Page 1 :
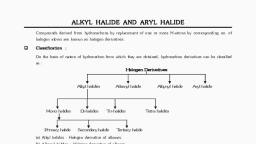
HALO ALKANES AND HALOARENES, , Chapter–01, , HALO-ALKANES AND HALO-ARENES, SESSION 1, AIM - To classify haloalkane and nomenclature of haloalkanes., Introduction: Compounds derived from hydrocarbons by the replacement of one or more, hydrogen atoms by the corresponding number of halogen atoms are termed as halogen, derivatives., Many halogen containing organic compounds occur in nature and some of these are, medicinally useful., For exp, the chlorine containing antibiotic chloromycetin or chloramphenicol, produced by, soil microorganism, is very effective for the treatment of typhoid fever., Our body produces an iodine containing hormone called thyroxine, the deficiency of which, causes the disease goiter., Some synthetic halogen containing compounds are very useful in health–care and medicine., For example, chloroquine is used for the treatment of malaria fever., , Classification: Halogen derivation of hydrocarbons broadly classified in to two types:, A., , Aliphatic halogen compounds:, These are obtained by replacement of one or more hydrogen atoms of an aliphatic, hydrocarbon by an equal number of halogen atoms. Depending upon the nature of the, aliphatic hydrocarbon, whether alkane, alkene, alkyne aliphatic halogen compounds are of, the following three types:, a) Haloalkanes: are the halogen derivatives of alkanes. They derived from alkanes by the, replacement of one or more hydrogen atoms by the corresponding number of halogen, atoms (fluorine, chlorine, bromine or iodine) are called as halo–alkanes., These are classified as fluro, chloro, bromo or iodo compounds according to the type of, halogen present in them., Ex:, CH3Cl, C2H5I, C3H7Br, Methyl chloride, Ethyl iodide, Propyl bromide, Depending on the number of halogen of atoms present in the halogen derivative, these, are termed as mono-, di-,tri-and tetra halogen derivatives., Monohalogen derivatives, These derivatives are formed by the replacement of one hydrogen atom by one halogen, atom ., CH3Cl, C2H5I, C3H7Br, Methyl chloride, Ethyl iodide, Propyl bromide, , Page number, , 1, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 2 :
HALO ALKANES AND HALOARENES, , Monohalogen derivatives or alkyl halides are classified as primary (1°), secondary (2°) or, tertiary (3°) depending upon whether the halogen atom is attached to primary, secondary or, tertiary carbon atoms., i], Primary (1o) alkyl halide, When the carbon atom containing halogen, atom is linked to only one carbon atom (i.e.,, primary carbon atom)., , ii], , e.g., ,, , etc, o, Secondary(2 ) alkyl haide, When the carbon atom having halogen, atom is linked to two carbon atoms (i.e.,, secondary carbon atom), e.g, , ,, iii], , Tertiary, , , etc., , alkyl halide, , When the carabon atom having halogen atom is killed to three carbon atoms (i.e.,tertiary, carbon atoms)., e.g, , ,, , , etc., , Dihalogen derivatives, These are formed by the replacement of two hydrogen atoms by two halogen atoms., The dihalogen derivatives are mainly three types., a] Geminal or Gem-dihalides, Here both the halogen atoms are attached to the same carbon atom. These are also, called alkylidene halides, , Page number, , 2, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 3 :
HALO ALKANES AND HALOARENES, , b] Vicinal or vic-dihalides, Here both the halogen atoms are attached to adjacent carbon atom. These are also, called alkylene halides, , c], , Halides (terminal dihalides), Here both the halogen atoms are attached to terminal carbon atoms. These are also, called polymethylene halides, , Trihalogen derivatives, These are formed by the replacement of three hydrogen atoms from three halogen, atoms. These are also known as haloforms, , Tetrahalogen derivatives, These are formed by the replacement of four hydrogen atoms from four halogen atoms, , (b) Haloalkenes or Alkenyl halides are the halogen derivates of alkenes. The mono halogen, derivates of alkenes with formula is CnH2n–1X ., where X = F, Cl, Br or I and n = 2,3,4….., etc., , Page number, , 3, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 4 :
HALO ALKANES AND HALOARENES, , (c) Haloalkynes of Alkynyl halides are the halogen derivatives of alkynes. The monohalogen, derivatives of alkynes have the general formula CnH2n–3X, where X = F, Cl, Br or I and n = 2, 3,, 4…., etc. For example,, , B) Aromatic Halogen Compounds, These are obtained by replacement of one or more hydrogen atoms of an aromatic, hydrocarbon by an equal number of halogen atoms., These have been further classified into the following two major categories :, (i) Nuclear halogen derivatives (aryl halides). Halogen derivatives of aromatic hydrocarbons in, which the halogen atom (F, Cl, Br or I) is directly attached to an aromatic ring are called, aryl halides. Their general formula is Ar–X where Ar (short name for aryl) represents a, phenyl, a substituted phenyl or any other aryl group such as naphthyl, etc. Some examples, of aryl halides are:, , (ii) Side chain halogen derivatives (aralkyl halide). Halogen derivatives of aromatic, hydrocarbons in which the halogen atom is linked to one of the carbon atoms of the side, chain carrying the aryl group are called aralkyl halides. For example,, , Classification based on hybridization of the carbon atom linked to the halogen:, The halogen derivatives are of three types:, (A) A compound containing C–X bond, where carbon is sp3-hybridized., (i) Alkyl halides or Haloalkanes: In alkyl halides, the halogen atom is bonded to an alkyl, group., , Page number, , 4, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 5 :
HALO ALKANES AND HALOARENES, , The homologous series is represented by CH2n+1X., They are further classified as primary (1°), secondary (2°) tertiary (3°) halides depending, upon whether the halogen atom is attached to a primary, secondary and tertiary carbon, atom., , Examples are:, (ii) Allylic halides: In these halides, the halogen atom is attached to allylic carbon, i.e., carbon atom next to C=C. Examples are:, , like, , Allylic halides may be primary secondary or tertiary., (iii) Benzylic halides: In these halides, the halogen atom is attached to a benzylic carbon, ,, i.e.,the carbon atoms of the side chain carrying the aryl group., Examples are: Benzyl chloride;Benzo trichloride;…. etc.,, (B) Compounds containing C–X bond, where carbon is sp2 hybridized, (i) Arylhalides: In these halides, the halogen atom is directly attached to the carbon, atom of an aromatic ring. These halides are also called haloarenes., Cl, , I, , Br, CH 3, , 2-bromotoluene, Iodobenzene, Examples are: Chlorobenzene, (ii) Vinylic halides:In these halides, the halogen atom is attached to vinylic, , carbon, , Page number, , 5, , i.e., one of the carbon atoms of C= C. Examples are:, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 7 :
