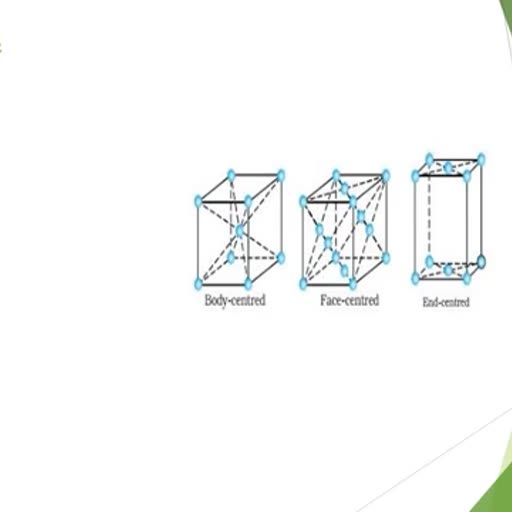
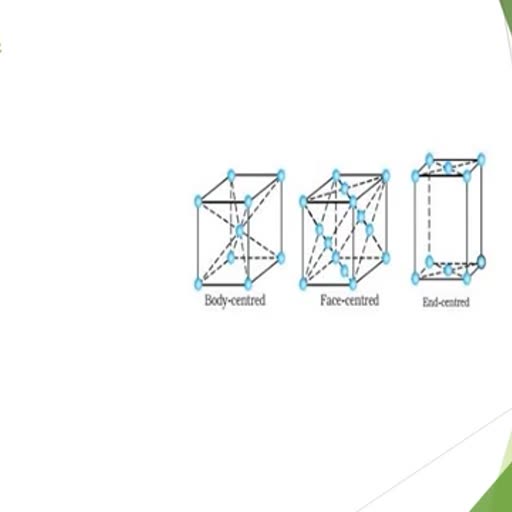
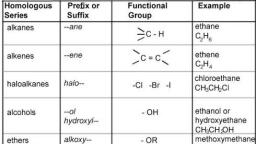
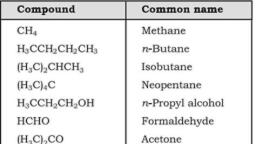
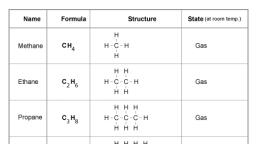
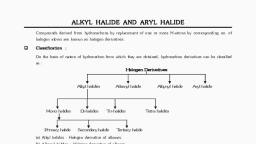
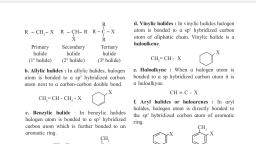
Page 1 :
Halogen Derivatives, , , , Multiple Choice Questions (1 Mark), , , , 1. The type of monohalogen derivative in which a halogen atom is bonded, to sp> hybridized carbon atom next to carbon-carbon double bond is, , (A) alkyl halide (B) _ allylic halide, (C) vinylic halide (D) benzylic halide, , , , 2. Aromatic electrophilic substitution with iodine can be carried out using, , (A) HNO, (B) HCl, (C) HI (D) H;PO,, , , , 3. For the isomeric dihalobenzenes, melting point of, (A) _ ortho isomer is higher, (B) meta isomer is higher, (C) para isomer is higher, (D) all isomers is nearly same, , 4. Optical activity of a molecule is associated with, (A) _ plane polarized light, (B) 3-D structure of a molecule, (C) achiral molecule, (D) superimposable mirror images, , 5. Propane nitrile can be prepared by heating, (A) ethyl bromide with alcoholic KCN, (B) propyl bromide with alcoholic KCN, (C) ethyl bromide with alcoholic AgCN, (D) propyl bromide with alcoholic AgCN, Hint: Propane nitrile can be prepared by heating ethyl bromide with alcoholic, , KCN., CH;CH,Br —“©8:4_, CH,;CH,CN, Ethyl bromide Propane nitrile, , , , Std. XII Sci.: Chemistry
Page 3 :
4., , Ans:, , Ans:, , ii., , Ans:, , Ans:, , Write IUPAC name of the product ‘B’ in the following reaction, sequence., , C,H;0H —“1201_, 4 —“_, B, , The IUPAC name of the product ‘B’ formed in the following reaction, sequence is iodoethane., , CoHs — OH S225, C,H; — Br —“4—> C,Hs —1 + NaBrY, , Ethanol Bromoethane lodoethane, (A) (B), , Nucleophilic substitution reaction of 2,4-dinitrochlorobenzene is, faster than p-nitrochlorobenzene. Give reason., , The presence of electron withdrawing (-NO>) group at ortho and para, position greatly increases the reactivity of 2,4-dinitrochlorobenzene, towards substitution of halogen atom., , Greater the number of electron withdrawing (-NO>) groups at o and p, position, greater is the reactivity. Therefore, nucleophilic substitution, reaction of 2,4-dinitrochlorobenzene is faster than p-nitrochlorobenzene., , Name the reagent used to convert alkyl halide to ester., , The reagent used to convert alkyl halide to ester is silver carboxylate, (RCOOAg)., , Write the CORRECT order’ of increasing ease of, dehydrohalogenation., , CH;, , |, CH; — CH; — CH; — Cl CH; — CH(Cl) — CH; CH; — i -Cl, , CH;, (D) (ID (IID), , The correct order of increasing ease of dehydrohalogenation is,, > >.
