3. (a), 13. (d), 23. (c), , 4. (a), 14. (c), 24. (b), , 5. (d), 15. (d), 25. (c), , 6. (a), 16. (c), 26. (a), , 7. (a), 17. (b), 27. (d), , 8. (d), 18. (c), 28. (d), , 9. (c), 19. (a), 29. (d), , 10. (b), 20. (a), 30. (d)
Page 169 :
6.18, , 1. (c, d), 2. (a, c, d), 3. (a, c, d) 4. (a, c) 5. (a, b, c) 6. (a, b ), 7. (a, b, c, d), 9. (a,b,c), 10. (a,b,c,d) 11. (a, b, d) 12. (b), 13. (c), 14. (b), 15. (b), 17. (d), 18. (b), 19. (b), 20. (6), 21. (29), 22. (6), 23. (12), 24. A " R; B" P, Q,R,S; C " P, R; D " Q, 25. A " S; B " Q, R; C " Q; D " P, , , 1. A " p. s; B " r; C " p, q; D " p, 2. (c), 3. (6), 4. (a, c, d), 5. (a), 11. (b) 12. (a), 13. (a), 14. (b), , 6. (a, b, c) 7. (a, b) 8. (a), 15. (d), 16. (d), 17. (d), , 9. (d), 18. (a), , 8. (a, b, c), 16. (b), , 10. (b), 19. (d), , 20. (c), , Hints and Solutions, 13., 1., , (d) Unlike p-block elements, the various oxidation, states of d-block elements differ by one unit., 2., (d) M(s) " M+n(aq) + ne The above change involves sublimation,, ionization and hydration., 3., (b) Sc3+ ([Ar] 4s0 3d0) has no electrons in d-sub, shell and hence, d-d transitions are not possible., 4., (b) Brass (Cu and Zn), 5., (c) Due to lanthanide contraction, elements in 5dseries have almost equal atomic and ionic radii, with 4d- series elements., 6., (b) CuF2 (Cu+2 = [Ar] 4s0 3d9), Due to d-d transitions, this compound is, coloured., 7., (c) Stainless steel (Fe + Cr + Ni), 8., (d) Transition metals and their compound show, catalytic activity because they can show, variable oxidation state and they have tendency, to form complex., 9., (a) Zn + 2NaOH " Na2ZnO2 + H2 10., (d) B4C is a covalent network carbide., 11., (b) Interstitial compound do not have high, chemical reactivity., 12., (d) K2MnO4 can be converted into KMnO4 by, passing Cl2 (oxidizing agent) or by electrolytic, oxidation or by disproportionation in acidic or, in neutral medium., , 14., 15., , (c) Sc and Zn show fixed oxidation state +3 and, +2 respectively., (a) Zn and Co are present in insulin and vitamin, B12 respectively., pH < 7, , , → Cr O 2, (b) CrO42- ←, 2 7, pH > 7, , , X will be less than 7 and Y will be more than 7., 16., (d) +7, MnO4(VII group), +6, , Cr2O72-, , (VI group), , +5, , VO43-, , (V group), , +6, , FeO42-, , (VIII group), , 17., , (d) S-block elements (Ca) are not formed, interstitial compounds., , 18., , (a) MnF6 does not exist., , 19., , (c) Mn2O7 and CrO3 are acidic oxides., , 20., , (a) Solder (Pb + Sn), , 21., , (b) Chromium (+3, +6), , 22., (b) Co+3 = [Ar] 4s0 3d6 (4 unpaired e-), Fe+2 = [Ar] 4s0 3d6 (4 unpaired e-), 23., (c) SO2 can decolorize acidified KMnO4 but CO2, cannot decolorize acidified KMnO4., 24., (d) Fe2(SO4)3 [Ferric sulphate] does not behave as, reducing agent.
Page 173 :
6.22, , 15., , (d) All the members of lanthanoids exhibit +3, oxidation state not + 4 oxidation state., 16., (d) The outer electronic configuration of Lu is 4f14, 5d1 6s2, 17., (d) As the positive oxidation state increases,, tendency of hydrolysis increases., Ferric salts (Fe+3) are more easily hydrolysed, than the corresponding ferrous salts (Fe+2)., , 18, (a) Ions , Number of unpaired e–, , V+2 , 3, +2 , Cr, 4, Mn+2 , 5, +2 , Fe, 4, Order of paramagnetic behaviour :, V+2 < Fe+2 = Cr+2 < Mn+2, 19., (d) CO acts as a reducing agent to reduce FeO into, Fe., 20., (c) Cause of colour of KMnO4 is L " M charge, transfer.
Page 174 :
Chapter, , Key Concepts, BF3 is hydrolysed as follows:-, , Group 13 contains boron (B), aluminium (Al), gallium, (Ga), indium (In), and thallium (Tl). These elements have, outer electronic configuration (ns)2 (np)1, where n varies, from 2 to 6. Boron is nonmetal while others are metals., The atomic radii of Ga, In and Tl are smaller than expected, values due to d-block contraction. The atomic radius of Tl, is a little larger than In due to lanthanide contraction. On, descending the group, +1 oxidation state becomes more, stable than +3 state due to the inert pair effect., The very high melting point of boron is due to its covalent, network structure. In boron family, gallium has the lowest, melting point. The ionization energies do not follow the, expected trend of decreasing values on descending the, group. All elements burns in oxygen at high temperatures, forming M2O3. The reaction of aluminium with oxygen, (known as thermite reaction) is strongly exothermic., Aluminium is amphoteric. It dissolves in dilute minerals, acids and in aqueous sodium hydroxide., The acidic character of hydroxides decreases on, descending the group., Boric acid is a very weak monobasic acid. It does not, liberate hydrogen ion but accepts a hydroxyl ion. In the, presence of cis-diol (glycerol, mannitol or sugars), boric, acid behaves as a strong acid and can be titrated with, NaOH in the presence of phenolphthalein indicator., , , , The fluorides of Al, Ga, In and Tl are ionic while the other, halides are generally covalent and exist as dimer., The trihalides of boron are electron-deficient compounds., Du to back bonding, the electron density on boron in, increased. The tendency to form pp-pp bond is maximum, is BF3 and falls rapidly on passing to BCl3 to BBr3. The, increasing order of acid strength follows the order BF3 <, BCl3 < BBr3., , Boron belongs to Group 13 of the periodic table. The chief, minerals of boron are borax (Na2[B4O5(OH)4].8H2O), i.e.,, Na2B4O7. 10H2O, colemanite (Ca2[B3O4(OH)3]2.2H2O), i.e., Ca2B6O11.5H2O and kernite (Na2[B4O5(OH)4].2H2O), i.e., Na2B4O7.4H2O., Boron is isolated by converting its mineral into boron, trioxide followed by its reduction with magnesium., Na2(B4O5(OH)4).8H2O + 2HCl, 2NaCl +, , borax , 5H2O + 4H3BO3, , orthoboric acid
Page 175 :
7.2, , 2H3BO3, , B2O3 + 3H2O, , B2O3 + Mg, , 2B + 3MgO, , Crystalline boron is obtained by the reduction of boron, trichloride with zinc or dihydrogen at the high temperatures., K, 2BCl3 + 3Zn 1200, , → 3ZnCl2 + 2B, K, 2BCl3 + 3H2 1200, , → 2B + 6HCl, , Boron is an extremely hard refractory solid of high melting, point, low density and very low electrical conductivity., Boron is quite inert to chemical attack at ordinary, temperature. It reacts with strong oxidising agents such as, fluorine and concentrated HNO3 at room temperature. At, elevated temperatures, it combines with metals (forming, borides) and nonmetals., Diborane is B2H6. It has two coplanar BH2 groups and the, remaining two hydrogen atoms lie centrally between BH2, groups in a plane perpenticular to the plane containing, BH2 groups., In borax two boron atoms are in a trianglular geometry, and two boron atoms are in tetrahedral geometry., , H, , H, B, H, , OH, –, B, , H, , O, , H, , O, O, , HO–B, , B, , O, , O, , H, , B–OH, , B–, OH, 2–, , B2H6, , [B4O5(OH)4] ion in borax, , Boric acid (H3BO3) is obtained by treating borax with, minerals acids., Na2[B4O5(OH)4].8H2O + 2H+, 4H3BO3 +, , 5H2O + 2Na+, Boric acid is a white crystalline substance, soft and soapy, to touch. It is moderately soluble in cold water. On heating, it decomposes as follows:, K, H3BO3 −375, , → HBO2, H O, 2, , Metaboric acid, K, 4HBO2 −435, , → H2B4O7, H O, 2, , , Tetraboric acid, , Group 14 contains carbon (C), silicon (Si), germination, (Ge), tin (Sn) and lead (Pb). Their outer electronic, configuration is (ns)2 (np)2, where n varies from 2 to 6. The, metallic character of elements increases on descending the, group; C and Si are nonmetals, Ge is nonmetal but also has, some metallic characterstics, and Sn and Pb are metals., The melting points decreases on descending the group,, with the exception of Pb whose melting point is slightly, higher than that of Sn. Carbon has extremely high melting, point. This is due to the stronger C–C bonds in the network, of carbon atoms. The ionization energies decrease from C, to Si, but then change in an irregular way because of the, effects of filling d and f sub-shells., Carbon forms single, double and triple bonds with carbon, itself and with other elements. The tendency to form, multiple bond by other elements is rare. However, silicon, can form double bond due to back bonding in which the, lone pair in p orbitals of an atom is extended to an empty, orbital of Si. One of the examples of back bonding is, trsilylamine, N(SiH3)3., The chemical reactivity of elements decreases down the, group. The inert effect becomes increasingly effective on, descending the group., The stability of +4 oxidation state decreases while that, of the +2 oxidation state increases on descending the, group., 4Sn + 10HNO3, 3Pb + 8HNO3, , 4Sn(NO3)2 + NH4NO3 + 3H2O, 3Pb(NO3)2 + 2NO + 4H2O, , C is not affected by alkalis, Si reacts forming silicates, while Sn and Pb form stannate [Sn(OH)6]2–, and plumbate, [Pb(OH)6]–2, respectively., All the elements of Group 14 from tetrahalides with, the exception of PbI4 which is not known. The stability, of halides decreases down the group. CCl4 is stable, while other halides are hydrolysed. The hydrolysed. The, hydrolysis of SiCl4 produces SiO2 while SiF4 produces, SiO2 as well as (SiF6)–2., , heat, H2B4O7 red, , → 2B2O3, −H O, , The acidic nature of the dioxdes of carbon family decreases, down the group; CO2 and SiO2 are acidic, GeO2 is weakly, acidic and SnO2 and PbO2 are amphoteric., , Boric acid is a very weak monobasic acid. It does liberate, hydrogen but accepts a hydroxyl ion, i.e., it behaves as a, Lewis acid., , Silicones are organosilicon polymers with general, formula (R2SiO)n, where R may be methyl, ethyl or phenyl, group., , , , 2, , Boric oxide
Page 176 :
7.3, , Because of the directed lone pair of electrons on carbon,, the molecule forms carbonyls with a number of metal, in which the coordinate bond is formed through carbon, atom and not through oxygen atom. With nickel, it forms, tetracarbonyl which decomposes at higher temperature., The burning of carbon in a limited supply of air or in a, deficiency of oxygen produces carbon monoxide. A few, reactions producing carbon monoxide are as follows:, Fe2O3 + 3C, , 2Fe + 3CO, , , COOH, concentrated H 2 SO 4, , → CO + CO2 + H2O, , Ω, COOH, , , 325 345 K, ������, �, Ni + 4CO �, �����, � [Ni(CO)4], 450 K, , The poisonous nature of carbon monoxide is due to its, ability to form a bond with iron atom in the haemoglobin, of blood. In the form of producer gas (CO + N2), water, gas (CO + H2) or semiwater gas (mixture of producer and, water gases), is used as fuel., , concentrated H SO, , 2, 4, HCOOH , → CO + H2O, , → CO + ZnO, CO2 + Zn heat, , K4Fe(CN)6 + 6H2SO4, , , 2K2SO4 + FeSO4 +, , concentrated 3(NH4)2SO4 + 6CO, , Carbon monoxide is an extremely poisonous gas. A, concentration of one in 800 volume of air will lead to, death in 30 minutes. It combines with haemoglobin of, the blood to give more stable carboxyhaemoglobin and, thus render it useless as an oxygen carrier. In air, it burns, with a blue flame to give carbon dioxide. The gas readily, dissolves in ammonical or acidic solution of cuprous, chloride giving the additional product CuCl. CO. 2H2O., Some the reactions shown by carbon monoxide are given, below., CO + NaOH pressure, , → HCOONa, heat, , sodium formate, CO + Cl2 hv, → COCl2, + Cu powder, CO + 2H2 ZnO, , → CH3OH, 425 – 675 K, − 1175 K, Fe2O3 + 3CO 875, , → 2Fe + 3CO2, , I2O5 + 5CO, , I2 + 5CO2, , The structure of carbon monoxide may be represented as, – +, C O, , or, C O, Carbon atom is considered to be sp hybridized. One sp, orbital used to form a single bond with oxygen atom while, the other sp orbital which points away from the C–O bond, contains a lone pair of electrons. The sideways overlap, of singly filled 2p orbitals on carbon and oxygen atoms, produces a p bond. The second p bond is formed by the, overlap of doubly filled 2p orbitals on carbon and oxygen, atoms produces a p bond. The second p bond is formed by, the overlap of doubly filled 2p orbital on oxygen with the, vacant 2p orbital on carbon. But once the bond is formed,, it is not possible two distinguish the two p bonds., , Carbon dioxide can be prepared by any of the following, reactions., CaCO3 + 2HCl, , CaCl2 + H2O + CO2, , 2NaHCO3 heat, , → Na2CO3 + H2O + CO2, MgCO3 heat, , → MgO + CO2, Solid carbon dioxide is known as dry ice and is used as a, refrigerant., Carbon dioxide is an acidic oxide., CO2 + H2O, , H2CO3, , With reactive metals, it is reduced to CO., 2Na + 2CO2, , Na2CO3 + CO, , 2Mg + CO2, , 2MgO + C, , Carbon dioxide is absorbed by green plants in the presence, of sunlight and is ultimately transformed into starch and, cellulose in the chloroplast. This process is known as, photosynthesis., Carbon dioxide is a linear molecule with carbon-oxygen, ond equal to 115 pm, which is intermediate between those, calculated for carbon-oxygen double and triple bond. It is, thus cosidered to the resonance hybrid of the following, structures., O C O, , +, , O C O, , –, , –, , +, , O C O, , Carbon in CO2 is sp hybridized. The two sp orbitals form, two bonds wiith two oxygen atoms. The two p orbital not, included in hybridization give rise two p bonds., , The are prepared by direct combination of metals with, carbon at elevated temperature or indirectly, the heating of, metallic oxide with carbon. The carbides may be classified, into three groups, namely, ionic, covalent and interstitial., Ionic carbides are formed by metals of Group 1, 2 and 3.