HALO ALKANES AND HALOARENES, , SESSION 2, AIM - To explain methods of preparation of haloalkane, (1) From Alkanes: [Halogenation of Alkanes], Alkanes react with halogen (Cl2, Br2) in presence of sunlight at ordinary temperature or by, heating at 400–500° or heating in presence of free radical initiators such as peroxides, TEL,, etc give RX., R-H, , R-X + HX, , The reactivity of halogens decreases in the order: F2> Cl2> Br2> I2, This is chain process and direct halogenation proceeds through free radical mechanism. This, is not a suitable laboratory method since polyhalogen derivatives are formed along with, monohalogen derivatives., For example, chlorination of methane gives four products,, i.e.,, Mechanism: The reaction proceeds via free radical formation., It takes place in the following steps:, , Initiation Step: The reaction is initiated by the breaking of chlorine molecule into, chlorine-free radicals in presence of UV light., fission, Cl2 Homolytic, Cl* + Cl* …..… (i), Hv or heat, , Propagation Step:The chlorine-free radicals attach methane molecule (, is a, substituent), *, CH4 + Cl*, CH3 + HCl …….… (ii), Each methyl-free radicals, in turn, reacts with chlorine molecule to form methyl chloride, and at the same time chlorine-free radical is produced., *, , CH3Cl + *Cl…….… (ii), , CH3 + Cl2, , The chlorine-free radical can react with fresh methane molecule as in next step or methyl, chloride.This process may extend further till all the replaceable hydrogen atoms in the, methane have been substituted by chlorine atoms., Chain termination on step:, The chain reaction is terminated when any two free radicals., Cl*+ Cl*, Cl2, *CH +, 3, , Page number, , 7, , Cl*, , For any queries, , CH2 Cl, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 8 :
HALO ALKANES AND HALOARENES, Direct iodination is not possible as the reaction is slow, reversible and endothermic., Hence it is done by heating alkane with I2 in presence of oxidising agents such as conc., HIO3 of HNO3 or HgO., , Fluorination of alkanes is highly exothermic or explosive hence it cannot be controlled, under ordinary conditions., In case of higher alkanes, even monohalogenation gives a mixture of all the possible, isomeric haloalkanes indicating the replacement of all the types of possible H atoms, present in a given alkane., Monohalogenation does not depend upon the type of carbon atoms present in given, alkanes, but on type of replaceable H atoms in the given structures., The relative amounts of the isomeric haloalkanes depends upon the nature of halogen, and the number type of hydrogens (1°, 2°, 3°) being substituted. The ease of substitution, of various H atoms generally follows the order 3° > 2° > 1°, but their relative rate of, abstraction varies with the nature of halogens., , The abstraction of H is in the order:, Benzylic allylic > 3° > 2° > 1° > CH4> Vinylic = aryl, Chlorination of n–alkanes (C4 and above) gives a mixture of d– and l–optical isomers., i.e., racemic mixture (optically inactive), , Page number, , 8, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 11 :
HALO ALKANES AND HALOARENES, , b., , Propagation: During the first step, a Br adds to the double bond in such a way to give, the more stable free radical. In the second step, the free radical thus produced, abstracts a H from HBr to complete the addition., , c. Termination: It involves the following three steps:, , Exceptional behavior of HBr:, Peroxide effect is effective only in the case of HBr, because HF, and HCl are held by strong electrostatic force hence they cannot be broken into, free radicals., H–I also give corresponding halogen free radicals. I° free radical being, larger in size is less reactive towards C= C bond but readily combines with, another I° to give I2 molecule., , Page number, , 11, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 12 :
HALO ALKANES AND HALOARENES, , * From Alcohols:, Alkyl halides are prepared from alcohols by replacing –OH group of alcohols by a halogen, atom., R–OH + H–X R–X + H2O, The replacement of –OH group can be done with HX, phosphorus halides and SOCl2., a. By action of halogen acids:, This reaction is carried out in presence of a dehydrating agent as ZnCl2 or conc. H2SO4., The1:1 ratio of of HCl and anhydrous ZnCl2 is known as Lucas reagent., This reaction is also known as Groves process., , The reactivity of halogen acids is in the order: HI > HBr > HCl., The order of reactivity of alcohols for halogen acid is as: 3° > 2° > 1°., Reason: This reaction is an example of a nucleophilic substitution reaction in which the, nucleophile i.e., halide ion attacks the protonated alcohol molecule with the expulsion of, water–a good leaving group., , R OH, , , , +, , H, , ROH, , S N1, H 2O, , R, , +X, , H, X, , RX ( Some, , rearranged, product, if possible), , SN2, , R X + H 2O, the reaction of alcohol with HX proceeds via the formation of a carbocation, hence, attacks the most stable carbocation., , Ex:, and not, Because of the strong tendency of neopentyl cation to rearrange to the more stable 3°, carbocation, neopentyl chloride cannot be prepared by the action of HCl on neopentyl, alcohol. Instead 2–chloro–2–methylbutane is formed as shown below:, (b) By action of Phosphorus halides, , Page number, , 12, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 14 :
HALO ALKANES AND HALOARENES, , SESSION 4, AIM, , , , To explain physical properties isomers of alcohol, To explain mechanism of nucleophilic substitution reaction., Properties of Alkyl Halides:, , Physical Properties:, 1. CH3F, CH3Cl, CH3Br and C2H5Cl are gases at room temperature, while CH3I is a liquid at, room temperature. The RX s up to C18 are colorless liquids while higher members are, colorless solids., 2. RXs being polar in nature are water insoluble., 3. They burn on Cu wire with green edged flame (Beilstein test for halogens), 4. R-Br and RI are heavier than water, RCl and RF are lighter than water., 5. RIs become brown or violet in color on exposure to light., The I2 thus liberated dissolves in RX to impart dark color., 6. The B.Ps of RXs are in the order: RI > RBr > RCl > RF., Greater the molecular mass, stronger the Vander Waal’s forces of attraction and hence, higher is the M.P and B.P. For a given halogen, the B.Ps of RXs increases with the increase, of the size of ‘R’ group., In isomeric RXs, as branching increases, surface area is decreased and hence B.P is, decreased. This it is in the order: 1° > 2° > 3°., , 7. Decreasing order of density among RXs is in the order: RI > RBr > RCl > RF., Highest density is observed for CH3I. Thus, for RI, the decreasing order of density is as, follows:, CH3I > CH3CH2I > CH3CH2CH2I > ……, 8. Stability of C–X bond decreases as the strength of C–X bond decreases. Bond strength of, C–X bond decreases as the size of halogen atom increases., bond strength of C–X bond is in the order: CH3–F > CH3–Cl > CH3–Br > CH3–I, 9. Dipole moment of RXs decreases as E.N. of halogen atom decreases from Cl to Br to I., fluorides have lower, than chlorides due to very small size of F which out weights the, effect of greater E.N. The actual order is: CH3Cl > CH3F > CH3Br > CH3I, , Page number, , 14, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 15 :
