Page 1 :
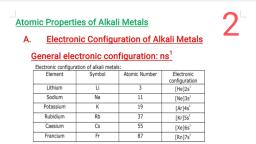
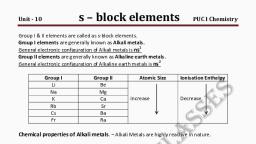
S BLOCK ELEMETS, , , , Che s-block a A fsa, ‘ ck elements ‘ ele ; stron enters the outermost S, k elements of the Periodic Table are those in which the last electron enters the ot, , orbital., Grou Sapa sod ) : a ae, a l ot the Periodic Table consists of the elements: lithium, sodium, potassium, aot:, and tranciu IMA TAgR R, um. They are collectively known as the alkali metals. she, and radium. Thes', , The elements of Gr, ‘ 7 e, ements of Group 2 include beryllium, magnesium, calcium, strontium, barium, , elements wi siattaicat: ; * See etals., , ments with the exception of beryllium are commonly known as the alkaline earth metals, , GROUP 1 ELEMENTS, , Symbol | Electronic configuration, Li, , Lithium 1322s!, , : ALKALI METALS:, , , , , , , , , , , , , , , , , , , , , , , , Sodium Na 1s22s?2p*3s!, Potassium K 13225°2p®3s?3p*4s', Rubidium 1 $22.9?2p°3s?3 p83 dA SA pos, , , , 13229°2p°3s23p°3d4s>, 4p®4d'°5s*5p*6s! or [Xe] 6s!, , [Rn]7s!, , Caesium Cs, , , , , , , Francium Fr, , , , , Atomic and Ionic Radii, The alkali metal atoms have the largest sizes in a particular period of the periodic table. : : l, The monovalent ions (M+) are smaller than the parent atom. The atomic and ionic radii of alkali metals, , increase on moving down the group., , Ionization Enthalpy ;, The ionization enthalpies of the alkali metals are considerably low and decrease down the group from Li, , to Cs., , , , Hydration Enthalpy, , The hydration enthalpies of alkali metal ions decrease with increase in ionic sizes., , Li’> Na* > K* > Rb’ > Cs*, , Chemical Properties, i) Reactivity towards air: They burn vigorously in oxygen forming oxides. Lithium forms monoxide,, sodium forms peroxide, the other metals form superoxides. The superoxide O2~ ion is stable only in the, , , , presence of large cations such as K, Rb, Cs., , 4Lit+ Q 21,0 (oxide), , 2Na + O,—Na,0O, (peroxide), , M + 0, MQ, (superoxide), , (M = K, Rb, Cs), , towards water: The alkali metals react with water to form hydroxide and dihydrogen., , 2M + 2H20 —2M’ + 20H" + H2, , (M = an alkali metal), , , , ii) Reactivi