Page 177 :
7.4, , These compounds, in general, occur as transparent crystals, and in the solid state they are nonconductors of electric, current. They give hydrocarbons when treated with water, or acids. On the basis of anions, these have been classified, as methanides (C4–), acetylides (C22–) and allylides. (C34–)., The examples are:, , are shared with other tetrahedra resulting in a threedimensional lattice. The formula of such silicates is SiO2., Silicones, , CaC2 + 2H2O, , Ca(OH)2 + C2H2, , Silicones are polymeric organosilicon compounds, containing individual or cross-linked Si–O chains or, rings in which some of the oxygens of SiO4 tetrahedron, are replaced by –OH, –CH3, –C2H5 groups. For example,, dialkyldichlorosilane (R2SiCl2), which is produced by the, reaction, , Al2C6 + 6H2O, , 2Al(OH)3 + 3C2H2, , 2RCl + Si(Cu), , Be2C + 4H2O, , 2Be(OH)2 + CH4, , Al4C3 + 12H2O, , Al(OH)3 + 3CH4, , Mg2C3 + 4H2O, , 2Mg(OH)2 + CH3C∫CH, , R2SiCl2, , reacts with water producing dialkyldihydrosilane. This, in, turn, may be dehydrated to give a linear polymer., R2SiCl2 + 2H2O, , Silicon belongs to Group 14 and is classified as metalloid., It exists in two allotropic forms; the amorphous silicon, and the crystalline or admantine silicon. Silicon does not, occur freely in nature. It occurs as silica (SiO2) or as, silicates like feldspar, kaolinite, mica, etc. in rocks and, clays., Silicon is produced by the reduction of sand with coke in, an electric arc furnace., SiO2 + 2C, Si + 2CO, Silicon is a hard solid having melting point 1793 K and, boiling point 3550 K. It reacts with fluorine at room, temperature to form SiF4. With other elements, it reacts at, elevated temperatures., Silicates and silica contains SiO44– tetrahedra differing in, the way the tetrahedra are linked together as described in, the following., Orthosilicates, These contain individual discrete SiO44– tetrahedra., Examples are phenacite (Be2SiO4) and zircon (ZrSiO4)., Pyrosilicates, These contain discrete Si2O76– ions and are formed, when one oxygen atom of two SiO4 tetrahedra is shared., Example is thorteveitite (Sc2Si2O7)., Chain and Cyclic Silicates, In these silicates two oxygen atoms per tetrahedron are, shared., Sheet Silicates, These are formed by the sharing of three oxygen atoms by, each tetrahedron giving an infinite two-dimensional sheet, of the empirical formula (Si2O5)n–2n, , R2Si(OH)2 + 2HCl, , nR2Si(OH)2 −, → (R2SiO)n, H O, 2, , , , Silicone, , Silicones have good thermal and oxidative stability., These are excellent water repellants and chemically inert, substances. Silicon rubber is not attacked by ozone. Liquid, silicones are used as excellent lubricants. These are mixed, with paints and enamels to increase the resistance to the, effects of high temperatures, sunlight and chemicals., , Group 15 contains nitrogen (N), phosphorus (P), arsenic, (As), antimony (Sb) and bismuth (Bi). Their outer, electronic configuration is (ns)2 (np)3, where n varies from, 2 to 6. The metallic character of these elements increases, on descending the group; N and P are nonmetals, As and, Sb are metalloids and Bi is a metal. The melting and, boiling points follows the order, melting point, , N < P < As > Sb > Bi, , boiling point, , N < P < As < Sb > Bi, , Phosphorus has two common allotropic forms; white and, red. White phosphorus is more reactive than red form due, to highly strained structure (P–P–P angle is 60°)., Black phosphorus is a highly polymerised form and is, most stable., Nitrogen form triple bond in dinitrogen because bond, enthalpy e(N∫N) is greater than three times bond enthalpy, e(N–N). In phosphorus, the reverse is true, hence, it, involves single bonds., The melting points of hydrides follow the order, , Three-Dimensional Silicates, , NH3 > PH3 < AsH3 < SbH3., , In these silicates, all the four oxygen atom of a tetrahedron, , All the five elements of Group 15 form trihalides. Of these
Page 179 :
7.6, , It is a strong dehydrating agent. For example, H2SO4 and, HNO3 are converted into corresponding anhydride., 2H2SO4 + P4O10, , 2SO3 + 4HPO3, , 4HNO3 + P4O10, , 2N2O5 + 4HPO3, , In the structure of P4O10, each P atom forms three bonds, to oxygen atoms and also an additional coordinate bond, with an oxygen atom. Terminal coordinate P–O bond is, 143 pm which is much shorter than the expected bond, length of 162 pm. The shows the presence of considerable, pp-dp back bonding because of the leteral overlap of full, p orbitals on oxygen with empty d orbitals on phosphorus., , Cyclictrimetaphosphoric acid, (HPO3)3 or H3P3O9, O, , OH, P, , , HO, O, , O, , O, , P, , P, O, , O, OH, , Oxoacids of Phosphorus : , Hypophosphorus acid, H3PO2, O, P, , H, OH, H, Phosphosrus acid, H3PO3, O, , P, , H, , The metallic character of these elements increases on, descending the group; O and S are nonmetallic, Se and Te, are weaker nonmetallic and Po is metallic., , OH, , OH, , Pyrophosphorus acid, H4P2O5, O, , O, , P, P, H, O, H, OH HO, , Hypophosphoric acid, H4P2O6, , , HO, , O, , O, , P, , P, , OH, , HO, , HO, , OH, , OH, , Pyrophosphoric, , O, , acid, H4P2O7, , O, , P, P, , HO, O, OH, OH HO, Metaphosphoric acid, HPO3 or (HPO3)n, O, O, O, P, , ~O, O, OH, , P, OH, , O, , P, OH, , Electron affinity of sulphur is larger than that of oxygen,, this due to the more repulsion experienced by the incoming, electron from the smaller, more compact electronic cloud, of oxygen atom., Oxygen is diatomic with the unpaired electrons. Sulphur, exists in two allotropic forms – rhombic and monoclinic, sulphur. Rhombic sulphur is stable at room temperature, while monoclinic sulphur is stable above 369 K. Selenium, exists in six allotropic forms. Tellurium has only one, crystalline form and polonium has two allotropic forms, (cubic and rhombohedral)., Ozone is another allotropic form of oxygen. It is very, reactive. It is formed in the upper layer of atmosphere, (about 20 km from the earth) by the action of ultraviolet, radiation on oxygen. Ozone is a strong oxidizing agent., In organic chemistry, ozone is used to locate the carbon, double and triple bonds. The ozone molecule is angular, with bond angle about 117° and bond length 127.8 pm., , OH, , Phosphoric acid, H3PO4, O, P, , Group 16 contains oxygen (O), sulphur (S), selenium, (Se), tellurium (Te) and polonium (Po). Their electronic, configuration is (ns)2 (np)4, where n varies from 2 to 6., , O~, , The melting and boiling point of hydrogen compounds, of elements of Group 16 follow the order H2O > H2S <, H2Se < H2Te. The exceptional high values of H2O is due, to hydrogen bondings., H2O2 is a strong oxidizing agent. With stronger oxidizing, agents such as KMnO4, KIO3, and O2, hydrogen peroxide, acts as a reducing agent., Oxoacids of sulphur may be classified into four series.