HALO ALKANES AND HALOARENES, , Chemical Properties:, Nature of C–X Bond, The high reactivity of RXs can be explained in terms of the nature of C–X bond which is, highly polarized covalent bond due to large difference in E.N of C and halogen atoms., This polarity gives rise to 2 types of reactions namely nucleophilic substitution reactions, and elimination reactions., , Such reactions in which a stronger nucleophile displaces a weaker nucleophile are called, nucleophilic substitution reactions and the atom or group (halide ion in the present case), which departs with its bonding pair of electrons is called the leaving group. Better the, leaving group, more facile is the nucleophilic substitution reaction., Mechanism of nucleophilic substation reactions:, 2 types of nucleophilic substitution reaction namely, , and, , 1] Substitution nucleophilic bimolecular reactions, If the rate of the reaction depends on the concentration of alkyl halide As well as, nucleophile, then the reaction is known as, reaction., Rate, , [alkyl halide] [nucleophile], , Reactions are occur in one step. During the reaction the nu attacks from the back, side (opposite side) of the halogen atom, the carbon-halogen band starts breaking and a, new carbon–nu bond starts forming. These two process takes place simultaneously in a, single step and a transition state is formed., In the transition state, the carbon atom is simultaneously bonded to the incoming, nucleophile and the outgoing leaving group.This is unstable stste,it ultimately, decomposes to form the product (CH3OH) and the leaving group (Cl– ion)., HO:, , —, , C, , Br, , —, C, , —, , HO, , C, , + Br—, , (Inversion), , Page number, , 15, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 17 :
HALO ALKANES AND HALOARENES, , Thus reactivity of different alkyl halides towards, Alkyl halide>, , Alkyl halide>, , reaction is:, , Alkyl halide > Methyl halide., , Stereochemistry of nucleophilic substitution reactions:As the reaction involves the, formation of a planar carbocation, it allows the attack of nucleophile from both sides and, leads to the formation of enantiomers.So optical property observed is racemization, COMPARISON OF SN1 AND SN2, SN1, (a) Number of steps, , SN2, , 2 steps :, , 1 step :, R : L + : Nu R:Nu + :L, , slow, , (i) R : L R+ + :L, , Or, , fast, , (ii) R+ + :NuH , R:Nu + H+, , R : L + :NuH R : N+uH +:L, , (b) Reaction rate & order, , Rate = k1[RL]; first order, , Rate = k1[RL] [:Nu]; second order, , (c) Molecularity, , Unimolecular, , Bimolecular, , , , , , (d) TS of slow step, , , , R …… L ……HNu:, , +, , Inversion, and, racemization), , (f) Reacting nucleophile, , Nucleophilic solvent; stable R+ may react, with added nucleophile, , Added nucleophile, , (g) Structure of R, , 3° > 2° > 1° > Me, , Me > 1° > 2° > 3°, , Weakest base is best leaving group, i.e., I > Br > Cl > F, , Weakest base is best leaving group, i.e., I > Br > Cl > F, , For HNu: (solvent),, , In protic solvents,, , rate basicity of HNu:, , (i) Within a periodic table group, rate , polarizability of Nu, , of, , Leaving, , (i) Nature of nucleophile, , (Partial, , HNu……C….L (with : HNu), , (e) Stereochemistry, , (h) Nature, group, , retention, , Nu……C…… L (with : Nu), , Inversion of configuration (backside, attack), , (ii) For same nucleophilic site, rate , basicity of Nu, In polar aprotic solvents, rate basicity, of Nu, (j) Solvent effect, , Page number, , 17, , Rate Hbonding ability and dielectric, constant, , For any queries, , ACTIVE SITE EDUTECH:, , Depends on charge type. Polar aprotic, solvents leave “freest” most reactive, Nu., , Contact No. : 9844532971
Page 18 :
HALO ALKANES AND HALOARENES, (k) Determining factor, , Stability of R+, , Steric hindrance, , (l) Rearrangement, , Observed, , Not observed, except for allylic, , (m) Catalysis, , Lewis and Bronsted acids:, , No specific catalyst, , +, , Ag , AlCl3 , ZnCl2 etc., , SESSION 5, AIM-Discuss reactions in nucleophilic substitution & combination of reaction, The nucleophilic substitution reactions of haloalkanes are discussed below., 1. Substitution by hydroxyl group :, Haloalkanes on treatment with boiling aqueous alkalies or moist, hydrolysis to form alcohols., , undergo, , (boil), R X HOH , R OH X, , R X KOH aq. , R OH KBr, , R X Ag2 O moist , R OH AgBr, , 2., , Substitutionby alkoxy group:, Haloalkanes on treatment with sodium or potassium alkoxides or dry, If the attacking nucleophile is, , from ethers., , , the reaction is called Williamson’s ether synthesis, , Ex:, Ex:, 3], , Substitution by hydrosulphide group:, Haloalkanes on Heating with aqueous alcoholic sodium or potassium hydrogen Sulphide, gives thioalchols., , Ex:, , Page number, , 18, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 19 :
HALO ALKANES AND HALOARENES, 4], , Substitution by cyano group :, Haloalkanes on heating with alcoholic solution of KCN (ionic natured) gives alkyl cyanides, as the major product along with a small amount of alkyl isocyanide., , EX5], , Substitution by isocyanide group :, Alkyl halides on heating with aqueous ethanolic solution of AgCN(covalent natured),gives, alkyl isocyanides (carbylamines) as the major product along with a small amount of alkyl, cyanide, , Ex:, Explanation: The formation of cyanides or isocyanides from alkyl halides involves, nucleophilic substitution reaction., Alkali metal cyanides (KCN or NaCN) are predominantly ionic. Therefore, both carbon and, nitrogen atoms are free to donate electron pair. Since C–C bond is relatively stronger than C–, N bond, therefore, in this case attack mostly occurs through the carbon atom of the cyanide, group and alkyl cyanides are the major product., Silver cyanide is predominantly covalent. Consequently only nitrogen electron pair is available, for bond formation, and the attack mostly occurs through the nitrogen atom of cyanide group, giving alkyl isocyanides as the major product., , 6], , Substitution by nitrite group:, Alkyl halides react with aq,ethanolic solution of KNO2, alkyl nitrite major product, formed along with a small amount of nitro alkane., , Ex:, 7], , Substitution by nitro group:, Haloalkanes react with aqueous ethanolic solution of, major product with a small amount of alkyl nitrite, , Page number, , 19, , For any queries, , ACTIVE SITE EDUTECH:, , gives nitro alkane as the, , Contact No. : 9844532971
Page 20 :
HALO ALKANES AND HALOARENES, Ex:, Explanation: Nitrite ion (–O–N=O) like cyanide ion is an ambident nucleophile since it has, two sites (oxygen and nitrogen) through which it can attack an alkyl halide. Whereas, attack through nitrogen gives nitro compounds, attack through oxygen gives nitrites., , Alkali metals nitrites are ionic compounds and hence have a negative charge on one of the, oxygen atoms.Hence it gives alkyl nitrites., In contrast, silver nitrite is a covalent compound and hence does not have a negative charge, on the oxygen atom,therefore, lone pair of electrons on the nitrogen atom is more easily, available for bond formation or nucleophilic attack occurs through nitrogen and hence, silver nitrite predominantly gives nitro compounds, 8] Substitution by carboxylate group:Alkyl halides on heating with ethanolic solution of silver salts of fatty acids give esters., , Ex:, 9], , Substitution by aminogroup:, Haloalkanes on heating with ethanolie solution of, , in a sealed tube at, , , gives, , a mixture of, amines and quaternary ammonium salts . This reaction is called, Hoffmann ammonolysis., Ex:, , Page number, , 20, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 21 :
HALO ALKANES AND HALOARENES, , 10] Substitution of chlorine or bromine by iodine:, Alkyl chlorides or bromides on treating with NaI or KI in acetone give alkyl iodides. This, reaction is called Finkelstein reaction., , SESSION 6, AIM- To discuss other reactions of haloalkane., , Elimination reaction, , Reaction with metals, , Reduction., A] Elimination Reaction: (Dehydrohalogenation), When alkyl halide boiled with conc.Alc KOH, they undergo dehydrohalogenation to give, alkenes. These reaction are called, - elimination reactions because the hydrogen atom of haloalkane which is eliminated, comes from a, , carbon, , Orientation of dehydrohalogentaion: If the halogen is present on the terminal carbon, atom of the chain, dehydrohalogenation can occur only in one direction to give only the, terminal alkene., For example,1-chlorobutane on dehydrohalogenation gives but-1-ene, , Page number, , 21, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 22 :
