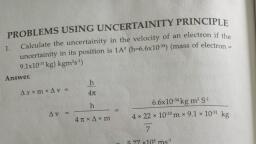Page 1 :
Dual nature of matter, French scientist Louis de Broglie proposed that like photon, all material particle possess wave, nature as well as particle nature, that is possess dual nature. The wave associated with the, particle is called matter wave or de Broglie wave., de Broglie relation, The wavelength associated with any material particle is calculated by using de Broglie relation, which is, λ=h/mv, or λ=h/p, In this equation, h is Planck’s constant, m is the mass of the particle in kg, and v is the velocity, of the particle in m/s. p is the momentum, Since wavelength λ is inversely proportional to mass of the particle, the wavelength associated, with the macroscopic particle with large mass is very short whereas the wavelengths associated, with the microscopic objects having small mass like electron is large., Heisenberg Uncertainty Principle, Heisenberg’s uncertainty principle states that it is impossible to measure simultaneously, both, the position and the momentum of a small moving particle with absolute accuracy. This, principle is based on the wave-particle duality of matter., Mathematical statement, Mathematical statements of uncertainty principle are, , Where 𝛥x is the uncertainty in position, 𝛥p is uncertainty in the momentum, h is Planck’s, constant, m is the mass of the particle, and 𝛥v is the uncertainty in the velocity., This implies that the position and velocity of small particle like electron cannot be measured, simultaneously with certainty.
Page 2 :
Significance of Uncertainty Principle, The important implication of Uncertainty Principle is that it rules out the existence of definite, paths of electrons and similar particles. So Bohr’s concept of fixed circular paths with definite, position and momentum of electron have been replaced by stating that the electron has a, probability of having fixed position and momentum. Hence a new concept based on, probability was introduced to describe path of electron, which is known as orbital. An orbital, is three dimensional space around the nucleus within which the probability of finding an, electron is maximum.