Page 180 :
7.7, , Sulphurous Acid Series, , Peroxodisulphuric acid (H2S2O8), , , Sulphurous acid (H2SO3), HO, S O, HO, Di-or pyrosulphurous acid (H2S2O5), O O, HO S, , , S, , Sulphuric Acid Series, , Sulphuric acid (H2SO4), O, OH, , Thiosulphuric acid (H2S2O3), S, OH, , O, Di-or pyrosulphuric acid (H2S2O7), O, O, HO S, , , O, , S, , O, , OH, , O, , Thionic Acid Series, , Dithionic acid (H2S2O6), O O, , HO S, , S, , O, , O, , OH, , Polythionic acid (H2Sn+2O6), O, O, HO S, , , (S)n, , O, , S, , OH, , O, , Peroxoacid Series, , Peroxomonosulphuric acid (H2SO5), O, , H O, , O, , O, , O, , O, , S, , OH, , O, , Group 17 contains fluorine (F), chlorine (Cl), bromine, (Br), iodine (I) and astatine (At). Their outer electronic, configuration is (ns)2(np)5, where n varies from 2 to 6. The, trends in this group are as follows., Covalent and ionic radii – increases down the group., Electronegativity and ionization energy – decreases down, the group., Electron affiinity – Cl > F > Br > I, Melting and boiling points – increases down the group., , O, , , HO S, , , HO S, , O, , OH, , O, Dithionous acid (H2S2O4), O O, , HO S S OH, , , HO S, , O, , S, O, , OH, , Bond enthalpy (X–X)– Cl2 > Br > F2 > I2, Oxidizing ability – decreases down the group., Halogens are very reactive and do not occur in free, state. Fluorine is most electronegative atom, there exists, hydrogen bondings in gaseous HF., HF is a weak acid and HCl, HBr and HI behave as strong, acids. In the glacial acetic acid medium, the acid strength, follows the order HI > HBr > HCl > HF., Halogens with the exception of F form a number of, oxoacids– hypohalous acids (HOX), halous acids (HXO2),, halic acid (HXO3) and perhalic acid (HXO4). The acid, strength follows the order HXO4 > HXO3 > HXO2 > HXO., Fluorine forms only hypofluorous acid (HOF)., Halogens also form interhalogen compounds AX, AX3,, AX5 and AX7. The compounds AX and AX3 are formed, where the electronegativity is not very large. The, compounds AX5 and AX7 are formed by large Br and I, atoms surrounded by small atom F. The molecule AX3 is, T-shaped with two lone pair of electrons at the equilateral, positions. The orientations of five pair of electrons around, the atom A is trigonal bipyramidal. Interhalogens are more, reactive than elemental halogens except fluorine., The six pairs of electrons around atom A in AX5 acquire, octahedral orientation. The seven pairs of electrons around, atom A in AX7 acquire pentagonal bipyramidal orientation., Halogens are nonmetallic and have high electron affinity., The nonmetallic character decreases down the group and, iodine shows some metallic character. With metals, they, form ionic compounds by accepting one electron and with
Page 181 :
7.8, , nonmetals covalent compounds are formed by sharing an, electron., , are formed. Halogens react with metals and nonmetals to, form halides, the reactivity decreases down the group., , All halogens exhibit-1 oxidation state. Except fluorine, rest, of the halogens also exhibit +1, +3, +5 and +7 oxidation, states. Fluorine is the most electronegative and thus there, exists hydrogen bonding in HF with the result that it has, exceptionally high melting and boiling points as compared, to those of HCl, HBr and HI., , All the halogens react with hydrogen to form hydrogen, halides (HX). The reaction between flourine and hydrogen, is violent while that between iodine and hydrogen is, very slow at room temperature. The acidic character of, hydrogen halides increases in the order, HF < HCl < HB2, < HI., , Because of high reactivity, halogens do not exist in the, free state. The chief ore of flurine are fluorspar (CaF2),, cryolite (Na3AlF6) and fluoroapatite (Ca2(PO4)F). Other, halogens mainly occur in seawater as salt. Some sea weeds, and sponges contain iodine as iodides. Chile slatpeter, (NaNO3) contains 0.02 – 1% iodide in the form of sodium, iodate., , Oxoacids of Chlorine, , Fluorine was obtained by electrolysis of KF dissolved, in anhydrous HF. The products H2 and F2 are collected, separately so as to avoid explosion caused by combination, of these two gases., , Cl, HO, O, Chloric acid, HClO3, , Chlorine, in the laboratory, can be prepared by the, following methods:, 1. Action of concentrated hydrochloric acid on, manganese dioxide., 2. Oxidation of HCl by strong oxidizing agents such, as KMnO4 and K2Cr2O7., Bromine can be obtained by the oxidation of bromide, with chlorine gas or manganese dioxide in the presence of, concentrated sulphuric acid. Iodine can also be obtained, by the oxidation of iodide., Halogens are oxidizing agents, oxidizing power decreases, down the group. Flourine is the most oxidizing in nature, and can oxidize water to oxygen and ozone. With dilute, alkalis, fluorine forms oxygen difluoride (OF2) and with, hot concentrated alkaliis flouride and oxyen are formed., The other halogens reacts with cold and dilute alkali, solution to give hypohalites, (XO–) and with hot and concentrated alkali, halates (XO3–), , Four oxoacids of chlorine are known. These are:, Hypochlorous acid, HOCl, , Cl, , O, , H, , Chlorous acid, HClO2, , Cl, O, OH, O, Perchloric acid HClO4, O, Cl, O, O, , OH, , The Group 18 contains helium (He), neon (Ne), argon, (Ar), krypton (Kr), xenone (Xe) and radon (Rn). Their, outer electronic configurations is (ns)2(np)6 with the, exception of the electronic configuration of He which is, 1s2, all elements exist as monatomic gas., Xenon forms a number of fluorides –XeF2, XeF4 and, XeF6. The other compounds are XeO3, XeOF4, XeO2F2,, XeO4 and [XeO6]4–, , Solved Examples, 1. Which species does not exist 3–, , (a) [BF6], , 3–, , (c) [GaF6], , 3–, , (b) [AlF6], , (d) [InF6]3–, , Sol.(a) [BF6]3– does not exist because boron does not, have vacant d-subshells., 2. Orthoboric acid when heated to red hot gives, , (a) Metaboric acid (b) Pyroboric acid, (c) Boron and water (d) Boric anhydride, C, C, Sol.(d) H3BO3 100°, , → HBO2 160°, , → H2B4O7 + H2O, , , 2B2O3 + H2O
Page 182 :
7.9, , 3. Alumnium vessels should not be washed with, materials containing washing soda because (a) Washing soda is expensive., (b) Washing soda is easily decomposed., (c) Washing soda reacts with aluminium to form, soluble aluminate., (d) Washing soda reacts with aluminium to form, insoluble aluminium oxide., , Gas (A) burns with blue flame and is oxidised to, gas (B). Gas (B) turns lime water milky., , Sol.(c) Na2CO3 + 2H2O, , COO H, (A), , 2NaOH + H2O + CO2, , 2NaOH + 2Al + 2H2O, , 2NaAlO2 + 3H2., , , , soluble, , 4. When Al is added to KOH solution (a) No action takes place, (b) Oxygen gas is evolved, (c) Water is produced, (d) Hydrogen gas is evolved, Sol.(d) 2Al + 2KOH + 2H2O, , 2KAlO2 + 3H2, , 5. Carbon forms carbon monoxide when burnt in (a) Absence of air or oxygen, (b) Excess of air or oxygen, (c) Limited supply of air or oxygen, (d) Moist air, 1, Sol.(c) C + O2 (limited ) ∆, → CO, 2, 6. CCl4 does not act as Lewis acid, while SiCl4 and, SnCl4 acts as Lewis acid as well as their aqueous, solution is acidic. Explain why?, Sol. SiCl4 and SnCl4 are hydrolysed to form acidic, solution as well as they can act as Lewis acid, because they can increase their co-ordination, number greater than four due to availability of, d-orbitals., SnCl4 + 4H2O, Sn(OH)4 + 4HCl, SiCl4 + 4H2O, Si(OH)4 + 4HCl, SiCl4 + 2Cl–, (SiCl6)–2, 7. PbCl4 exists while PbBr4 and PbI4 do not exist., Explain why?, Sol. Pb4+ is an oxidising agent and readily changes, into Pb2+ (due to inert pair effect) while Br– and, I– ions are reducing agents. Thus, redox reaction, occurs indicating that PbBr4 and PbI4 are unstable, compounds., 8. H2C2O4 ∆, → gas(A) + gas(B) + liquid(C), oxalic acid, , 3 ,∆, (D) NH, , → (E), , Gas(A) + Cl2, 3 ,∆, (B) NH, , → (E), , Identify (A) to (E) and explain the reactions., CO OH, , Sol., , ∆, → CO + CO2 + H2O, (B), , (C), , O, ||, NH 3 , ∆, COCl2 , → NH2–C–NH2, , CO + Cl2, , (A) (D) , (A) is CO, , (B) is CO2, , (C) is H2O, , (D) is COCl2, , (E) Urea, , (E) is NH2CONH2, 9. In P4O6 the number of oxygen atoms bonded to, each P atom is –, (a) 1.5, (b) 2, , , (c) 3, , (d) 4, , Sol.(c) Each P in P4O6 is bonded to 3 oxygen atoms., O, O, , P, O, , P, , P, O, O, , O, P, , 10. Which trihalides is not hydrolysed by water (a) NF3, (b) NCl3, (c) PCl3, (d) AsCl3, Sol.(a) In the first stage of hydrolysis, an extra bond is, formed by water molecule. While chlorine and, the group 15 elements (except nitrogen) can, expand their octet by using vacant d-orbitals of, the valence shell, F and N cannot. As a result, NF3, is extremely stable., NF3 + H2O (l), No hydrolysis, NCl3 + 3H2O (l), NH3 + 3HOCl, PCl3 + 3H2O (l), H3PO3 + 3HCl, 2AsCl3 + 3H2O, 6HCl + As2O3, 11. Derivatives of nitrogen (III) act as –, (a) Oxidizing agent only, (b) Reducing agent only, (c) Both Oxidizing and Reducing agent, (d) Nitrating agent, Sol.(c) Deriatives of nitrogen (III) have both oxidizing, and reducing properties.
Page 187 :
7.14, , 11. (NH4)2Cr2O7 on heating liberates a gas. The same, gas will be obtained by:, (a) Heating NH4NO2, (b) Heating NH4NO3, (c) Treating H2O2 with NaNO2, (d) Treating Mg3N2 with H2O, 12. When Cl2 reacts with NH3 of low concentration, and of high concentration, then oxidised, products obtained from NH3 are ____ and _____, respectively:, (a) N2, NH2Cl, , (b) NCl3, N2, , (c) N2H4, N2, , (d) N2, NH4Cl, , 13. The incorrect statement(s) about O3 is (are):, (a) O–O bond lengths are equal., (b) Thermal decomposition of O3 is endothermic., (c) O3 is diamagnetic in nature., (d) O3 has a bent structure., 14. Compounds A and B are treated with dilute, HCl separately. The gases liberated are Y and Z, respectively. Y turns acidified dichromate paper, green while Z turns lead acetate paper black. So,, A and B compounds are respectively:, (a) Na2SO3, Na2S, (b) NaCl, Na2CO3, (c) Na2S, Na2SO3, (d) Na2SO4, K2SO3, 15. When H2SO4 reacts with Cl2 gas then X is, produced. X is a good chlorinating agent and, given H2SO4 after hydrolysis. Then [X] is:, (a) SOCl2, , (b) SO2Cl2, , (c) SCl2, , (d) S2Cl2, , 16. Gaseous products formed when Zn react with dil., H2SO4 and conc. H2SO4 respectively:, (a) H2S, SO2, , (b) H2 and SO2, , (c) SO2 and H2, , (d) SO3, H2S, , 17. Which of the following process is not feasible, spontaneously?, (a) F2 + H2O " HF + O2, (b) Cl2 + H2O " HCl + HOCl, (c) Br2 + H2O " HBr + HOBr, (d) I2 + H2O " HI + HOI, 18. Molecular size of I-Cl and Br2 is nearly same but, boiling point of I-Cl is about 40°C higher than, Br2. This might be due to:, , (a) I–Cl bond is stronger than Br–Br–bond, (b) Ionisation energy of I < ionisation energy of, Br, (c) I-Cl is polar whereas Br2 is non polar, (d) Size of I > size of Br, 19. When chlorine is passed slow over dry slaked like, Ca(OH)2 at room temperature, the main product, is:, (a) CaCl2 only, , (b) CaOCl2, , (c) Ca(ClO2)2, , (d) Ca(OCl)2 only, , 20. Which of the following is not correct?, (a) Among halogens, radius ratio between iodine, and fluorine is maximum, (b) All halogens have weak X–X bond than X–X', bond in interhalogens, (c) Among interhalogen compounds maximum, number of atoms are present in iodine, fluroide., (d) Interhalogen compounds are more reactive, than halogen compounds., 21. Which of the following order is not correct ?, (a) F– < Cl– < Br– < I– reducing nature, (b) F– > Cl– > Br– > I– hydration energy, (c) Cl2 > F2 > Br2 > I2 bond dissociation energy, (d) F2 > Cl2 > Br2 > I2 reactivity, 22. What should be the correct statement with respect, to XeF5–?, (a) Central atom Xe has sp3d2 hybridisation, (b) It is square planar, (c) There are two non bonding electron pairs, one, above the plane and the other below the plane, (d) It is an odd electron species, 23. XeF4 act as fluoride acceptor with:, (a) PF5, , (b) SbF5, , (c) KF, , (d) All of these, , 24. What is not true for ozone?, (a) The two O—O bond lengths are not equal., (b) O—O bond order is between 1 and 2., (c) O—O—O angle is approximately 117°., (d) It is light blue gas with pungent odour., 25. The formation of which of the substance is known, as tailing of mercury?, (a) Hg2O, , (b) HgO, , (c) Hg2O3, , (d) Hg(NO3)2