HALO ALKANES AND HALOARENES, In the dehydrohalogenation of secondary and tertiary haloalkenes, when there is a, possibility of formation of two isomers, the hydrogen atoms is preferentially eliminated, from the adjacent carbon atom with lesser number of hydrogen atoms. This, generalization is known as Saytzeff’s rule, i.e., the more substituted alkene is more, stable. For example,, CH 3, H 3C, , H 3C CH CH, But- 2-ene, (Major ) (80%), , alc. KOH, , H 3C, , Br, , B], 1], , CH 2 CH, , CH 3, , CH 2, , But- 1-ene, (Minor) (20%), , The ease of dehydrohalogenation is in the order :, Tertiary alkyl halide >Secondary alkyl halide >Primary alkyl halide., Other reaction:, Reduction: Alkyl halides on reduction with ZN/HCL, No/alcohol,, couple/alcohol gives corresponding alkanes., , or, , Ex:, 2], , 3], , Reaction with sodium (Wurtz reaction):, Alkyl halides react with metallic sodium in presence of dry ether to form symmetrical, alkanes containing double the number of carbon atoms present in the alkyl halide., 2RX, , 2Na, , 2CH 3 I, , 2Na, , ether, ether, , R R, H 3C, , 2NaX, CH 3, , 2NaI, , Reaction with magnesium:, Alkyl halides form Grignard reagents when treated with dry magnesium powder in dry, ether., RX, , Mg (powder), , Dry ether, , R, , Mg X, , Grignard reagent, , Grignard reagents are used for making a very large number of organic compounds. Reactivity, order is RI > RBr > RCl., 4. Reaction with other metals: Organometallic compounds are formed., When heated with zinc powder in ether, alkyl halides form dialkyl zinc compounds., These are called Frankland reagents., , Page number, , 22, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 24 :
HALO ALKANES AND HALOARENES, 1., , 2., , From glycols :, Glycols on reacting with halogen acids ,, , or, , gives Vic-dihalides., , From alkenes:, Addition of halogens to alkenes gives ric-dihalides., , Trihalogen derivatives, Chloroform, It is was discovered by Liebig and named chloroform by Dumas. Earlier it was extensively, used as anesthesia for surgery. But now it is rarely used as it causes liver damage., Preparation of chloroform, By distillation of ethyl alcohol and bleaching powder:, Chloroform is prepared by the distillation of ethyl alcohol with bleaching powder and, water .The yield is about 40% the available chlorine from bleaching powder serves as, oxidising agent and chlorinating agent., The reaction takes place 4 types, a] Hydrolysis of bleaching powder, b] Oxidizing of ethyl alcohol to acetaldehyde by, c] Chlorination of acetaldehyde to chloral, d] Hydrolysis of chloral with, , Physical properties:, 1], 2], 3], 4], , It is a colorless sweet-smelling liquid, It is a heavier than water. (d= 1.485 g/cc), It is less soluble in water but more soluble in organic solvents, It is a non-inflammable liquid. But vapors burn with green flame, , Chemical properties:, , Page number, , 24, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 25 :
HALO ALKANES AND HALOARENES, 1], , Oxidation: When chloroform is exposed to sunlight and air it is slowly oxidized to, phosgene a colorless poisonous gas., , The oxidation of chloroform is prevented by, a] Strong chloroform in dark brown colored bottles filled up to the brim., b] Adding 1% ethyl alcohol, Ethyl alcohol prevents the oxidation chloroform and converts phosgene into harm less, ethyl carbonate., Phosgene is extremely poisonous gas. To use chloroform as an anesthetic agent, it is, necessary to prevent the above reaction. The following precautions are taken when, chloroform is stored., (a) It is stored in dark blue or brown colored bottles which are filled up to the brim., (b) 1% ethyl alcohol is added. This retards the oxidation and converts the phosgene, formed into harmless ethyl carbonate., , Carbylamine reaction (isocyanide test): This reaction is actually a test of primary amines., Chloroform, when heated with primary amine in presence of alcoholic potassium, hydroxide forms a derivative called isocyanide (carbylamines) which has a very offensive, smell., , This reaction is also used for the test of chloroform., Uses:, (i) It is used as a solvent for fats, waxes, rubber, resins, iodine, etc., (ii) It is used for the preparation of chloretone (a drug) and chloropicrin (insecticide)., (iii) It is used in laboratory for the test of primary amines, iodides and bromides., (iv) It can be used as anaesthtic but due to harmful effects it is not used these days for this, purpose. It causes liver damage when inhaled in excess (SO is CCl4)., (v) It may be used to prevent putrefaction of organic materials, i.e., in the preservation of, anatomical species., , Page number, , 25, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 26 :
HALO ALKANES AND HALOARENES, Test of chloroform:, (i), It gives isocyanide test (carbylamines test)., (ii), It forms silver mirror with Tollen’s reagent., (iii), Pure chloroform does not give white precipitate with silver nitrate., Iodoform: Iodoform reaction: Organic compounds having methyl ketone, Or gives methyl ketone group on oxidation, when heated with in presence of alkali, gives iodoform. Basic requirement for a compound to give iodoform reaction is, , presence of, , (or), , Group. Compounds having, , undergo oxidation with halogens to give, , group, , group ., , Other components which give idoform reaction are, , p - p - Dichloro diphenyl tri chloro ethane (DDT), It is manufactured by condensation of chloro benzene with trichloro acetaldehyde (chloral) in, the presence of sulphuric acid., CCl3, Cl, , CCl3, Cl, , Chlorobenzene, , H 2SO 4, , Cl, , CH, , Cl, , CHO, Chloral, , Properties, It is a white powder insoluble in water but soluble in oils., Uses, It is a powerful insecticide. However it is highly stable & is not easily decomposed in the, environment. Therefore its long term effect could be potentially dangerous & its use is banned, in many countries, , Page number, , 26, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 27 :
HALO ALKANES AND HALOARENES, , SESSION 9 AND 10, AIM - To discuss preparation and properties of haloarenes., Haloarenes - Compounds in which halogen is directly attached to an aromatic ring are, known as aryl halides or haloarenes and are represented as Ar –X, where Ar is phenyl,, substituted phenyl or a group derived from other aromatic system., General methods of preparation:, 1. By direct halogenation of aromatic hydrocarbons: This method is used for the, preparation of chloro and bromo derivatives. Halogens react with aromatic, hydrocarbons in presence of catalysts or halogen carriers such as iron, iodine or, anhydrous ferric or aluminium chloride (Lewis acid) at room temperature in absence of, direct sunlight., , Ar – H + X2, , Lewis acid, , , Ar – X+HX, ((X = Cl, Br), , Cl, Cl 2, , FeCl 3, , HCl, (Chlorobenzene), , in dark, Br, , Br 2, , Fe or, , CH 3, , CH 3, Cl 2, , HBr, (Bromobenzene), , FeBr 3, , CH 3, Cl, , FeCl 3, dark, (o-chlorotoluene), , Cl, (p-chlorotoluene), , For further halogenation, more halogen is used,, , The function of the Lewis acid is to carry the halogens to the aromatic hydrocarbon., If toluene is used instead of benzene, a mixture of o–and p–chlorotoluenes is obtained, since –CH3 group is o, p–directing., , Page number, , 27, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 29 :
