Page 1 :
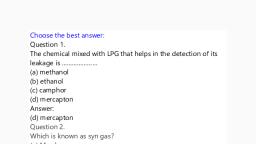
Green Chemistry and Alternative Energy Resources, By Dr. Prasad Puthiyillam, For Video Class Visit Youtube Channel: My Intuition, , 3. Green Fuel, 3.1 Photosynthesis, In biological H2 production, the electrons produced by the photo-system are taken, up by hydrogenases, resulting in the formation of hydrogen. A bioreactor produces this, bio-hydrogen. Figure given below represents a natural photosynthesis., , The hydrogen produced in this process is used for carbohydrate production., By biomimetics an artificial photosynthesis system are introduced., Artificial photosynthesis is also known as, •, , photocatalytic water splitting, and, , •, , photoelectrocatalytic water splitting., , 3.3 Hydrogen Production by Photocatalytic Water Splitting, Splitting of water to get hydrogen by using a photocatalyst and by using solar, energy is called photocatalytic water splitting., This process can be more efficient if the photocatalyst is directly suspended in, water so that the reaction takes place in one step. Figure given below represents a, photocatalyst with a cocatalyst (catalyst nanoparticles)., , 1
Page 2 :
Green Chemistry and Alternative Energy Resources, By Dr. Prasad Puthiyillam, For Video Class Visit Youtube Channel: My Intuition, , Figure: Representation of a photocatalytic water splitting, The light energy excites an electron in the semiconducting photocatalytic material., Thus produced hole (h+) will react with the neighbouring water molecule., H2O (l) + [hn] + 2 h+ ® 2 H+ (aq.) + ½ O2 (g), The H+ ions combine to produce hydrogen at the surface of the cocatalyst., 2 H+ + 2 e- ® H2 (g), The produced hydrogen can be used as the fuel in hydrogen fuel cell., , 3.2 Hydrogen Production by Photo-Electro Catalytic Water Splitting, Photoelectrocatalytic (PEC) water splitting is one of the most promising artificial, photosynthesis approaches for solar fuel production., PEC water splitting involves photogenerated carriers on the surface and reactants, adsorbed on the surface or in the electrolyte. The reaction takes place at the, electrode−electrolyte interface., , Figure: Schematic representation of a typical photoelectrochemical cell, , 2
Page 3 :
Green Chemistry and Alternative Energy Resources, By Dr. Prasad Puthiyillam, For Video Class Visit Youtube Channel: My Intuition, The light energy excites an electron in the semiconducting material (electrode)., Thus produced hole (h+) will react with the neighbouring water molecule., H2O (l) + [hn] + 2 h+ ® 2 H+ (aq.) + ½ O2 (g), These H+ ions formed will then bond with one other proton and combine with two, electrons to form hydrogen gas., 2 H+ + 2 e- ® H2 (g), The produced hydrogen can be used as the fuel in hydrogen fuel cell., , 3.1 Hydrogen Fuel Cells, A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, and an oxidizing agent into electricity through a pair of redox reactions. If the fuel taken, is hydrogen and the oxidizing agent is oxygen such fuel cells are called hydrogen fuel, cells (H2-O2 fuel cells). Figure given below represents a hydrogen fuel cell., , Figure: Schematic of a hydrogen fuel cell, , 3.4 Construction, Working and Applications of Methanol-Oxygen Fuel Cell (H2SO4 as, Electrolyte), Construction, Both the anodic and cathodic compartments contain Nickel electrodes coated, with Platinum and Palladium catalyst. Methanol containing H2SO4 is passed through, anodic compartment. Oxygen is passed through the cathodic compartment. The, , 3
Page 4 :
Green Chemistry and Alternative Energy Resources, By Dr. Prasad Puthiyillam, For Video Class Visit Youtube Channel: My Intuition, electrolyte is sulfuric acid. A membrane is provided which prevents the diffusion of, methanol into the cathode., , Working of Methanol-Oxygen Fuel Cell, In methanol-oxygen fuel cells, methanol acts as the fuel (at anodic compartment), and oxygen acts as an oxidant (at cathodic compartment)., Reaction at anode surface:, , → CO2 + 6 H+ + 6e-, , CH3OH + H2O, , Reaction at cathode surface: 3/2 O2 + 6H+ + 6e- → 3 H2O, Net Cell Reaction:, , CH3OH + 3/2 O2, , → CO2 + 2 H2O, , Applications of Methanol-Oxygen Fuel Cell, The methanol-oxygen fuel cell can produce limited power, but can still store high, energy content in a small space. This means they can produce a small amount of power, over a long period of time., This makes them ill-suited for powering large vehicles, but ideal for smaller, vehicles and consumer goods such as mobile phones, digital cameras or laptops., Military applications of methanol-oxygen fuel cell are an emerging application, since they have low noise and thermal signatures and no toxic effluent. These, applications include power for man-portable tactical equipment, battery chargers, and, autonomous power for test and training instrumentation., [Note: KOH electrolyte can be used in methanol-oxygen fuel cell. The use of alkali as, electrolyte presents problems. CO2 produced is absorbed by the electrolyte and, electrolyte is gradually converted into carbonates.], , 4