Page 188 :
7.15, , 26. Heating of which of the following nitrate produces, a gaseous substance which is used as anaesthetic, in dental surgery?, (a) NH4NO2, (b) Pb(NO3)2, (c) NH4NO3, (d) NaNO3, 27. Which allotropic form of phosphorus is good, conductor of electricity?, (a) Yellow phosphorus, (b) Red phosphorus, (c) Black phosphorus, (d) None of these, 28. The inertness of nitrogen is due to:, (a) Its intermediate electronegativity., (b) High bond dissociation energy of nitrogennitrogen bond., (c) Stable configuration of N atom., (d) Small atomic size., 29. Which is not true for phosphorus?, (a) Phosphorus exists in different allotropic, forms., (b) Black phosphorus has layer type structure., (c) White phosphorus is less reactive than red, phosphrous., (d) White phosphorus exists in tetrahedral, molecular solid., 30. What is not true about N2O5?, (a) It is anhydride of HNO3., (b) In solid stable it exists as NO2+ NO3–., (c) It is structurally similar to P2O5., (d) It can be prepared by heating HNO3 over, P2O5., 31. Sulphur does not exist as S2 molecule because:, (a) it is less electronegative., (b) it is not able to constitute pp-pp bond., (c) it has ability to exhibit catenation., (d) of tendency to show variable oxidation states., 32. Among the oxo-acids of chlorine, the correct, order of increasing acid strength is:, (a) HClO4 < HClO < HClO2 < HClO3, (b) HClO3 < HClO2 < HClO4 < HClO, (c) HClO4 > HClO3 > HClO2 > HClO, (d) HClO4 < HClO3 < HClO2 < HClO, 33. The ease of liquification of noble gases decreases, in the order, , (a), (b), (c), (d), , Xe > Kr > Ar > Ne > He, He > Ne > Ar > Kr > Xe, Xe > Ar > Kr > Ne > He, Xe > He > Kr > Ar > Ne, , 34. XeF6 on complete hydrolysis gives:, (a) XeO4, , (b) XeOF2, , (c) XeOF4, , (d) XeO3, , 35. XeF6 on reaction with CsF gives:, (a) [XeF5]+ [CsF2]–, (b) XeF8, (c) [XeF4]2+ [CsF3]2–, (d) Cs+[XeF7]–, , ONE OR MORE THAN ONE OPTIONS CORRECT, TYPE, 1. Select the correct statement(s), (a) Graphite is diamagnetic and diamond is, paramagnetic in nature., (b) Graphite acts as a metallic conductor along, the layers of carbon atoms., (c) Graphite is less denser than diamond., (d) C60 is called as Buckminster fullerene., 2. Borax bead test is given by:, (a) An aluminium salt, , (b) A cobalt salt, , (c) A copper (II) salt, , (d) A nickel salt, , 3. Which of the following species exists:, (a) [BF6]3–, , (b) [AlF6]3–, , (c) [GaF6]3–, , (d) [lnF6]3–, , 4. A complex cross-linked polymer (silicone) is, formed by:, (a) Hydrolysis of (CH3)3SiCl, (b) Hydrolysis of a mixture of (CH3)3SiCl and, (CH3)2SiCl2, (c) Hydrolysis of CH3SiCl3, (d) Hydrolysis of SiCl4, 5. White phosphorus be removed from, phosphorus by:, (a) Sublimation under reduced pressure, (b) Dissolving in water, (c) Dissolving in CS2, (d) Heating with an alkali solution, , red
Page 189 :
7.16, , 6. A gas is obtained on heating ammonium nitrate., Which of the following statements are incorrect, about this gas?, (a) Causes laughter, (b) Brings tears to the eyes, (c) Is acidic in nature, (d) Is basic in nature, 7. Which of the following represents correct, dissociation of nitrate salts on heating?, (a) 2LiNO3, , 1, O, 2 2, 1, PbO + 2NO2 + O2, 2, , Li2O + 2NO2 +, , (b) Pb(NO3)2, (c) NH4NO3, , N2O + 2H2O, , (d) NH4NO2, , N2 + 2H2O, , 8. Which of the following is/are correct regarding, nitrogen family?, (a) Nitrogen is restricted to a maximum, covalency of 4 as only four orbitals are, available for bonding., (b) The single N–N bond is weaker than the, single P–P bond., (c) The catenation tendency is weaker in nitrogen, as compared to phosphorous., (d) Nitrogen forms pp-pp bond as well as pp-dp, bonds., (a) H2SO4, , (b) HNO3, , (c) HClO4, , (d) HPO3, , 11. In the reaction 2Br– + X2, are:, , Br2 + 2X–, X2 is/, , (b) F2, (d) N2, , 12 Among the following which reactions are possible, (a) F2 + H2O, , HF + O2, , (b) Cl2 + H2O, , HCl + HClO, , (c) Br2 + H2O, , HBr + HBrO, , (d) I2 + H2O, , (b) IF5, , (c) FCl3, , (d) BrF5, , PASSAGE-BASED QUESTIONS, Passage # 1 (Q. 14 and 15), Species having X—O—H linkage (X = non metal with, positive oxidation state) are called oxy acids and parent, acid of a non-metal may exist in two form (a) –ic form of, parent oxy acid (b) -us form of parent oxy acid., 14. Which of the following parent oxy acid does not, have its pyro-oxy acid?, (a) H2SO3, , (b) HNO3, , (c) H3PO3, , (d) H4SiO4, , 15. X—O—X bond (where X = central atom) is not, present in species., (a) Cl2O7, , (b) H2N2O2, , (c) N2O5, , (d) H2S2O7, , Passage # 2 (Q. 16 and 17), The property of hydrides of p-block elements mostly, depends on:, (i) electronegativity difference between central, atom and hydrogen,, (iii) number of valence electrons in central atom., , 10. Which statements are correct about halogen?, (a) They are all diatomic and form univalent ions., (b) Halogen have the smallest atomic radii in, there respective periods., (c) They are all diatomic and form diatomic ions., (d) They are all reducing agents., , (c) I2, , (a) ClF3, , (ii) size of central atom, and, , 9. P2O5 can dehydrate:, , (a) Cl2, , 13. Which of the following inter-halogen compounds, is/are possible?, , Hl + HIO, , Some undergo hydrolysis in which central atom is, less electronegative, react with OH– to give hydrogen., While acidic property of hydride in a period depends on, electronegativity of central atoms, i.e. more electronegative, the atom, more acidic is the hydride. In a group, acidic, property is proportional to size of the central atom. Some, electron deficient hydrides behave as Lewis acid while, only one hydride of an element in p-block behaves as, Lewis base with lone pair of electrons. Hydrides in which, central atom’s electronegativity is close to hydrogen has, no reaction with water., 16. Which one is the weakest acid among the, following?, (a) HF, (c) HBr, , (b) HCl, (d) HI, , 17. Which one is strongest base?, (a) OH–, , (b) HS–, , (c) HSe–, , (d) HTe–
Page 190 :
7.17, , Passage # 3 (Q. 18 and 19), An orange solid (A) on heating gives a green residue (B),, a colourless gas (C) and water vapours. The dry gas (C), on passing over heated Mg gave a white solid (D), (D) on, reaction with water gave a gas (E) which formed black, precipitate with mercurous nitrate solution., 18. Select the incorrect statement., (a) The central atom of the anion of solid (A) has, d3s hybridisation., (b) The orange solid (A) is diamagnetic in nature., (c) The anion of orange solid (A) is oxidising in, nature., (d) All metal oxygen bond lengths are equal in, anion of solid (A)., 19. Which of the following is false for the gas (E)?, (a) It gives a deep blue colouration with CuSO4, solution., (b) It is oxidised to a colourless gas (neutral, oxide) at 1200 K in presence of a catalyst Pt/, Rh in air., (c) It gives the same gas (C) with potassium, permanganate solution., (d) It gives black precipitate with HgCl2., INTEGER VALUE TYPE QUESTIONS, 20. Consider a prototypical fullerence C60., Let, a = Number of 5-membered rings;, b = Number of 6-membered rings;, c = Number of p-bonds in C60., , 23. What is the number of oxygen atoms which are, shared between tetrahedrons in Si3O96– ?, 24. How many of the following properties increase, down the group for nitrogen family?, (a) Atomic size, (b) Acidic character of oxides, (c) Boiling point of hydrides, (d) Reducing power of hydrides, (e) Extent of pp-pp overlap, (f) Metallic character, 25. Number of gaseous oxides among the following, at room temperature is:, (a) N2O, (b) NO, (c) N2O3, (d) NO2, (e) N2O5, (f) P4O6, , (g) P4O10, (h) SO2, (i) SO3, , 26. The number of mixed anhydride among the, following are:, Cl2O; ClO2; Cl2O6; Cl2O7; N2O5; NO2; N2O, 27. How many ion in following behave like, pseudohalide?, CN–, SCN–, I3–, O–2, N3–, CNO–, S2O3–2, C2O4–2, MATCH THE COLUMN TYPE QUESTIONS, 28., Column I, , Find the value of (3a – 2b + c), 21. Central atom may exhibit sp3 hybridisation in, how many of the following species:, (a) CO2, , (b) Graphite, , (c) Diamond, , (d) CO, , (e) H3BO3(aq), , (f) Zeolites (Si-central), , (g) Silicones (Si), , (h) Chlorosilane (Si), , (i) Borax (Boron), , (j) Al2Cl6, , (k) B2H6, , , (l) SiO2(solid), , (m) H2CO3, , (n) COCl2, , (o) CH4, , , (p) CCl4, , Column II, , (A) Hypo phosphoric, acid, , (P), , All hydrogen, are ionizable in, water, , (B) Pyro phosphorus, acid, , (Q), , Lewis acid, , (C) Boric acid, , (R), , Monobasic in, water, , (D) Hypo phosphrous, acid, , (S), , sp3 hybridized, central atom, , 29., Column I, Silicates, , Column II, Number of oxygen atoms, shared per tetrahedron, , 22. Which of the following compounds are amphoteric, in nature?, , (A), , Ortho silicate, , (P), , 4, , (B), , Pyro silicate, , (Q), , 1, , PbO, PbO2, SnO, SnO2, Al2O3, ZnO, BeO, Ga2O3,, B2O3., , (C), , Cyclic silicate, , (R), , 0, , (D), , 3-D silicate, , (S), , 2
Page 191 :