HALO ALKANES AND HALOARENES, N 2 Cl, , -, , Cl, CuCl, , N 2 Cl, , N2, , -, , Br, CuBr, , N2, , CuCl, , (ii) By Gattermann reaction: Haloarenes particularly chloro-and bromoarenes can also be, prepared by Gattermann reaction. It is a modification of the Sandmeyer, reaction. In this reaction, a mixture of freshly prepared copper powder in the, presence of HCl or HBr is used. The yields are often around 40%. Thus,, , , , From silver salt or aromatic acids–Hunsdiecker reaction: Like alkyl bromides, aryl, bromides can also be prepared by refluxing the silver salt of aromatic acids with, bromine in boiling carbon tetrachloride., C 6 H 5 COOAg, CCl 4 / Xylene, X 2 , AgX, , C 6 H 5 X CO 2, (Cl 2 or Br 2 ), , Chemical properties of haloarenes:Aryl halides are less reactive than that of alkyl halides towards nucleophilic substitution, reactions., This can be explained as follows:, (i) Resonance Effect: In haloarenes (e.g., chlorobenzene), the lone pairs of electrons on, the halogen atom are delocalized on the benzene ring as shown below:, , Page number, , X, , X, , (I), , (II), , 29, , For any queries, , X, , X, , X, , (III), , (IV), , (V), , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 30 :
HALO ALKANES AND HALOARENES, As a result, C–Cl acquires some double bond character. On the other hand, in case of alkyl, halides (say methyl chloride) carbon is attached to chlorine by a pure single bond., Consequently, C–X bond in aryl halides is little stronger than in alkyl halides, and hence, cannot be easily broken., (ii) Bond energies due to difference in hybridization:, In alkyl halides, the carbon holding halogen is, hybridized. In aryl halides, carbon is, SP2 hybridized; the carbon halogen bond is shorter and stronger and the molecule is, more stable., (iii) Polarity (or Nature) of the carbon halogen bond: Another reason for the low reactivity, of aryl/vinyl halides over alkyl halides is their lesser polar character., , The sp2–hybrid carbon due to greater s–character is more electronegative than a sp3–, hybrid carbon. Therefore, the sp2–hybrid carbon of C–X bond in aryl halides or vinyl, halides has less tendency to release electrons to the halogen than a sp3–hybrid carbon in, alkyl halides. As a result, the C–X bond in aryl halides or vinyl halides is less polar than in, alkyl halides. This is supported by the observation that the dipole moment of, chlorobenzene is just 1.69D as compared to the dipole moment of methyl chloride i.e.,, 1.86 D. Consequently, the halogen atom present in aryl halides cannot be easily displaced, by nucleophiles., 1] Substitution reactions:, Nucleophilic substitution reactions of chlorobenzene are given below, (a) Replacemet of –Cl by –OH: When chlorobenzene is heated at 350°C under high, pressure with caustic soda, phenol is formed (Dow process)., Cl, , OH, , ONa, , NaOH, , 623 K, 300 atm, , H, , Na, , (b) Replacement by methoxy group: Ether is formed when chlorobenzene is heated with, sodium methoxide at 200°C in presence of copper salts., Cl, , OR, Cu salt (catalyst), , NaOR, High pressure, 573 K, Ether, , Page number, , 30, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 42 :
HALO ALKANES AND HALOARENES, NaNH2, , CH3CH2CH(Br)CH2Br, , CH3CH2CCH, , 2HBr, , 12bromobutane, (C), , (D), , CH3CH2CCH + Ag2O, But1yne, , CH3CH2CCAg, , Tollen’s, reagent, , White ppt., , Example 6: Give reasons for the following observations., CH3, , Br, , CH3CBr, , Aq. C2H5OH, , Aq. C2H5OH, , acidic solution ;, , (a), , neutral solution, , CHMe2, F, , OH, , F, , NaOH, , (b), NO2, , + F, , CH3, , NaOH, , ;, , CH3, NO2, , No reaction, , NO2, CH3, , Solution: (a) In aqueous solution, the given alkyl bromide ionizes to give corresponding resonance stabilised, tertiary benzylic carbocation and Br. This carbocation is attacked by ethanol followed by abstraction of proton by, Br to produce corresponding ether and HBr. Due to HBr, the resultant solution becomes acidic., But the aryl bromide cannot ionise even in aqueous solution due to partial double bond between, carbon and bromine as a result of resonance. Therefore no reaction will take place., (b), , In the first aryl fluoride, attack of nucleophile (OH) is favoured by the presence of a strong electron, withdrawing group (NO2) at para position. On the other hand in second aryl fluoride, attack of, nucleophile (OH) is opposed by the presence of an electron donating methyl group at para position., , Example 7: Explain the fact that a small amount of NaI catalyzes the general reaction:, RCl + RO: Na+ ROR + NaCl, Solution: With I ion, the overall reaction occurs in two steps, each of which is faster than the, uncatalyzed reaction., Step 1. RCl + I RI + Cl. This step is faster because I, a soft base has more, nucleophilicity than OR, a hard base., Step 2. RI + RO: ROR + I . This step is faster because I is a better leaving group, than Cl., Example 8: Account for the following observations: (a) tBuF is solvolyzed only in very acidic solutions., (b) tBuCl is solvolyzed more slowly than 2chloro2,3,3trimethylbutane (A). (c) tBuCl is, solvolyzed much faster than 2chloro1,1,1trifluoro2methylpropane (B). (d) tBuCl is, solvolyzed more slowly in 90% D2O10% dioxane than in 90% H2O- 10% dioxane solution., Solution:, (a) F is a poor leaving group but Hbonding with a strong acid encourages its departure. This, is, an example of electrophilic catalysis., , Page number, , 42, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 43 :
HALO ALKANES AND HALOARENES, Me, , (b) Formation of Me3CC, , +, , alleviates some of the steric crowding in (A) induced by the two, , Me, , Me’s and the tBu on the carbon. This is an example of steric acceleration., Me, , (c) F3CCH+ is destabilized by the strongly electronwithdrawing CF3 group, making the, solvolysis of (B) slower., (d) Dbonds are not as stabilizing as Hbonds., , Example 9: Compare the rates of SN1 and SN2 reactions of (a) cyclopropyl and cyclopentyl chloride and, (b) 1chlorobicyclo[2.2.2]octane and 9chlorodecalin (A)., Solution:, (a) Cyclopropyl chloride is much less reactive than cyclopentyl chloride in each type of, reaction because the sp2 hybridised carbon (120° bond angle) created in each transition, state augments the ring strain., (b) The bridgehead halide is inert by both reaction types. A flat R+ cannot be formed at the, bridgehead carbon, making SN1 reaction impossible and the three bridges prevent the, backside attack necessary for SN2 reaction. Furthermore, inversion is impossible. (A) is a, typical 3° halide and reacts rapidly via SN1 reaction, but poorly via SN2 reaction., Example 10: Provide the products of the reactions of the following substrates with NaNO2 in EtOH:, (i) nBuCl and (ii) ClCH2OCH2CH3., Solution: (i) nBuNO2 and (ii) ONOCH2OCH2CH3 + EtOCH2OCH2CH3., The less the positive charge on the attacked carbon, the more likely it will bond to the, less electronegative nucleophilic site of the ambident ion (N). This happens in the SN2 reaction in, (i), where a CN bond forms. The greater the positive charge on the attacked carbon, the more likely it, will bond to the more electronegative nucleophilic site of the ambident ion (O). This happens in the, SN1 reaction in (ii), where a CO bond forms. Since the R+ in (ii) is so stable, it has a long enough, halflife to react with any added nucleophile as well as nucleophilic solvent., , Page number, , 43, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 44 :