Page 5 :
Green Chemistry and Alternative Energy Resources, By Dr. Prasad Puthiyillam, For Video Class Visit Youtube Channel: My Intuition, , 4. Solar Energy, 4.1 Introduction, The large magnitude of solar energy available makes it a highly appealing source, of electricity. Solar energy has been cheaper than fossil fuels since 2021., Solar power is the conversion of renewable energy from sunlight into electricity,, either directly using photovoltaics (PV), indirectly using concentrated solar power, or a, combination., Concentrated solar power systems use lenses or mirrors and solar tracking, systems to focus a large area of sunlight into a small beam., Photovoltaic cells convert light into an electric current using the photovoltaic, effect. The photovoltaic effect is the generation of voltage and electric current in a, material upon exposure to light. The importance of photovoltaics is listed below:, · They provide suitable energy system, · Solar energy is unlimited, · It is renewable source of energy., · There are no harmful emissions and pollution is absent, · It can be used in remote areas where transmission lines are not available, · By using photovoltaic cells, global warming can be avoided, · They provide power for spacecrafts & satellites, · Installation is quick, · Safety record is excellent, 4.2 Solar cell or Photovoltaic Cell (PV Cell), They are semiconductor devices that convert solar energy into direct current, electricity. As long as light is present a solar cell will generate electricity. When light stops, power generation stops. It does not require recharging. They are having a long life time., Photovoltaic cell is based on photoelectric effect. When light fall on a, semiconductor, electrons jumps into the conduction band from the valence band. In the, valence band holes are created. Thus we get an electron-hole layer. Electrons in the, , 5
Page 6 :
Green Chemistry and Alternative Energy Resources, By Dr. Prasad Puthiyillam, For Video Class Visit Youtube Channel: My Intuition, conduction band and holes in the valence band are able to move. A photo cell does two, functions:, 1] Photo generation of charge carries [+ve and -ve] in a light absorbing material., 2] Separation of charges by a potential gradient (p-n junction) within the material., , 4.3 Construction of a Photovoltaic Cell, A silicon photovoltaic cell is composed of a thin wafer which contains a very thin, layer of phosphorous doped silicon which is kept above boron doped silicon. Phosphorus, doped silicon acts as the n-type layer and boron doped silicon functions as a p-type layer., Thus a p-n junction is formed between these two layers., Metal grids are placed above P-doped silicon, and a metal layer is connected to Bdoped silicon. The anti-reflective layer containing silicon nitride increases the amount of, light transmitted to the semi conductor., A solar cell is represented in the figure given below:, , 4.4 Working of a Photovoltaic Cell, Semiconductors have the capacity to absorb light and a part of the energy of the, absorbed photons is given to charge carries [electrons and holes]. A semiconductor, diode separates and collects the charge carries and conduct the generated electricity in, a specific direction. The electrons moves towards n-type end (metal grids) and holes, migrate towards the p-type end (metal layer). When these two ends are electrically, connected, current flows through the external circuit. Thus photo electric current is, , 6
Page 7 :
Green Chemistry and Alternative Energy Resources, By Dr. Prasad Puthiyillam, For Video Class Visit Youtube Channel: My Intuition, produced. The anti-reflective layer containing silicon nitride increases the amount of light, transmitted to the semi conductor., , 4.5 Applications of Photovoltaic Cells, The photovoltaic systems can be used to supply electricity for:, · telecommunication repeater stations, · water pumps, · navigational aids, · laptop computers, · cottages and remote residences, · parks in remote regions, · supplying occasional power, , Kindly Subscribe the YouTube Channel “My Intuition”, , 7