7.18, , 30. Match the reactions island in column-I with, characteristic(s) type of reactions listed in, column-II., Column-I, (A), , Moist, Air, , PCl5 , →, , Column-II, (P), , Hydrolysis, , (B) P4 + NaOH(conc.) + H2O (Q), Warm, , →, , At least one of, the products has, tetrahedral geometry, , 200° C, , (C) H3PO3 , →, , (R), , Disproportionation, , (D), , C, P4O6 + H2O 200°, , →, , (S), , At least one of the, products has pp-dp, bonding, , 6. Amongst H2O, H2S, H2Se and H2Te, the one with, the highest boiling point is:, (a) H2O because of hydrogen bonding, (b) H2O because of higher molecular weight, (c) H2S because of hydrogen bonding, (d) H2Se becuase of lower molecular weight, [IIT-2000], 7. The number of P—O—P bonds in cyclic, metaphosphoric acid is:, (a) zero, (b) two, (c) three, (d) four, [IIT-2000], 8. The number of S—S bonds in sulphur trioxide, trimer, (S3O9) is:, (a) three, (b) two, (c) one, (d) zero, [IIT-2001], , 1. KF combines with HF to form KHF2. The, compound contains the species:, (a) K+, F– and H+, (b) K+, F– and HF, (c) K+ and [HF2]–, (d) [KHF]+ and F–, [IIT-1996], 2. The following acids have been arranged in the, order of decreasing acidic strength. Identify the, correct order., ClOH (I), BrOH (II), IOH (III), (a) I > II > III, , (b) II > I > III, , (c) III > II >I, , (d) I > III > II, [IIT-1996], , 3. Which one of the following species is not a, pseudo halide?, (a) CNO–, , (b) RCOO–, , (c) OCN–, , (d) NNN–, [IIT-1997], , 4. In compounds of type ECl3, where E = B, P, As or, Bi, the angles Cl — E — Cl for different E are in, the order:, (a) B > P = As = Bi, (b) B > P > As > Bi, (c) B < P = As = Bi, (d) B < P < As < Bi, [IIT-1999], 5. Ammonia can be dried by:, , 9. The set with correct order of acidic strength is:, (a) HClO < HClO2 < HClO3 < HClO4, (b) HClO4 < HClO3 < HClO2 < HClO, (c) HClO < HClO4 > HClO3 > HClO2, (d) HClO4 < HClO2 < HClO3 < HClO, [IIT-2001], ASSERTION AND REASON TYPE QUESTIONS, (Q. 10 and 11), Read the following questions and answer as per, the direction given below:, (a) Statement I is correct; Statement II is correct, Statement II is the correct explanation of, Statement I., (b) Statement I is correct; Statement II is correct, Statement II is not the correct explanation of, Statement I., (c) Statement I is correct; Statement II is incorrect., (d) Statement I is incorrect; Statement II is, correct., 10. Statement I: Pb4+ compounds are stronger, oxidzing agents than Sn2+ compounds., , Statement II: The higher oxidation states for the, group 14 elements are more stable for the heavier, members of the group due to ‘inert pair effect’., [IIT-2008], 11. Statement I: Between SiCl4 and CCl4, only SiCl4, reacts with water., , (a) conc. H2SO4, , (b) P4O10, , (c) CaO, , (d) anhydrous CaCl2, [IIT-2000], , , Statement II: SiCl4 is ionic and CCl4 is covalent, [IIT-2001]
Page 192 :
7.19, , 12. H3BO3 is:, (a) monobasic acid and weak Lewis acid, (b) monobasic and weak Bronsted acid, (c) monobasic and strong Lewis acid, (d) tribasic and weak Lewis acid, [IIT-2003], 13. Me2SiCl2 on hydrolysis will produce:, (a) (Me)2Si(OH)2, (b) (Me)2 Si = O, (c) [—O—(Me)2Si — O—]n, (d) Me2SiCl(OH), , 20. A pale blue liquid obtained by equimolar mixture, of two gases at –30°C is, (a) N2O, (b) N2O3, (c) N2O4, (d) N2O5, [IIT - 2005], 21. Which of the following allotropes of phosphorus, is thermodynamically most stable?, (a) Red, (b) White, (c) Black, (d) Yellow, [IIT-2005], , [IIT-2003], , 14. For H3PO3 and H3PO4, the correct choice is:, (a) H3PO3 is dibasic and reducing, (b) H3PO3 is dibasic and non reducing, (c) H3PO4 is triabsic and reducing, (d) H3PO3 is tribasic and non reducing, [IIT-2003], , 22. B(OH)3 + NaOH NaBO2 + Na[B(OH)4] + H2O, How can this reaction be made to proceed in the, forward direction?, (a) Addition of cis 1, 2-diol, (b) Addition of borax, (c) Addition of trans 1, 2-diol, (d) Addition of Na2HPO4, [IIT-2006], , 15. When I– is oxidised by KMnO4 in alkaline, medium, I– converts into:, (a) IO–3, , (b) I2, , (c) IO–4, , (d) IO–, , Read the following questions and answer as per, the direction given below:, [IIT-2004], , 16. Which of the following has —O—O— linkage?, (a) H2S2O6, , (b) H2S2O8, , (c) H2S2O3, , (d) H2S4O6, , 17. Name the structure of silicates in which three, oxygen atoms of [SiO4]4– are shared:, (a) pyrosilicate, (b) sheet silicate, , (a) Statement I is correct; Statement II is correct., Statement II is the correct explanation of, Statement I., (b) Statement I is correct; Statement II is correct., Statement II is not the correct explanation of, Statement I., (c) Statement I is correct; Statement II is, incorrect., (d) Statement I is incorrect; Statement II is, correct., 23. Statement I : Boron always forms covalent bond., , (c) linear chain silicate, (d) three-dimensional silicate, [IIT-2005], , , Statement II : The small size of B3+ favours, formation of covalent bond., [IIT-2007], , [IIT - 2005], , 24. Statement I : In water, orthoboric acid behaves, as a weak monobasic acid., , In water, orthoboric acid acts as a, proton donor., [IIT-2007], , 19. Which gas is evolved when PbO2 is treated with, concentrated HNO3?, (a) NO2, (b) O2, (c) N2, (d) N2O, [IIT - 2005], , 25. The percentage of p-character in the orbitals, forming P—P bonds in P4 is:, (a) 25, (b) 33, (c) 50, (d) 75, [IIT - 2007], , 18. Which of the following is not oxidised by O3?, (a) KI, , (b) FeSO4, , (c) KMnO4, , (d) K2MnO4
Page 193 :
7.20, , (c) moist O2, (d) O2 in the presence of aqueous NaOH, There are some deposits of nitrates and phosphates in, earth’s crust. Nitrates are more soluble in water. Nitrates, are difficult to reduce under the laboratory conditions but, microbes to it easily. Ammonia forms large number of, complexes with transition metal ions. Hybridisation easily, explains the case of sigma donation capability of NH3 and, PH3. Phosphine is a flammable gas and is prepared from, white phosphorus., [IIT-2008], 26. Among the following, the correct statement is:, (a) Phosphates have no biological significance in, humans., (b) Between nitrates and phosphates, phosphates, are less abundant in earth’s crust., (c) Between nitrates and phosphates, nitrates are, less abundant in earth’s crust., (d) Oxidation of nitrates is possible in soil., 27. Among the following, the correct statement is:, (a) Between NH3 and PH3, NH3 is a better, electron donor because the lone pair of, electrons occupies spherical ‘s’-orbital and is, less directional., (b) Between NH3 and PH3, PH3 is a better, electron donor because the lone pair of, electrons occupies sp3 orbital and is more, directional., (c) Between NH3 and PH3, NH3 is a better, electron donor because the lone pair of, electrons occupies sp3 orbital and is more, directional., (d) Between NH3 and PH3, PH3 is a better, electron donor because the lone pair of, electrons occupies spherical s-orbital and is, less directional., 28. White phosphorous on reaction with NaOH gives, PH3 as one of the products. This is a:, (a) dimerisation reaction, (b) disproportination reaction, (c) condensation reaction, (d) precipitation reaction, 29. The reaction of P4 with X leads selectively to, P4O6. The X, is:, (a) dry O2, (b) a mixture of O2 and N2, , [IIT - 2009], 30. Extra pure N2 can be obtained by heating:, (a) NH3 with CuO (b) NH4NO3, (c) (NH4)2Cr2O7, , (d) Ba(N3)2, , [IIT - 2011], , 31. The reaction of white phosphorus with aqueous, NaOH gives phosphine along with another, phosphrous containing compound. The reaction, type, the oxidation states of phosphorus in, phosphine and the other product respectively are:, (a) redox reaction, –3 and –5, (b) redox reaction, +3 and +5, (c) disproportionation reaction, –3 and + 1, (d) disproportionation reaction, –3 and + 3, 32. Which ordering of compounds is according to, the decreasing order of the oxidation state of, nitrogen?, (a) HNO3, NO, NH4Cl, N2, (b) HNO3, NO, N2, NH4Cl, (c) HNO3, NH4Cl, NO, N2, (d) NO, HNO3, NH4Cl, N2, 33. The shape of XeO2F2 molecule is:, (a) trigonal bipyramidal, (b) square planar, (c) tetrahedral, (d) see-saw, [IIT-2012], The reactions of Cl2 gas with cold-dilute and hotconcentrated NaOH in water give sodium salts of two, (different) oxoacids of chlorine, P and Q, respectively. The, Cl2 gas reacts with SO2 gas in the presence of charcoal,, to give a product R.R reacts with white phosphorus to, give a compound S. On hydrolysis, S gives an oxoacid of, phosphorus T., 34., , , , , , P and Q respectively, are the sodium salts of:, (a) hypochlorous and chloric acids, (b) hypochlorous and chlorous acids, (c) chloric and perchloric acids, (d) chloric and hypochlorous acids, [JEE Advanced - 2013]
Page 194 :
7.21, , 35. R, S and T, respectively, are:, (a) SO2Cl2, PCl5 and H3PO4, , 39. Which of the following properties in not shown, by NO?, (a) It is paramagnetic in liquid state., , (b) SO2Cl2, PCl3 and H3PO3, , (b) It is neutral oxide., , (c) SOCl2, PCl3 and H3PO2, (d) SOCl2, PCl5 and H3PO4, 36. The product formed in the reaction of SOCl2 with, white phosphorus is:, (a) PCl3, , (b) SO2Cl2, , (c) SCl2, , (d) POCl2, [JEE Advanced - 2014], , 37. Concentrated nitric acid upon long standing, turns, yellow-brown due to the formation of:, (a) NO, , (b) NO2, , (c) N2O, , (d) N2O4, , (c) It combines with oxygen to form nitrogen, dioxide., (d) Its bond order is 2.5., [JEE Main - 2014], 40. Among the following oxacids, the correct, decreasing order of acidic strength is, (a) HOCl > HClO2 > HClO3 > HClO4, (b) HClO4 > HOCl > HClO2 > HClO3, (c) HClO4 > HClO3 > HClO2 > HOCl, (d) HClO2 > HClO4 > HClO3 > HOCl, , [JEE Advanced - 2014], , [JEE-Main - 2014], 41. Which among the following is the most reactive?, , 38. Which of the following is the wrong statement?, (a) ONCl and ONO– are not isoelectronic, , (a) Cl2, , (b) Br2, , (b) O3 molecule is bent, , (c) I2, , (d) ICl, [JEE-Main - 2015], , (c) Ozone is violet-black in solid state, , 42. Which one has highest boiling point?, , (d) Ozone is diamagnetic gas, [JEE Main - 2013], , (a) He, , (b) Ne, , (c) Kr, , (d) Xe, [JEE-Main - 2015], , Answer Key, , 1. (a), 11. (a), 21. (d), 31. (a), , 2. (a), 12. (d), 22. (a), 32. (c), , 3. (d), 13. (a), 23. (a), 33. (a), , 4. (b), 14. (d), 24. (a), 34. (b), , 5. (c), 15. (c), 25. (a), 35. (b), , 6. (b), 16. (d), 26. (b), , 7. (c), 17. (d), 27. (c), , 8. (c), 18. (b), 28. (d), , 9. (b), 19. (a), 29. (d), , 10. (b), 20. (b), 30. (c), , 1. (c), 11. (a), 21. (c), 31. (b), , 2. (c), 12. (b), 22. (c), 32. (c), , 3. (d), 13. (b), 23. (c), 33. (a), , 4. (c), 14. (a), 24. (a), 34. (d), , 5. (c), 15. (b), 25. (a), 35. (d), , 6. (b), 16. (b), 26. (c), , 7. (c), 17. (d), 27. (c), , 8. (b), 18. (c), 28. (b), , 9. (a), 19. (b), 29. (c), , 10. (a), 20. (b), 30. (c)
Page 195 :