HALO ALKANES AND HALOARENES, , SOLVED OBJECTIVE EXAMPLES, Example 1: An optically active 3bromo3methyl hexane on hydrolysis gives, (a) 3methyl3hexanol with retention of configuration., (b) 3methyl3hexanol with inversion of configuration., (c) a partially racemic mixture of 3methyl3hexanol., (d) optically inactive 3methyl3hexanol., Solution: 3bromo3methyl hexane, on ionization gives a 3° carbocation, which can be attacked by nucleophile, (H2O) to give both dextro and levo in unequal amounts of 3methyl3hexanol., , (c), , Example 2:, The halide, which undergoes nucleophilic substitution (by SNAr mechanism) most readily is, (a) pMeC6H4Cl (b), pMeOC6H4Cl, (c) pClC6H4Cl, (d), pO2NC6H4Cl, Solution:, The reaction proceeds by carbanion formation, which can be stabilized by electronwithdrawing groups, present at ortho and/or para positions. The most electronwithdrawing group amongst, all is NO2., (d), , Example 3:, Identify the set of reagents/ reaction conditions ‘X’ and ‘Y’ in the following set of transformations., CH3CH2CH2Br, , (X), , , Product, , (Y), , , CH3CHCH3, Br, , (a), (b), (c), (d), , (X) = dilute aqueous NaOH, 20°C ; (Y) = HBr /acetic acid, 20°C, (X) = concentrated alcoholic NaOH, 80°C ; (Y) = HBr /acetic acid, 20°C, (X) = dilute aqueous NaOH, 20°C ; (Y) = Br2/CHCl3, 0°C, (X) = concentrated alcoholic NaOH, 80°C ; (Y) = Br2 / CHCl3, 0°C, , Solution:, The product obtained in the first reaction can be either CH3CH2CH2OH or CH3CH=CH2, which is a result of, substitution or elimination respectively. If reaction occurs at 20°C, substitution would dominate, elimination, as elimination is favoured only at high temperature. At 80°C, the elimination product is, predominant and the subsequent reaction of addition of HBr to alkene at low temperature would be, greatly favoured., (b), , Example 4:, Treatment of obromofluorobenzene with one equivalent of Mg in presence of ether, generates, , Page number, , 44, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 45 :
HALO ALKANES AND HALOARENES, F, MgBr, , (a), , (b), MgF, Br, , (c), , (d), , Solution:, The reaction proceeds as, F, , F, + Mg(1 equivalent), , Br, , MgBr, unstable, dimerizes, , Biphenylene, , (d), , Example 5:, In nonpolar solvents, which of the following represents correct order of decreasing, nucleophilicity?, (a), OH > MeO > CH 3 CO 2 (b), OH > HS, (c), , F > Cl > Br, , (d), , CH3 > NH 2 > OH > F, , Solution:, In nonpolar solvents, the salts of nucleophile are present as ionpairs in which nearby cations diminish, the reactivity of the anion. The order in which largest nucleophile appears first, will be the right order., Ionpairing in NaOH is stronger than NaOMe or NaHS. In NaF, ionpairing is stronger than NaCl and NaCl, has stronger pairing than NaBr., , (d), , Example 6:, Successful SN2 reactions, (a), are endothermic., (b), proceed with retention of configuration., (c), are stereospecific., (d), occur rapidly in polar protic solvents., Solution:, , Page number, , 45, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 46 :
HALO ALKANES AND HALOARENES, Successful SN2 displacements are exothermic, proceed with inversion of configuration, are favoured in, nonpolar solvents and are stereospecific (because stereoisomeric reactants give stereochemically, different products)., (c), , Example 7:, Arrange following compounds in the decreasing order of their reactivity towards SN2, reaction., , (a), (c), , PhCH2Cl, PhCH(Cl)Me, (A), (B), (C), (b), (A) > (B) > (C), (d), (B) > (C) > (A), , (C) > (B) > (A), (B) > (A) > (C), , PhC(Cl)Me2, , Solution:, The rate of SN2 reaction depends on steric crowding in the transition state. More is the steric crowding,, less stable the transition state is, less will be the rate of SN2 reaction. Thus, the reactivity order of SN2, reaction would be (A) > (B) > (C)., (b), , Example 8:, , For the given compound, , , the nucleophilic substitution by C2H5OH occurs by, Cl, , (a), (c), , SN1 mechanism, SN1 & SN2 both, , (b), (d), , SN2 mechanism, no reaction will occur, , Solution:, In the given compound, SN2 reaction does not occur due to impossibility of attack of nucleophile from rear, side while SN1 reaction is also not possible because the carbocation formed after ionization is not stable, as the bridge head carbon having one carbon bridge cannot be sp2 hybridized., , , , (d), , Example 9:, A SN2 reaction at an asymmetric carbon of a compound always gives, (a), an enantiomer of the substrate. (b), a product with opposite rotation., (c), a mixture of diastereomers., (d), a single stereoisomer., Solution:, A typical SN2 reaction is shown as, , , Y + CX, , Page number, , 46, , RDS, , For any queries, , , , Y, , , , C, , X, , #, , YC + X, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 47 :
HALO ALKANES AND HALOARENES, It is evident that only one product is formed, which is not the enantiomer of the substrate. Moreover, it is, not necessary that the product has opposite optical rotation than that of the substrate; they differ in, configuration only. When the reactant is a stereoisomer, product will also be a single stereoisomer., (d), , Example 10:, Which of the aryl halide undergoes nucleophilic substitution by benzyne mechanism?, Br, , Br, , (a), , (b), CF3, NO2, Br, , Br, CF3, , (c), , NO2, , (d), , Solution:, Unsubstituted aryl halides and those containing electron releasing groups or electron withdrawing groups, at the meta position undergo nucleophilic substitution reactions by benzyne mechanism. Aryl halides, containing electron withdrawing groups at the ortho and/or para positions undergo nucleophilic, substitution by SN AR mechanism which does not involve benzyne intermediate., (a), , Page number, , 47, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 50 :
HALO ALKANES AND HALOARENES, (c) Chloromethane and chloroethane., , (d) Chloromethane and 1chloropropane., , 10., , In which of the following case, formation of butane nitrile is possible, (b) C4H9Br + KCN, (a) C3H7Br + KCN, (c) C3H7OH + KCN, (d) C4H9OH + KCN, , 11., , The compound, added to destroy phosgene gas formed in the oxidation of chloroform, is, (b) CH3COOH, (a) C2H5OH, (c) CH3COCH3, (d) CH3OH, , 12., , Among the following, the one which reacts most readily with ethanol is, (a) pnitrobenzyl bromide., (b) pchlorobenzyl bromide., (c) pmethoxybenzyl bromide., (d) pmethylbenzyl bromide., , 13., , Chloropicrin is obtained by the reaction of, (a) Chlorine on picric acid., (c) Steam on carbon tetrachloride., , (b) Nitric acid on chloroform., (d) Nitric acid on chlorobenzene., , 14., , Benzyl chloride cannot be prepared from toluene by chlorination with, (a) SO2Cl2, (b) SOCl2, (c) Cl2/h, (d) (CH3)3COCl, , 15., , Ethylene dibromide on heating with metallic sodium in ether solution yields, (a) cyclobutane, (b) ethyne, (d) 1butene, (c) 2butene, , 16., , What is the end product (C) of the following sequence of reactions?, NBS, , HBr / ROOR, , Mg / , , CH3CH=CH2 (A) (B) (C), (a) Cyclopropane, (b) Propane, (c) BrMgCH2CH2CH2CH2MgBr, (d) None of these, 17., , Ethylene dichloride and ethylidine chloride are isomeric compounds. The false statement about, these isomers is that they, (a) react with alcoholic potash and give the same product., (b) are position isomers., (c) contain the same percentage of chlorine., (d) are both hydrolysed to the same product., , 18., , Why is chloroform put into dark coloured bottles?, , Page number, , 50, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 51 :
