Page 1 :
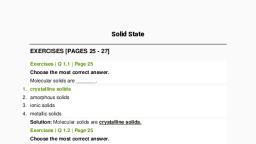
& myCBSEguide.com, , A Complete guide for CBSE students, , CBSE Class 12 Chemistry, Quick Revision Notes, Chapter 1, The Solid State, , Solid: Solid is a state of matter in which the constituting particles are arranged very closely., The constituent particles can be atoms, molecules or ions., , Properties of solids:, , 1. They have definite mass, volume and shape., , 2. They are incompressible and rigid., , 3. Intermolecular distances are very short and hence the intermolecular forces are strong., , 4. Their constituent particles have fixed position. sand can only oscillate about their mean, positions., , Classification of on the basis of the arrangement of constituent particles:, , Amorphous Solids, , , , e Properties of crystalline solids:, , e They have a definite geometrical shape., , e They have a long range order., , e They have a sharp melting point., , e They are anisotropic in nature i.e. their physical properties show different values, when measured along different directions in the same crystal., , Material downloaded from myCBSEguide.com. 1/16
Page 2 :
& myCBSEguide.com, , A Complete guide for CBSE students, , e They have a definite and characteristic heat of fusion., , e They are called true solids., , e When cut with a sharp edged tool , they split into two pieces and the newly generated, surfaces are plain and smooth., , , , Anisotropic nature of crystalline solids, , ¢ Polymorphic forms or polymorphs:, , The different crystalline forms of a substance are known as polymorphic forms or, polymorphs. For example: graphite and diamond., , e Characteristics of amorphous solids:, , They have an irregular shape., , They have a short range order., , They gradually soften over arrange of temperature., , They are isotropic in nature i.e. their physical properties are the same in all directions., When cut with a sharp edged tool, they cut into two pieces with irregular surfaces., They do not have definite heat of fusion., , They are called pseudo solids or super cooled liquids. This is because they have a, , Nem Pw PP, , tendency to flow,though very slowly., e Types of crystalline solids:, , A. Molecular Solids, Constituent Particles: Molecules, , , , Material downloaded from myCBSEguide.com. 2/16
Page 3 :
& myCBSEguide.com, A Complete guide for CBSE students, , , , , , , , Type of Constituent | Bonding/ Electrical physical | Melting | Examples, solid Particles Attractive Forces | conductivity | nature | point, Non-polar Dispersion or Vel Ar, CCly,, P Molecules P Insulator Soft y, solids London forces low H2; lo, co2, HCl, solid, Polar Dipole- dipole ‘, . Molecules |. : . » Insulator Soft low $02, solid, solids interactions, NH3, Hydrogen Hydrogen ;, yeroeeD | Molecules ae Insulator Hard low H20 (ice), bonded bonding, , , , , , , , , , , , , , , , , , , , B. Ionic Solids, , Constituent Particles: Ions, , Bonding/Attractive Forces: Coulombic or Electrostatic, , Electrical Conductivity: Insulators in solid state but conducts in molten state and in, aqueous solutions, , Physical Nature: Hard but brittle, , Melting Point: High, , Examples: CaF», ZnS, MgO, NaCl, , C. Metallic Solids, , Constituent Particles:Positive ions in a sea of delocalized electrons, Bonding/Attractive Forces: Metallic bonding, , Electrical Conductivity: Conductors in solid state as well as in molten state, Physical Nature: Hard but malleable and ductile, , Melting Point: Fairly high, , Examples: Fe ,Cu, Ag, Mg, , D. Covalent or NetworkSolids, , Constituent Particles:Atoms, , Bonding/Attractive Forces: Covalent bonding, , Electrical Conductivity: Bad Conductors in solid state as well as in molten state, Physical Nature: Hard but malleable and ductile, , Material downloaded from myCBSEguide.com. 3/16
Page 4 :
& myCBSEguide.com, A Complete guide for CBSE students, , , , Melting Point: Fairly high, Examples: SiO2, (quartz), SiC, C (diamond), C(graphite), 141.5 pm, ali Patel, 1 Fy Poa, 154 pm} He Ph Oe ik, Network structure of diamond, , e Crystal lattice: A regular ordered arrangement of constituent particles in three, dimensions is called crystal lattice., , , , , , , , , , , , , , , , Diagram showing a portion of 3Dcubic, lattice and its unit cell, , ¢ Lattice points or lattice sites:the fixed positions on which the constituent particles, are presentare called lattice points or lattice sites. A group of lattice points which, when repeated over and over againin3dimensions give the complete crystal lattice., , © Unit cell: It is defined as the smallest repeating unit in space lattice which when, repeated over and over again generates the complete crystal lattice. The crystal can, consist of an infinite number of unit cells., , e Parameters which characterize a unit cell:, , 1. Dimensions of the unit cell along the three edges ,a, b and c:these edges may or may not, be mutually perpendicular., , 2. Inclination of the edges to each other:this is denoted by the angle between the edges a6,, andrespectively.aisthe angle between the edges b and c,fisthe angle between the edges a, and c ,andyis the angle between a and b., , Material downloaded from myCBSEguide.com. 4/16
Page 5 :
& myCBSEguide.com, A Complete guide for CBSE students, , , , , , , , , , , , Parameters which characterise a, unit cell, , e Seven crystal systems:, , Cubic: a=f=7=90° ,a=b=c, , Tetragonal: a={=y=90° ; a=b¢c, Orthorhombic: a =$=y=90°; a¢bA~c, Monoclinic: a==90°,8490°; a#b#éc, Hexagonal: a=$=90°,y=120°; a=bA¢c, Rhombohedral or trigonal: a= B=y#90°;a=b=c, Triclinic: af#PAyF£90°;ax-DAc, , e Types of unit cells:, , Neue wnrn pp, , 1. Primitive or simple unit cells have constituent particles only at its corners., 2. Centered unit cells are those unit cells in which one or more constituent particles are, present at positions in addition to those present at the corners., , e Types of centered unit cells:, , 1. Face centered unit cell: It consists of one constituent particle present at the centre of each, face in addition to those present at the corners., , 2. Body centered unit cell: It consists of a one constituent particle is present at its body, centre in addition to those present at the corners., , 3. End centered unit cell: It consists of one constituent particle present at the centre of any, two opposite faces in addition to those present at the corners., , e Number of particles at different lattice positions:, , Material downloaded from myCBSEguide.com. 5/16