7.22, , 1. (b, c, d) 2. (b, c, d), 3. (b, c, d), 4. (c), 5. (a, c, d), 9. (a, b, c) 10. (a, b), 11. (a, b), 12. (a, b, c), 13. (a, b, d), 17. (a), 18. (d), 19. (d), 20. (26), 21. (11), 25. (5), 26. (3), 27. (4), 28. A, P, S; B, S; C Q, R; D R,S, 29. A R; B Q; C S; D P, 30. A, P, Q, S; B P, Q, R, S; C Q, R, S; D P, Q, R, S, , 1. (c), 11. (c), 21. (c), 31. (c), 41. (d), , 2. (a), 12. (a), 22. (a), 32. (b), 42. (d), , 3. (b), 13. (c), 23. (a), 33. (d), , 4. (b), 14. (a), 24. (a), 34. (a), , 5. (c), 15. (a), 25. (d), 35. (a), , 6. (a), 16. (b), 26. (c), 36. (a), , 6. (b, c, d) 7. (a, b, c, d) 8. (a, b, c), 14. (b), 15. (b), 16. (a), 22. (8), 23. (3), 24. (3), , 7. (c), 17. (b), 27. (c), 37. (b), , 8. (d), 18. (c), 28. (b), 38. (*), , 9. (a), 19. (b), 29. (b), 39. (a), , 10. (c), 20. (b), 30. (d), 40. (c), , (*) No answer , , Hints and Solutions, P4 + excess Cl2, SO, 1. (a) Borax H, → H3BO3 ∆, → B2O3 Al, →, 2, , 4, , B, , , , ( Boron ), , 2. (a) In borax, anion [B4O5(OH)4]–2 is present., OH, , O, , O, B, �, �, HO – B – O – B – OH, O, , B, , In this, hybridisation of B is sp2 and sp3., ν, 3. (d) CO + Cl2 h, → COCl2, , ( phosgene ), , 4. (b) H 2 C2 O 4 , → CO + CO 2, conc. H 2 SO4, , (A), , ( B), , CO is absorbed by ammonical cuprous chloride, while CO2 is absorbed by KOH., 5. (c) SiC is a covalent network solid., 6. (b) NH3 + O2 pt→ NO + H2O (ostwald process), 7. (c) NO is paramagnetic in gaseous state., 8. (c) P4 + Cl2, , PCl3(A), , PCl3 + H2O, , 10. (b) Pb(NO3)2 ∆, → PbO + NO2 + O2 , 11. (a) Single N–N bond is weaker than the single P–P, bond., O, O, , O, , ( Oxalic acid ), , PCl5 + H2O H3PO4 + HCl, 9. (b) Holme’s signal can be given by using (CaC2 +, Ca3P2), , 12. (d), , OH, , H3PO3 + HCl, , PCl5 (B), , N, , O, , N, , O, O, , 13. (a) Phosphine (PH3) is combustible due to presence, of P2H4., 14. (d) Extra pure N2 can be obtained by heating metal, azides Ba(N3)2., 15. (c) Ammoia can be dried by anhydrous CaO., 16. (d) 2Hg + O3, Hg2O + O2, 17. (d) Polythionic acid, H2SnO6, O, , , , O, , HO—S—(s)n–2 — S — OH, O, , O, , Total S–S bond = n – 1, Number of S with zero oxidation state = n – 2, Number of S with + 5 oxidation state = 2, Number of dp – pp bond = 4
Page 197 :
7.24, , 21. (c) Correct order of bond dissociation energy: Cl2 > Br2 > F2 > I2, 22. (c) Shape of XeF5– is pentagonal planar. Hybridisation, of Xe is sp3d3., 23. (c) XeF4 acts as fluoride acceptor with KF., 24. (a) Due to resonance both O—O bond lengths are, equal., 25. (a) 2Hg + O3, , Hg2O + O2, , 26. (c) NH4NO3 ∆, → N2O+ 2H2O, N2O acts as anaesthesia., 27. (c) Black phosphorus has layery structure. It behaves, as conductor of electricity., 28. (b) N2 is almost chemically inert. It is due to very, high bond dissociation energy of N/N., 29. (c) Order of reactivity :, White P > Red P > Black P, 30. (c) N2O5 has different structure from P2O5., P2O5(P4O10) has cage like structure., 31. (b) Third period elements can not form stable 3pp –, 3pp bond., 32. (c) Correct order of acidic strength is: HClO < HClO2 < HClO2 < HClO4, , CH3, |, HO—Si—OH, , |, OH, , , CH3, |, HO—Si—OH, |, OH, , OH, |, HO—Si—OH, |, CH3, , OH, |, HO—Si—OH, |, CH3, , CH3, CH3, |, |, ~ Si—O—Si—O ~, |, |, O, O, |, |, ~ Si—O—Si—O ~, |, |, CH3, CH3, Cross linked silicones, , In white phosphorus P4 molecules are attached, with weak vander waal’s force of attraction while, red phosphorus is polymeric. White phosphorus is, insoluble in water but soluble in organic solvents, like CS2. White phosphorus disproportionates in, alkali solution., NH4NO3 ∆, → N2O + 2H2O, N2O is also called as laughing gas. N2O is a, neutral oxide. It does not bring tears to the eyes., All decompositions are correct., Nitrogen is restricted to a maximum covalency, of 4 because in its valence shell only 4 orbitals, are available for bonding. N–N bond is weaker, than P—P bond hence, catenation tendency of P, is stronger than N., , 33. (a) The ease of liquification µ intermolecular bonding, order of case of liquification :, , N can not form p -d bonding., , Xe > Kr > Ar > Ne > He, , , P2O5 (P4O10) is an acidic dehydrating agent. It, dehydrate acidic substances., , 34. (d) XeF6 + 3H2O, , XeO3 + 6HF, , 35. (d) XeF6, +, CsF, Cs+[XeF7]–, , Lewis acid Lewis base, , LEVEL III, , 10. (a, b), , They (halogens), all are diatomic, form univalent, ions and are oxidizing agents., 11. (a, b) F2 and Cl2 can oxidize Br¯ into Br2, 12. (a, b, c), , 1. (b, c, d), , Both graphite and diamond are diamagnetic in, nature., Co+3, Cu+2 and Ni+2 give borax bead test., [BF6]–3 does not exist because due to unavailability, of d-orbital B can not form more than 4 bonds., CH3, |, Cl—Si—Cl +, |, Cl, , 9. (a, b, c), , 3H2O, , CH3, |, HO—Si—OH +, |, OH, , , I2 + H2O, , HI + HIO, , 13. (a, b, d), , F can not form 3 bonds. It can form only one, bond., 14. (b) To form pyro-oxy acid atleast two OH groups, must present in parent oxyacid., 15. (b) Structure of H2N2O2, OH, N, ||, N, , 3HCl, HO
Page 200 :
Chapter, , Key Concepts, Qualitative analysis involves the detection of basic radicals, (cations) and acidic radicals (anions) of a salt or a mixture, of salts., Acid + Base " Salt + H2O + Heat of Neutralisation, HCl + NaOH" NaCl + H2O + Heat, , , Chlorine gas smell, , Hypochlorites (ClO-), , Bitter almond smell, , Cyanides, , Physical appearance (Coloured substance):, Light pink, , Hydrated salt of Mn, , Reddish Pink, , Hydrated salt of Co(II), , Red, , HgO, HgI2, Pb3O4, , Orange – red, , Sb2S3, Some dichromates and, ferricyanides, , Reddish brown, , Fe2O3, , Dark brown, , PbO2, Bi2S3, CdO, Ag2O, CuCrO4, SnS, , Light yellow or brown, , Chromates, As2S3, As2S5, AgBr, AgI,, PbI2, CdS, SnS2, a few iodides and, ferrocyanides., , Green, , K2MnO4, Ni salts, hydrated ferrous, salts, some Cu (II) Compound, , , " Borax bead test, , Dark green, , Salt of Cr(III), , , " Charcoal cavity test, , Blue, , Hydrated CuSO4, anhydrous CoSO4, , , " Cobalt nitrate test, , Black, , Sulphides of Ag+, Cu+, Cu+2, Fe+2, Ni+2,, Co+2, Hg+2, and Pb+2. MnO2, Fe3O4,, FeO, CuO, Co3O4, Ni2O3, , , Na+ , Cl(Basic radical because (Acidic radical because it, it comes from base), comes from acid), The systematic procedure for qualitative analysis is:, Preliminary tests:, , " Physical appearance (Colour and smell), , " Dry heating test, , " Flame test, , Wet tests for acidic radicals, Wet tests for basic radicals, Physical appearance (Smell):, , Physical appearance (Solution is coloured):, Green or blue, , Ni+2, Fe+2, Cr+3 and Cu+2, , Smell, , Inference, , Pink, , Co+2 and Mn+2, , Ammonical Smell, , NH4+, , Yellow, , CrO42-, Fe+3, [Fe (CN) 6]4-, , Vinegar like Smell, , CH3COO-, , Orange, , Dichromates, , Smell like that of rotten eggs, , S-2, , Purple, , Permanganates
Page 201 :
8.2, , Dry heating:, 1., , Observation, , Inference, , Substance decrepitates (Crackling noise), , NaCl, KI,, Pb(NO3)2,, Ba(NO3)2, , 2., , Substance Melts (or, fuses), , Alkali metal, salts or salt, containing water, of crystallization, , 3., , Substance swells (due to loss of water of, crystallization), , Alums, borates, and Phosphates, , 4., , The substance Sublimes and the colour of, sublimate is:-, , 5., , (iii) Coloured gas, NO2 (Reddish brown, turns ferrous, sulphate solution brownish black), , NO2- or NO3-, , Br2 (Reddish brown turns starch paper, orange-red or yellow, turns starch, iodide paper blue), , Br-, , Cl2 (Greenish yellow, turns starch, Cliodide paper blue, bleaches moist, litmus paper, bleaches indigo solution), I-, , I2 (violet, turns starch paper blue), , a. White, , Hg2Cl2, NH4X,, AlCl3, HgCl2,, As2O3,Sb2O3, , b. Yellow, , As2S3 and HgI2, (turns red when, rubbed with, glass rod), , c. Blue black and violet vapours, , Iodides, , Flame test: The chlorides of the metals are more volatile, as compared to other salts and these are prepared by mixing, the compounds with a little concentrated HCl. On heating, in a non- luminous Bunsen flame, they are volatilized and, impart a characteristic colour to the flame., , A residue (generally oxides) is left and, its colour is:, , Metal, , Colour of flame, , Li, , Crimson red, , Na, , Golden yellow, , K, , Violet/lilac, , White (Cold) and Yellow (Hot), , ZnO, , Rb, , Red violet, , Reddish brown (Hot); Yellow (Cold), , PbO, , Cs, , Blue violet, , HgO, Pb3O4, , Ca, , Brick red, , Black (Hot); Red brown (Cold), , Fe2O3, , Sr, , Crimson red, , Original salt Blue becomes White on, heating, , Hydrated CuSO4, , Ba, , Apple green, , c. Black (Hot); Red (Cold), , 6., , CH3COO-, , Acetic acid vapours (characteristic, vinegar like smell), , Gas is evolved:, , Ammonium, nitrate, , Borax bead test:, , CO3-2, , , , (i) Colourless and odourless, CO2 (Turns lime water milky), , C2O4-2, O2 (Rekindles a glowing splinter), , N2, , Nitrates,, permanganate,, Dichromate,, chlorate, Amonium, Nitrate, , (ii) Colourless gas with odour, NH3 (Characteristic smell, turns, NH4+, nesseler’s solution brown and turns red, litmus blue), H2S (Smell of rotten eggs, turns lead, acetate paper black), , S-2 or Hydrated, S-2, , SO2 (suffocating or irritating smell, of burning sulphur, turns acidified, K2Cr2O7 paper green), , SO32-, , HCl (Pungent smell, white fumes, with ammonia, white ppt with AgNO3, Solution), , Hydrated Cl-, , Na2B4O7.10H2O ∆, → Na2B4O7 ∆, → 2NaBO2 + B2O3, , , Colourless transparent, , glassy bead, , On heating with a coloured salt, the glassy bead forms a, coloured metaborate in oxidizing flame., Colour of bead, Metal, , Oxidising flame, , Reducing flame, , Hot, , Cold, , Hot, , Cold, , Cr, , Yellow, , Green, , Green, , Green, , Mn, , Violet, (Amethyst), , Amethyst, , Colourless, , Colourless, , Fe, , Yellowish, Brown, , Yellow, , Green, , Green, , Co, , Blue, , Blue, , Blue, , Blue, , Ni, , Violet, , ReddishBrown, , Grey, , Grey, , Cu, , Green, , Blue, , Colourless, , Red opaque
Page 212 :
8.13, , Exercise, 1. Which of the following salt on heating with conc., H2SO4 gives violet vapours?, Iodide salt, , Nitrate salt, , Sulphate salt, , Bromide salt, , 2. Salts of which of the following metal are white?, , Zinc, Chromium, , Cobalt, Fe, , 3. A glassy bead formed by heating borax on a, platinum wire loop is:, Sodium tetraborate, Sodium metaborate, Sodium metaborate and boric anhydride, Boric anhydride and sodium tetraborate, 4. An oxalate salt gives which of the following gas, in dry heating test:, , CO + CO2, Only CO, , Only CO2, Oxalic acid vapours, , 5. The salts of which of the following elements are, generally dark green coloured?, Chromium, , Copper(I), , Barium, , Cobalt, , 6. The chromyl chloride test is meant for which of, the following ion?, , Cl- ions, I- ions, , Both Cl- and Br- ions, Cl- and CrO42- ions, , 7. Which of the following gases turn lime water, milky?, (a) SO2, , (b) CO2, , (c) H2S, , (d) Both (a) and (b), , 8. Yellow ammonium sulphide solution can be used, for the separation of which of the following pair, of species?, , CuS and PbS, Bi2S3 and CuS, , PbS and Bi2S3, CdS and As2S3, , 9. Reddish-brown (chocolate) ppt. are formed by, mixing solutions containing respectively:, Cu2+ and [Fe(CN)6]4- ions, , Ba2+ and SO42- ions, Pb2+ and I- ions, Pb2+ and SO42- ions, 10. Which of the following gives black precipitate on, passing H2S through it?, Acidified zinc nitrate solution, Ammonical barium chloride solution, Magnesium nitrate solution, Copper nitrate solution, 11. All ammonium salts liberate ammonia gas when:, Heated with water, Heated with caustic soda, Heated with H2SO4, Heated with NaNO2, 12. Addition of solution containing C2O42- ions to an, aqueous solution containing Ba2+, Sr2+ and Ca2+, will precipitate., , Ca+2, Ba+2 and Sr2+, , Ca+2 and Sr2+, All three, , 13. Sodium sulphide react with sodium nitroprusside, to form a purple coloured Compound. During the, reaction the oxidation state of iron:, Changes from +2 to +3, Changes from +3 to +2, Changes from +2 to +4, Remains unchaged, 14. Which of the following sulphide is not soluble in, dil HNO3?, PbS, , HgS, , ZnS, , Bi2S3, , 2+, , 15. Cu ions will be reduced to Cu+ ion by addition, of an aqueous solution of:, (a) KF, , (b) KCl, , (c) KI, , (d) KOH, , 16. Precipitate of AgCl dissolves in liquid ammonia, due to the formation of:, [Ag(NH4)2]OH, [Ag(NH4)2]Cl, [Ag(NH3)2]OH, [Ag(NH3)2]Cl