HALO ALKANES AND HALOARENES, (a), (b), (c), (d), , To prevent evaporation., To prevent from moisture., To prevent it from oxidation to form phosgene., To prevent its reaction with glass., , 19., , Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled, A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution., The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution, was added. Substance B gives a yellow precipitate. Which one of the following statements is, true for this experiment?, (b) A was C6H5CH2I, (a) A was C6H5I, (c) B was C6H5I, (d) Addition of HNO3 was unnecessary, , 20., , Which of the following statements is incorrect regarding benzyl chloride?, (a) It gives white precipitate with alcoholic AgNO3., (b) It is an aromatic compound with substitution in the side chain., (c) It undergoes nucleophilic substitution reaction., (d) It is less reactive than vinyl chloride., , 21., , Alkyl halide can be converted into alkene by, (a) nucleophilic substitution reaction., (b) elimination reaction., (c) both nucleophilic substitution and elimination reaction., (d) rearrangement., , 22., , The major product formed in the following reaction is, CH3, CH3CCH2Br, H, CH3, , CH3O, CH3OH, , , (a) CH3CCH2OCH3, , (b) CH3CHCH2CH3, , H, , OCH3, CH3, , CH3, , (c) CH3C=CH2, , (d) CH3CCH3, OCH3, , 23., , Page number, , The major product obtained on treatment of CH3CH2CH(F)CH3 with CH3O / CH3OH is, (a) CH3CH2CH(OCH3)CH3, (b) CH3CH=CHCH3, , 51, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 53 :
HALO ALKANES AND HALOARENES, (c) The presence of an electronwithdrawing substituent at ortho and/or para position, decreases the reactivity of nucleophilic substitution of chlorine in the substituted, chlorobenzene., (d) The replacement of chlorine in chlorobenzene by strong bases proceeds, by SN1 mechanism., 4., , Which of the following compound undergoes replacement of Cl by OH group by merely, warming the compound with aqueous NaOH?, Cl, Cl, Cl, Cl, NO2, NO2, NO2, O 2N, (a), (b), (c), (d), , NO2, , NO2, , 5., , Aryl halides are less reactive towards nucleophilic substitution reactions as compared to alkyl, halides due to, (a) the formation of less stable carbonium ion., (b) partial double bond character of C–X bond due to resonance., (c) shorter carbonhalogen bond due to more s-character of carbon., (d) all of these., , 6., , In the reaction of pchlorotoluene with KNH2 in liquid NH3, the major product is, (a) otoluidine, (b) mtoluidine, (c) ptoluidine, (d) pchloroaniline, , 7., , Which of the following anion has the highest nucleophilicity in nonpolar solvent?, (a) F, (b) OH, (c) CH3, , (d) NH 2, , 8., , The order of reactivities of the following alkyl halides for an SN2 reaction is, (a) RF > RCl > RBr > RI, (b) RF > RBr > RCl > RI, (c) RCl > RBr > RF > RI, (d) RI > RBr > RCl > RF, , 9., , A compound (A) of formula C3H6Cl2 on reaction with alkali can give compound (B) of formula, C3H6O or compound (C) of formula C3H4 depending upon the conditions employed. Compound, (B) on oxidation gave a compound of the formula C3H6O2. Compound (C) with dilute H2SO4, containing Hg2+ ion gave compound (D) of formula C3H6O, which on reaction with bromine and, NaOH gave the sodium salt of C2H4O2. The most probable structure of compound (A) would be, (a) ClCH2CH2CH2Cl, (b) CH3CCl2CH3, , Page number, , 53, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 54 :
HALO ALKANES AND HALOARENES, (c) CH3CH2CHCl2, , (d) CH3CHClCH2Cl, , 10., , Which of the following compound shows highest reactivity towards SN1 reaction?, (b) C2H5Cl, (a) CH3Cl, (c) CH2=CHCl, (d) CH2=CHCH2Cl, , 11., , 1chloro1phenyl ethane undergoes alkaline hydrolysis to give 1phenyl ethanol with, (a) retention of configuration, (b) complete racemization, (c) complete inversion of configuration, (d) racemization plus some inversion, , 12., , For the reaction,, Br, , + CH3CH2ONa+, , , , The major product is formed by, (a) An SN1 reaction, (c) An SN2 reaction, 13., , (b) An E1 reaction, (d) An E2 reaction, , Rank the following species in order of decreasing nucleophilicity in a polar protic solvent., O, , CH3CH2CH2O, (1), , (a) (3) > (1) > (2), (c) (1) > (3) > (2), , CH3CH2CH2S, , CH3CH2CO, , (2), , (3), , (b) (2) > (3) > (1), (d) (2) > (1) > (3), , 14., , Which of the following statement is true?, (a) CH3CH2S is both a stronger base and more nucleophilic than CH3CH2O., (b) CH3CH2S is a stronger base but is less nucleophilic than CH3CH2O., (c) CH3CH2S is a weaker base but is more nucleophilic than CH3CH2O., (d) CH3CH2S is both a weaker base and less nucleophilic than CH3CH2O., , 15., , Which of the following alkyl halide would be most likely to give a rearranged product under SN1, conditions?, (a), , Br, , (b), , Br, (c), 16., , Page number, , Br, , (d), , Br, , Which of the following phrases are not correctly associated with an SN1 reaction?, 1. Rearrangement is possible., , 54, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 55 :
HALO ALKANES AND HALOARENES, 2., 3., 4., 5., (a), (c), 17., , Rate is affected by solvent polarity., The strength of the nucleophile is important in determining the rate., The reactivity series is tertiary > secondary > primary., Proceeds with complete inversion of configuration., 3, 5, (b) 5 only, 2, 3, 5, (d) 3 only, , Reaction of HX with alkene in presence of peroxide follows the mechanism., , ., O, , HX, , ., , hv, , OO, , 2O, , OH + X, ., X, , RCH=CH2, ., , RCHCH2X, ., , (I), ., , (II), , ., , RCHCH2X, HX, , (III), ., , RCH2CH2X + X, , X + X, , X2, , (V), , In case of HI, which step is unfavourable?, (a) (II), (c) (IV), , 18., , (IV), , ., , The major product of the reaction, , CH2CHCH2, , (a), , (b) (III), (d) (V), , (b), , C=CHCH2, , Br, , Br, , CH2CH2CH, , (c), , HBr, , CH=CHCH2, , CH2CH2CH2, , (d), , Br, , Br, 19., , An SN2 reaction at an asymmetric carbon of an optically pure compound always gives:, (a) an enantiomer of the substrate, (b) a product with opposite optical rotation, (c) a mixture of diastereomers, (d) a single stereo isomer, , 20., , Identify the product (Y) formed in the following sequence of reactions:, Benzene (1 eqv .) / Anh. AlCl, , Air / h, 3, CHCl3 , , (X) , , (Y), , O, (a) C6H5–C–Cl, , O, (b) C6H5–C–C6H5, , O, , O, (c) Cl–C–Cl, , Page number, , 55, , For any queries, , (d) C6H5–C–H, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 59 :