Page 4 :
Chapter 10: Halogen Derivatives Ta rget Publications” Pvt. Ltd., , , , , , iii., , Short Answer Questions (Type-!) (2 Marks), , , , Explain. Aryl halides are less reactive than alkyl halides towards, nucleophilic substitution reactions., , The low reactivity of aryl halides is due to resonance effect and sp, hybrid state of carbon to which halogen atom is attached., , In aryl halides, one of the lone pairs of electrons on halogen atom is in, conjugation with z-electrons of the ring. Due to resonance, the C—X, bond acquires partial double bond character. Thus, the C—X bond in aryl, halides is stronger and shorter than alkyl halides. Hence, it is difficult to, break C—X bond in aryl halides., , Further, the phenyl cation produced due to self-ionization of aryl halide, will not be stabilised by resonance. This rules out the possibility of Sy1, mechanism. Also, the back side attack of nucleophile is blocked by the, aromatic ring. This rules out the possibility of Sy2 mechanism., , As a result, nucleophilic substitution reaction involving cleavage of, C-X bond in haloarenes proceeds with difficulty., , Therefore, aryl halides are less reactive than alkyl halide towards, nucleophilic substitution reactions., , Explain reactions of haloarenes with sodium metal., , : Haloarenes undergo Wurtz-Fittig reaction and Fittig reaction on reaction, , with sodium metal., , The reaction of aryl halide with alkyl halide and sodium metal in dry, ether to give substituted aromatic compounds is known as Wurtz-Fittig, reaction., , e.g. Br CH;, , oO +CH;3Br + 2Na —22> + 2NaBr, , Bromobenzene Methyl Sodium Toluene, bromide metal, , If only aryl halide takes part in the reaction, the product is biphenyl (or, diphenyl) and the reaction is known as Fittig reaction., , e.g. cl, , C) + Na 285 (CS) CO + 2NaCl, , Chlorobenzene Biphenyl, , , , Std. XII Sci.: Chemistry
Page 5 :
Chapter 10: Halogen Derivatives Target Publications” Pvt. Ltd., , , , , , Give reason. Though alkyl halides are moderately polar they are, insoluble in water., , Alkyl halides cannot form hydrogen bonds with water., In addition to this, the attraction between alkyl halide molecules is, stronger than attraction between alkyl halide and water., , Hence, alkyl halides though moderately polar are insoluble with water., , 4., Ans:, 1,, , ii., , ili., , iv., , Ss., Ans:, , " df Na & ih, ‘ . / Xow, , Explain optical activity of 2-chlorobutane., , The property of a substance by which it rotates plane of polarization of, incident plane polarized light is known as optical activity., 2-Chlorobutane has one chiral carbon atom and thus, exists as a pair of, enantiomers which are non-superimposable mirror images of each other., These mirror images are referred as optical isomers; which differ from, each other in terms of a measurable property called optical activity., Structure of 2-chlorobutane and its mirror image can be represented as,, , CH; CH; CH;CH;, , , , , , , , , , * * *, oC Cas eC Can, , H, , C>Hs CyHs CoH; C2Hs, Mirror plane Non-superimposable mirror image, Thus, 2-chlorobutane will show optical activity and in accordance with, , the direction of optical rotation, one isomer will be dextrorotatory and, other will be laevorotatory., , Distinguish between Sy1 and S,2 mechanism., , , , _No., , , , , , , , Factor Sy2 Mechanism Sx1 Mechanism, , , , i., , Kinetics 2™ order 1* order, , , , ii., , Molecularity Bimolecular Unimolecular, , , , iii., , Number of steps | One step Two steps, , , , iv., , Bond making Simultaneous First the bond in the, , and bond reactant breaks and, , breaking then a new bond in, product is formed, , , , Transition state | One step, one transition Two steps, two, _ state transition states, , , , Std. XII Sci.: Chemistry, , 5