Page 214 :
8.15, Lead, NH 4 CI, 2 O2, , →, , → (A) Na, 3. CrCl3 NH, , → (B) acetate, OH, H, O, 4, 2, (C) ; compound (C) is:, (a) Na2CrO4, (b) Na2Cr2O7, (c) Cr(OH)3, (d) PbCrO4, , 4. 2Cu2+ + 5I- $ 2CuI. + [X], , , , , [X] + 2S2O32-$3[Y] + S4O62(a) I3- and I(b) I2 and I3(c) I2 and I(d) I3- and I2, , ; X and Y are:, , 5. Which of the following reagents can separate a, mixture of AgCl and AgI?, (a) KCN, (b) Na2S2O3, (c) HNO3, (d) NH3, , (a) BeCO3, , (b) MgCO3, , (c) CaCO3, , (d) BaCO3, , 14. A + Na2CO3 $ B + C,, CO, , 2, A , → (Milky) C, , The chemical formula of A and B are respectively:, (a) NaOH and Ca(OH)2, (b) Ca(OH)2 and NaOH, (c) NaOH and CaO, (d) CaO and Ca(OH)2, 15. Which of the following salt on heating with, concentrated H2SO4, coloured vapours do not, evolve?, , 6. FeSO4 is used in the brown ring test for a nitrate., What is the oxidation state of Fe in the compound, responsible for the brown colour of the ring?, (a) 0, (b) +1, (c) + 2, (d) + 3, , (a) NaBr, , (b) NaNO3, , (c) CaF2, , (d) KI, , 7. In an alkaline solution, sodium nitroprusside, gives a violet colour with:, (a) S2(b) SO32 (c) SO42(d) NO2-, , (a) Ferric oxalate, , 8. Which of the following sulphides is white?, (a) CdS, (b) PbS, (c) ZnS, (d) SnS, 9. A white sublimate substance, that turns black on, treatment with an NH3 solution can be:, (a) Hg2Cl2, (b) HgCl2, (c) As2O3, (d) NH4Cl, 10. Which of the following pairs of cations can be, separated by adding NH4Cl and NH4OH to the, mixture and then passing H2S through it?, (a) Fe3+, Al3+, , (b) Cr3+, Ni2+, , (c) Al3+, Cr3+, , (d) Fe3+, Cr3+, , 11. Which of the following pairs of sulphides are, insoluble in dilute HCl?, (a) CoS and NiS, (b) CoS and MnS, (c) NiS and MnS (d) NiS and ZnS, 12. On heating, a salt gives a gas which turns lime, water milky and an acidified dichromate solution, green. The salt may be:, (a) carbonate, (b) sulphide, (c) sulphate, (d) sulphite, 13. Which of the following has the highest value of, Kp?, , 16. A salt made of bi-bivalent ions X and Y each, of which is capable of decolourising acidified, KMnO4. The salt is likely to be:, (b) Ferrous oxalate, , (c) Ferrous sulphate (d) Stannic chloride, 17. When concentrated H2SO4 is added to dry KNO3,, brown fumes are evolved. These fumes are due to:, (a) SO2, , (b) SO2 + SO3, , (c) NO, , (d) NO2, , 18. Freshly prepared chlorine water is added to the, aqueous solution of some halide salt containing, some CS2. After shaking the contents, a violet, colour appeared in CS2 layer. The halide ion in, solution is:, (a) Iodide, (b) Bromide, (c) Chloride, (d) Iodide as well as bromide., 19. For the confirmatory tests of acid radicals, sodium, carbonate extract is prepared because:, (a) All anions react with Na, (b) Na is more reactive, (c) Na2CO3 is water soluble, (d) Sodium salts of almost all anions are water, soluble., 20. In the precipitation of the radicals of iron group, in qualitative analysis, NH4Cl is added before, adding NH4OH. This causes:, (a) Decrease in the concentration of OH- ions, (b) Removal of PO43- ions, (c) Increase in the concentration of Cl- ions, (d) Increase in the concentration of NH4+ ions
Page 215 :
8.16, , 21. The aqueous solution of which of the following, reagent will give Prussian blue coloured ppt. with, an aqueous solution containing iron (III) ions?, (a) Potassium thiocyanate, (b) Potassium hexacyanoferrate (II), (c) Potassium pyroantimonate, (d) All of these, 22. Aqueous solution of salt A gives yellow precipitate, with aqueous solution of K2CrO4. which of the, following series of cation may be present in A?, (a) Pb2+, Ag+, , (b) Pb2+, Ba2+, , +, , (d) Hg2+, Ag+, , (c) Ag , Cu2+, , 23. The reagent that can distinguish between silver, and lead salt is:, (a) H2S gas, (b) Hot dilute HCl solution, (c) NH4Cl (solid) + NH4OH (solution), (d) NH4Cl (solid) + (NH4)2 CO3 (solution), 24. A yellow turbidity, sometimes appears on passing, H2S gas even in the absence of the second group, radicals. Explain why?, (a) Sulphur is present in the mixture as an, impurity, (b) The fourth group radicals are precipitated as, sulphides, (c) The H2S is oxidized by some acidic radical, present in solution, (d) The third group radicals are precipitated, , 27. On addition of aqueous NaOH to a salt solution,, a white gelatinous precipitate is formed, which, dissolves in excess alkali. The salt solution, contains:, (a) Chromous ions, (b) Aluminium ions, (c) Barium ions, (d) Iron ions, 28. Dimethyl glyoxime in a suitable solvent was, refluxed for 10 minutes with pure pieces of nickel, sheet, it will result in:, (a) Red precipitate, (b) Blue precipitate, (c) Yellow precipitate, (d) No precipitate, 29. A metal X on heating in nitrogen gas gives Y., Y on treatment with H2O gives a colourless gas, which when passed through CuSO4 solution gives, a blue colour. Y is:, (a) Mg(NO3)2, , (b) Mg3N2, , (c) NH3, , (d) MgO, , 30. A light green coloured salt (X) does not react, with dilute and conc. H2SO4. Its aqueous solution, becomes dark brown when sodium nitrite solution, is added to it. X can be:, (a) Some salt of Ni (b) Some salt of copper, (c) FeSO4, , (d) Unpredictable, , 25. Colourless salt (A), , , Conc. H2SO4, Cu, , A, HCI, , Brown fumes, Blue + metal C, solution, White ppt. soluble in NH4OH, , The salt A can be:, (a) Cu(NO3)2, , (b) AgBr, , (c) AgNO3, , (d) Pb(NO3)2, , 26. Al3+, Cr3+, Fe3+ are grouped together for, qualitative analysis because:, (a) Their carbonates are insoluble in ammonia, (b) Their hydroxides are insoluble in ammonia, (c) Their sulphides are insoluble in acid, (d) They belong to same group of periodic table, , ONE OR MORE THAN ONE CORRECT TYPE, 1. Which of the following salts release reddish, brown gas when heated in a dry test tube?, (a) LiNO3, , (b) KNO3, , (c) Pb(NO3)2, , (d) AgNO3, , 2. When Borax is heated it forms a colourless glassy, bead because of formation of :, (a) B2H6, , (b) NaBO2, , (c) B2O3, , (d) Na2B4O7, , 3. Which of the following metal chloride will give, chromyl chloride test ?, (a) NaCl, , (b) KCl, , (c) AgCl, , (d) SbCl3
Page 216 :
8.17, , 4. Which of the following statement(s) is/are correct, with respect to bromide ions ?, (a) KBr on heating with MnO2 and concentrated, H2SO4 liberates Br2 and SO2 gases., (b) KBr on heating with concentrated H2SO4, liberates Br2 and SO2 gases., (c) KBr forms HBr with concentrated H3PO4., (d) KBr(s) liberates Br2 on gentile warming with, concentrated H2SO4 and K2Cr2O7(s)., 5. KI solution is the reagent for :, (a) Hg2+, , (b) Pb2+, , (c) Ag+, , (d) Cu2+, , 6. Which of the following cations form(s) black, precipitate(s) with H2S(g)?, (a) Cu2+, , (b) Sb3+, , (c) Pb2+, , (d) Bi3+, , 7. Which of the following is/are correct for, potassium ferrocyanide?, (a) It gives a brown precipitate with Cu2+ ions., (b) It gives a white preciptate of mixed salt with, Ca2+ ions., (c) It in excess gives a bluish white/white, precipitate with Zn2+, , (d) It develops a deep red colouration with Fe3+., 8. The following can be used to regulate the, concentration of OH– ions for the scheme of basic, radical analysis (III group)., (a) NH4NO3, , (b) NH4Cl, , (c) (NH4)2SO4, , (d) (NH4)2CO3, , 9. Which of the following statement(s) is/are, correct?, (a) Nickel salts give rosy red precipitate with, dimethyl glyoxime in excess of NH4OH., (b) Fe(III) salts give red colour with potassium, sulphocyanide, (c) In nitroprusside, the iron and NO exist as, Fe(III) and NO., (d) Mn(II) salts give white precipitate with NaOH, which turns brown on adding Br2 water., 10. Which statement(s) is/are correct with reference, to the ferrous and ferric ions?, (a) Fe2+ gives brown colour with potassium, ferricyanide, (b) Fe2+ gives blue colour with potassium, ferricyanide, , (c) Fe3+ gives red colour with potassium, thiocyanate, (d) Fe2+ gives brown colour with potassium, thiocyanate, 11. Which of the following sulphates are soluble in, water ?, (a) CuSO4, (b) PbSO4, (c) Ag2SO4, (d) BaSO4, 12. Which of the following substances on being, heated will give a gas that turns lime water milky?, (a) Na2CO3, (b) ZnCO3, (c) ZnSO3, (d) MgCO3, 13. A yellow precipitate is obtained when :, (a) lead acetate solution is treated with K2CrO4, (b) Pb(NO3)2 solution is treated with K2CrO4, (c) AgNO3 solution treated with KI, (d) H2S is passed through a solution of CdSO4, 14. Which of the following species will be, decomposed on acidification?, (a) [Ag(NH3)2]+, (c) [Zn(OH)4]2–, , (b) [Cu(NH3)4]2+, (d) [Pb(OH)4]2–, , PASSAGE BASED QUESTIONS, Passage # 1 (Q. 15 to 17), A colourless inorganic compound (A) imparts a green, colour to the flame. Its solution gives a white ppt. (B) with, H2SO4. When heated with K2Cr2O7 and conc. H2SO4, a, brown red vapour/gas (C) is formed. The gas/vapour when, passed through aqueous NaOH solution, it turns into a, yellow solution (D) which forms yellow precipitate (E), with CH3COOH and (CH2COO)2Pb, 15. The colourless inorganic compound (A) is:, (a) Ba(NO3)2, , (b) BaCl2, , (c) CuCl2, , (d) CrBr3, , 16. The liberated gas vapour (C) is:, (a) Br2, , (b) NO2, , (c) CrO2Cl2, , (d) Cl2, , 17. The yellow ppt. formed when (D) reacts with, CH3COOH and (CH2COO)2 Pb is:, (a) PbI2, , (b) PbCrO4, , (c) BaCrO4, , (d) AgBr, , Passage # 2 (Q. 18 to 20), + Air, 2, 4, Black solid KOH, , , → (A) , → (B) + (C), ∆, , (green), (purple), H SO
Page 217 :
8.18, , (i) KI on reaction with alkaline solution of (B), changes into a compound (D)., (ii) The colour of the compound (B) disappears on, treatment with the acidic solution of FeSO4, (iii) With cold conc. H2SO4 compound (B) gives (E),, which being explosive decomposes to yield (F), and oxygen., 18. Nature of compound (E) is:, (a) Acidic oxide, (b) Basic oxide, (c) Amphoteric oxide, (d) Neutral oxide, 19. Colour of the solution obtained, when ferrous, sulphate reacts with acidic solution of (B):, (a) Colourless, , (b) Pink, , (c) Green, , (d) Yellow, , 20. Which of the following options is correct ?, (a) (C) and (F) are same compounds having same, colour., (b) (C) and (F) are different compounds having, same colour., (c) Compound (B) forms similar compound (E), with hot and conc. H2SO4., (d) Compound (A) does not give same type of, reaction in acidic and neutral medium., Passage # 3 (Q. 21 to 23), When a crystalline compound X is heated with K2Cr2O7, and concentrated H2SO4, a reddish brown gas A is, evolved. On passing A into caustic soda, a yellow solution, of B is formed. A yellow precipitate of C is obtained when, a solution of B is neutralised with acetic acid and then, treated with a lead acetate solution. When X is heated, with NaOH, a colourless gas is evolved which, when, passed into a solution of K2[HgI4], gives a reddish brown, precipitate of D., 21. Compound (X) is:, (a) NH4Br, , (b) NH4Cl, , (c) NH4NO2, , (d) NH4NO3, , 22. If the solution B is colourless, which of the, following ions would not be present in the solid, X?, (a) Cl–, (c), , NO3–, , (b) Br–, (d) NO2, , –, , 23. Which of the following is the composition of the, brown precipitate (D)?, (a) HgI2, , (b) Hg(NH2)I, , (c) HgO, , (d) HgO.Hg(NH2)I, , Passage # 4 (Q. 24 to 26), (i) A white solid mixture of two salts containing a, common cation is insoluble in water. It dissolved, in dilute HCl producing some gases (with, effervescence) that turns an acidified dichromate, solution green. After the gases are passed through, the acidified dichromate solution, the emerging, gas turns baryta water milky., (ii) On treatment with dilute HNO3, the white solid, gives a solution which does not directly give a, precipitate with a BaCl2 solution but gives a white, precipitate when warmed with H2O2 and then, treated with BaCl2 solution., (iii) The solution of the mixture in dilute HCl, when, treated with NH4Cl, NH4OH and an Na2HPO4, solution, gives a white precipitate., 24. The gases evolved in (i) are:, (a) CO2 and HCl, , (b) SO2 and CO2, , (c) SO2 and H2S, , (d) NH3 and CO2, , 25. The white precipitate obtained in (ii) indicates the, presence of a:, (a) carbonate, , (b) sulphide, , (c) sulphite, , (d) chloride, , 26. The white precipitate obtained in (iii) consists of:, (a) Ba3(PO4)2, , (b) Sr3(PO4)2, , (c) Ca3(PO4)2, , (d) MgNH4PO4.6H2O, , INTEGER VALUE TYPE QUESTIONS, 27. How many compounds liberate NH3 on heating, from the following?, (a) (NH4)2 SO4,, , (b) (NH4)2 CO3,, , (c) NH4Cl,, , (d) NH4NO3,, , (e) (NH4)2 Cr2O7, 28. How many of the following reactions give yellow, ppt., (a) NaBr + AgNO3 $, (b) NaI + AgNO3 $, (c) NaBr + Pb(NO3)2 $, (d) NaI + Pb(NO3)2 $
Page 219 :
8.20, , 5. In blue solution of copper sulphate excess of, KCN is added then solution becomes colourless, due to the formation of:, (a) [Cu(CN)4]2–, (b) Cu2+ get reduced to form [Cu(CN)4]3–, (c) Cu(CN)2, (d) CuCN, [IIT-2006], 6. MgSO4 + NH4OH + Na2HPO4 $ white, crystalline precipitate. The formula of crystalline, precipitate is:, (a) MgCl2. MgSO4 (b) MgSO4, (c) Mg(NH4)PO4 (d) Mg(PO4)2, , The compound Y is:, (a) MgCl2, (b) FeCl2, (c) FeCl3, (d) ZnCl2, The compound Z is:, (a) Mg2[Fe(CN)6] (b) Fe[Fe(CN)6], (c) Fe4[Fe(CN6]3 (d) K2Zn3[Fe(CN)6]2, , 12. The equilibrium, 2CuI E Cu0 + CII in aqueous, medium at 25°C shifts towards the left in the, presence of:, (a) NO3–, (b) Cl–, (c) SCN–, (d) CN–, [IIT-2011], , [IIT-2006], , 7. A solution of a metal ion when treated with KI, gives a red precipitate which dissolves in excess, KI to give a colourless solution. Moreover, the, solution of metal ion on treatment with a solution, of cobalt (II) thiocyanate gives rise to a deep blue, crystalline precipitate. The metal ion is:, (a) Pb2+, (c) Cu2+, , 10., , , 11., , , , (b) Hg2+, (d) Co2+, [IIT-2007], , 8. A solution of colourless salt H on boiling with, excess NaOH produces a non-flammable gas., The gas evolution ceases after some time. Upon, addition of Zn dust to the same solution, the gas, evolution restarts. The colourless salts(s) H is, (are):, (a) NH4NO3, (b) NH4NO2, (c) NH4Cl, (d) (NH4)2SO4, [IIT-2008], Passage # 1 (Q. 9 to 11), p-Amino-N, N-dimethylaniline is added to a strongly, acidic solution of X. The resulting solution is treated with a, few drops of aqueous solution of Y to yield blue coloration, due to the formation of methylene blue. Treatment of, the aqueous solution of Y with the reagent potassium, hexacyanoferrate(II) leads to the formation of an intense, blue precipitate. The precipitate dissolves on excess, additon of the reagent. Similarly, treatement of the solution, of Y with the solution of potassium hexacyanoferrate(III), leads to a brown colouration due to the formation of Z., [IIT-2009], 9. The compound X is:, (a) NaNO3, (b) NaCl, (c) Na2SO4, (d) Na2S, , Passage # 2 (Q. 13 to 15), When a metal rod M is dipped into an aqueous colourless, concentrated solution of compound N the solution turns, light blue. Addition of aqueous NaCl to the blue solution, gives a white precipitate O. Addition of aqueous NH3, dissolves O and give an intense blue solution., [IIT-2011], 13. The metal rod M is:, (a) Fe, (b) Cu, (c) Ni, (d) Co, 14. The compound N is:, (a) AgNO3, (b) Zn(NO3)2, (c) Al(NO3)3, (d) Pb(NO3)2, 15. The final solution contains:, (a) [Pb(NH3)4]2+ and [CoCl4]2–, (b) [Al(NH3)4]3+ and [Cu(NH3)4]2+, (c) [Ag(NH3)2]+ and [Cu(NH3)4]2+, , (d) [Ag(NH3)2]+ and [Ni(NH3)6]2+, 16. Passing H2S gas into a mixture of Mn2+, Ni2+,, Cu2+ and Hg2+ ions in an acidified aqueous, solution precipitates:, (a) CuS and HgS (b) MnS and CuS, (c) MnS and NiS (d) NiS and HgS, [IIT-2011], 17. For the given aqueous reaction which of the, statement(s) is (are) true?, excess KI + K3[Fe(CN)6], , dilute H2SO4, , brownish-yellow solution, ZnSO4, , (white precipitate + brownish – yellow filtrate), Na2S2O3, , colourless solution, , (a) The first reaction is a redox reaction, (b) White precipitate is Zn3[Fe(CN)6]2
Page 220 :
8.21, , (c) Addition of filerate to starch solution gives, blue colour, (d) White precipitates is soluble in NaOH, solution, [IIT-2012], 18. Concentrated nitric acid, upon long standing,, turns yellow-brown due to the formation of:, (a) NO, (b) NO2, (c) N2O, (d) N2O4, [JEE Advanced - 2013], 19. Upon treatment with ammoniacal H2S, the metal, ion that precipitates as a sulphide is:, (a) Fe(III), (b) Al(III), (c) Mg(II), (d) Zn(II), [JEE Advanced - 2013], Passage # 3 (Q. 20 and 21), An aqueous solution of a mixture of two inorganic salts,, when treated with dilute HCl, gave a precipitate (P) and a, filtrate (Q). The precipitate P was found to dissolve in hot, water. The filtrate (Q) remained unchanged, when treated, with H2S in a dilute mineral acid medium. However, it, gave a precipitate (R) with H2S in an ammonical medium., The precipitate (R) gave a coloured solution (s), when, treated with H2O2 in an aqueous NaOH medium., [JEE Advanced - 2013], 20. The precipitate P contains:, (a) Pb2+, (b) Hg22+, +, (c) Ag, (d) Hg2+, 21. The coloured solution S contains:, (a) Fe2(SO4)3, (b) CuSO4, (c) ZnSO4, (d) Na2CrO4, 22. Among PbS, CuS, HgS, MnS, Ag2S, NiS, CoS,, , Bi2S3 and SnS2, the total number of BLACK, coloured sulphide is [JEE Advanced - 2014], 23. The pair(s) of ions where BOTH the ions are, precipitated upon passing H2S gas in presence of, dilute HCl, is (are):, (a) Ba2+, Zn2+, (b) Bi3+, Fe3+, 2+, 2+, (c) Cu , Pb, (d) Hg2+, Bi3+, [JEE Advanced - 2015], 24. Which one of the following statement is correct?, (a) From a mixed precipitate of AgCl and AgI,, ammonia solution dissolves only AgCl., (b) Ferric ions gave a deep green precipitate on, adding potassium ferrocyanide solution, (c) On boiling a solution having K+, Ca2+, and HCO3– ions we get a precipitate of, K2Ca(CO3)2., (d) Manganese salts give a violet borax bead test, in the reducing flame, [AIEEE - 2013], 25. A red solid is insoluble in water. However it, becomes soluble if some KI is added to water., Heating the red solid in a test tube results in, liberation of some violet coloured fumes and, droplets of a metal appear on the cooler parts of, the test tube. The red solid is, (a) (NH4)2Cr2O7, (b) HgI2, (c) HgO, (d) Pb3O4, [AIEEE - 2003], 26. Which of the following compounds is not, coloured yellow?, (a) Zn2[Fe(CN)6], (b) K3[Co(NO2)6], (c) (NH4)3[As(Mo3O10)4] (d) BaCrO4, [JEE Main - 2015], , Answer Key, , 1. (a), 11. (b), 21. (b), , 2. (a), 12. (d), 22. (a), , 3. (c), 13. (d), 23. (a), , 4. (a), 14. (b), 24. (d), , 5. (a), 15. (c), 25. (c), , 6. (a), 16. (d), 26. (a), , 7. (d), 17. (c), 27. (c), , 8. (d), 18. (b), 28. (c), , 9. (a), 19. (c), 29. (b), , 10. (d), 20. (c), 30. (c), , 1. (c), 11. (a), 21. (b), , 2. (d), 12. (d), 22. (b), , 3. (d), 13. (a), 23. (b), , 4. (a), 14. (b), 24. (c), , 5. (d), 15. (c), 25. (c), , 6. (b), 16. (b), 26. (b), , 7. (a), 17. (d), 27. (b), , 8. (c), 18. (a), 28. (d), , 9. (a), 19. (d), 29. (b), , 10. (b), 20. (a), 30. (c)
Page 221 :
8.22, , , , 1. (a, c, d), 2. (b, c), 3. (a,b), 4. (b,c,d ) 5. (a, b, c, d) 6. (a, c, d) 7. (a, b, c) 8. (a, b), 9. (a, b, d) 10. (b,c), 11. (a, c) 12. (b, c, d) 13. (a,b, c, d) 14. (a, b, c, d) 15. (b), 16. (c), 17. (b), 18. (a), 19. (d), 20. (a), 21. (b), 22. (a), 23. (d), 24. (b) , 25. (c), 26. (d), 27. (3), 28. (7), 29. (3), 30. (34 ), 31. (3), 32. A " a,b,c,d; B " a; C " a,b,c; D " b,c,d, 33. A " d; B " c; C " b; D " a, 34. A " a,c; B " a,b; C " a,d; D " b,d, , 1. (a), 2. (a), 11. (b) 12. (b, c, d), 21. (d) 22. (7), , 3. (a), 13. (b), 23. (c, d), , 4. (b), 14. (a), 24. (a), , 5. (b), 15. (c), 25. (b), , 6. (c), 16. (a), 26. (a), , 7. (b), 8. (a, b) 9. (d), 17. (a, c, d) 18. (b), 19. (d), , 10. (c), 20. (a), , Hints and Solutions, 13., (a) I- ions are oxidized by H2SO4 to violet coloured, I2., (a) Due to electronic configuration of Zn+2 is [Ar], 4s03d10, salts of zinc are white (colourless)., heating, (c) Na2 B4 O7. 10H2O Strong, , →, , , , 2NaBO2 B2 O3 + 10H2O, �������, galssy bead, , (a) Dry heating of oxalate slats give CO and CO2., (a) Chromium salts are in general green in colour., (a) Chromyl chloride test is applied for the, detection of Cl- ion., (d) Both CO2 and SO2 turn limewater (Ca(OH)2), milky., Ca(OH)2 + CO2 " CaCO3. + H2O, Ca(OH)2 + SO2 " CaSO3. + H2O, , , 8., , White, , (d) As2S3 is soluble in YAS (yellow ammonium, sulphide) whereas CdS is not., , 9., (a) 2Cu2+ + [Fe(CN)6]4- " Cu2 [Fe(CN)6], , Chocolate ppt., 10., (d) Cu(NO3)2 + H2S " CuS. + 2HNO3, Black, , +, 11., (b) NH4 + NaOH " NH3 + H2O + Na+, 12., (d) All the three ions will precipitate as their, respective oxalates., , 2, , (d) Na2S + Na2 [ Fe (CN)5NO] " Na4, 2, , Sodium nitroprusside, , [ Fe (CN)5(NOS)], There is no change in oxidation state of Fe., 14., (b) HgS is not soluble in dil. HNO3. HgS is soluble, in aqua regia., 15., (c) I- ion acts as good reducing agent., 2Cu2+ + 4I- " Cu2I2 +I2, 16., (d) AgCl + NH3 " [Ag(NH3)2]Cl, Soluble complex, , , 17., , (c) BaCrO4 is precipitated first., , 18., , (b) Brown ring test is used to detect nitrate ion., , 19., , (c) I2 + S2O3-2 " I- + S4O6-2, , 20., (c) NaCl + K2Cr2O7 + H2SO4 " NaHSO4 +, , KHSO4 + H2O + CrO2Cl2, CrO2Cl2 is chromyl chloride., 21., (b) FeCl3 + KCNS " Fe(SCN)Cl2 + KCl, , , Blood red, , 22., (a) H3BO3 + 3MeOH " B(OMe)3 + 3H2O, Methyl borate, B(OMe)3 burns with green, flame., 23., (a) Ba+2 + K2CrO4 " BaCrO4. + 2K+, Yellow, , AgNO3 + Br " AgBr. + NO3 , , 24., , yellow, , (d) All three Zn+2, Cd+2 and Cu+2 form precipitate, with H2S but ZnS is white and