HALO ALKANES AND HALOARENES, OTs, , IV., , Mg / THF, , , , (D) Carbanion, , Br, , (E) Benzyne, REASONING TYPE, Directions: Read the following questions and choose, (A) If both the statements are true and statement-2 is the correct explanation of statement-1., (B) If both the statements are true but statement-2 is not the correct explanation of statement1., (C) If statement-1 is True and statement-2 is False., (D) If statement-1 is False and statement-2 is True., 1., , Statement-1: The dipole moment of CH3F is greater than CH3Cl., Statement-2: C-F bond is more polar than C–Cl bond., (a) (A), (b) (B), (c) (C), , (d) (D), , 2., , Statement-1:, Statement-2:, (a) (A), , Nucleophilic substitution of iodoethane is easier than chloroethane., Bond energy of C-I bond is less than that of C–Cl., (b) (B), (c) (C), (d) (D), , 3., , Statement-1:, , 2flourobutane, on reaction with C 2H5 O Na in ethanol gives 1-butene as major, , 4., , 5., , product., Statement-2: 1-Butene is more stable than 2butene., (a) (A), (b) (B), (c) (C), (d) (D), Statement-1: Benzonitrile is prepared by the reaction of chlorobenzene with KCN., Statement-2: Cynide ion is a strong nucleophile., (a) (A), (b) (B), (c) (C), (d) (D), Statement-1: PhNO2 and not C6H6 is used as a solvent for the Friedel-crafts alkylation of PhBr., Statement-2: C6H6 is more reactive than PhBr and would preferentially undergo alkylation., (a) (A), (b) (B), (c) (C), (d) (D), , LINKED COMPREHENSION TYPE, Nucleophilic aliphatic substitution reaction proceeds either by SN1 mechanism or by SN2, mechanism. The SN1 is a two step unimolecular reaction in which a carbocation is first formed as an, intermediate in a slow rate determining step followed by an attack by a nucleophile in a fast step. Since, , Page number, , 59, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 60 :
HALO ALKANES AND HALOARENES, carbocation is planar, the attack by a nucleophile can take place from either side of the plane. An, optically active substrate will give rise to the formation of both (+) and (–) forms of the product. In most, of the cases, the product usually coexists of 5–20% inverted product and 80–95% racemic mixture. The, more stable the carbocation, the greater is the proportion of racemisation., In solvolysis reaction, the more nucleophilic the solvent, the greater is the proportion of inversion., 1., , Which of the following compound will give SN1 reaction?, (I) C6H5–CH2–Br, (II) CH2=CH–CH2–Br, CH3, , (IV) CH3–C–CH2Br, , (III)CH3–CH2–Br, , CH3, , Select the correct answer from the codes given below:, (a) (I), (II) and (III), (b) (I), (II) and (IV), (c) (II), (III) and (IV), 2., , (d) (I), (III) and (IV), , Which one of the following substrates will give maximum racemisation in the following, reaction?, R', , R', H2O, , R–C–X R–C–OH, R' ', , R' ', , CH3, , CH3, , (b) CH2=CH–C–Br, , (a) C6H5–C–CH2CH3, , C2H5, , Br, , Br, , Br, , (c), , C6H5–C–, , (d), , –OCH3, , C6H5–C–, , NH3, , CH3, , 3., , –NO2, , Consider the following four reactions:, (I), , 95% Acetone, (+) C6H5–CH–Cl , C6H5–CH–OH, 5% water, , CH3, , (II), , CH3, , 90% Acetone, (+) C6H5–CH–Cl , C6H5–CH–OH, 10% water, , CH3, , CH3, , 80% Acetone, (III) (+) C6H5–CH–Cl C6H5–CH–OH, 20% water, , CH3, , Page number, , 60, , For any queries, , CH3, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 61 :
HALO ALKANES AND HALOARENES, 100% water, (IV) (+) C6H5–CH–Cl C6H5–CH–OH, , CH3, , CH3, , The correct decreasing order of proportion of inverted product would be, (a) (I) > (II) > (III) > (IV), (b) (II) > (I) > (III) > (IV), (c) (III) > (II) > (I) > (IV), (d) (IV) > (III) > (II) > (I), , EXERCISE – IV, SUBJECTIVE PROBLEMS, 1., , Treatment of Me3CCH=CH2 and Me3CCH(OH)Me with concentrated hydrochloric acid gives the, same two isomeric alkyl chlorides. What are these two products? Explain., , 2., , Propose a mechanism for the following reaction., , CH3, , CH3, CH3OH, , C CH3, , CH3, , Cl, 3., , Using the given starting material and any necessary organic or inorganic reagents, indicate how, the desired compounds could be synthesized., (a), , CH2CH3, , CH=CH2, , (b) CH3CH2CH=CH2 CH3CH2CH2CH2NH2, (c) HOCH2CH2CH=CH2 , O, , (d), OCH3, , (e) CH3CH2CH=CH2 CH2=CHCH=CH2, 4., , Two elimination products are obtained from the following E2 reaction., OH , , CH3CH2CH(D)CH2Br ? + ?, (a) What are the eliminations products?, (b) Which is formed in greater yield? Explain., 5., , Page number, , The rate law for the substitution reaction of 2bromobutane and OH in, 75% ethanol 25% water at 30°C is, Rate = 3.2 105 [2bromobutane] [OH] + 1.5 106 [2bromobutane], , 61, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 62 :
HALO ALKANES AND HALOARENES, , 6., , (a) What percent of the reaction takes place by the SN2 and SN1 mechanisms when, [OH] = 1.0 M?, (b) What percent of the reaction takes place by the SN2 and SN1 mechanisms when, [OH] = 0.001 M?, Which reaction in each of the following pairs will take place more rapidly?, , Cl, , (a), , Cl, (b), , O, , S, , + Cl, , (CH3)2CHS, , + Cl, , S, , Cl, , HO, H2O, , Cl, , HO, H2O, , Cl, , (c), , CH3S, , OH + Cl, , H2O, , OH + HCl, , H2O, , Cl, , OH + Cl, , O, , OH + HCl, , H O, , (d) (CH3)3CBr 2 (CH3)3COH + HBr, CH CH OH, , 3, 2, , (CH3)3COCH2CH3 + HBr, (CH3)3CBr , , 7., , Which of the following compound would you expect to be more reactive in an SN2 reaction?, H, H, , H, or, , H, , H, , Br, , CH3, CH3, , Br, , CH3, CH3, , H, , 8., , 3Bromo3methyl1butene forms two substitution products when it is added to a solution, of sodium acetate in acetic acid., (a) Give the structures of the substitution products., (b) Which is the kinetically controlled product?, (c) Which is the thermodynamically controlled product?, , 9., , Only one halo ether (ignoring stereoisomers) is obtained from the reaction of the following alkyl, dihalide with methanol. Give the structure of the halo ether., Cl, , 10., , Page number, , Br, Propose a mechanism for each of the following reactions:, , 62, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 63 :
HALO ALKANES AND HALOARENES, CH3, , CH3, OH, , H2O, , CHBr, , (a), , H, H2 O, , (b), H3C, , CHCH3, Br, , HO, , CH3, , H, +, , H3C, , CH3, , CH3, , OH, , ANSWERS, EXERCISE –I, JEE & NEET-SINGLE CHOICE CORRECT, , 1. (a), , 2. (a), , 3. (b), , 4. (a), , 5. (d), , 6. (b), , 7. (b), , 8. (c), , 9. (a), , 10. (a), , 11. (a), , 12. (c), , 13. (b), , 14. (b), , 15. (a), , 16. (a), , 17. (d), , 18. (c), , 19. (a), , 20. (d), , 21. (b), , 22. (c), , 23. (c), , 24. (a), , 25. (c), , Page number, , 63, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 64 :
HALO ALKANES AND HALOARENES, , EXERCISE – II, IIT-JEE SINGLE CHOICE CORRECT, , 1. (b), , 2. (d), , 3. (b), , 4. (d), , 5. (c), , 6. (b), , 7. (c), , 8. (d), , 9. (c), , 10. (d), , 11. (d), , 12. (d), , 13. (b), , 14. (c), , 15. (c), , 16. (a), , 17. (b), , 18. (c), , 19. (d), , 20. (a), , ONE OR MORE THAN ONE CHOICE CORRECT, , 1. (a,b,c), , 2. (a,b,c,d), , 3. (a,b,d), , 4. (a,b,c,d), , 6. (a,b,c,d), , 7. (b,c), , 8. (c,d), , 9. (b,c,d), , 5. (a,b), 10. (a,b,c), , EXERCISE – III, MATCH THE FOLLOWING, I (D), (C) ; II (A) ; III (B), (C) ; IV (E), , 1., , REASONING TYPE, 1. (d), , 2. (a), , 3. (c), , 4. (d), , 5. (a), , LINKED COMPREHENSION TYPE, 1. (b), , 2. (c), , 3. (a), , EXERCISE – IV, SUBJECTIVE PROBLEMS, 1. Both compounds give same carbonium ion initially., , Page number, , 64, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 66 :
HALO ALKANES AND HALOARENES, 3.2 10 5 [2 bromobu tan e], , % of SN2 reaction =, , 33.5 10 6 [2 bromobu tan e], , 100 = 95.5%, , (b) Rate = [2bromobutane] (3.2 108 + 1.5 106) = 1.532 ×106 [2bromobutane], % of SN1 reaction =, , % of SN2 reaction =, 6. (a), , (b), , 1.5 10 6 [2 bromobu tan e], 1.532 10 6 [2 bromobu tan e], 0.032 10 6 [2 bromobu tan e], 1.532 10 6 [2 bromobu tan e], , 100 98%, 100 2%, , CH3S, , + Cl, S, The nucleophile CH3S– is less sterically hindered than (CH3)2CHS–., Cl, , HO, , O, Cl H2O, OH + Cl, The electron–withdrawing oxygen increases the electrophilicity of the carbon that the, nucleophile attacks because of the stabilization of the carbocation produced (SN1, reaction), O, , O, , Cl, , –Cl, , , , .. CH2, O, .., , , , O, .., , CH2, , H2O, –H+, , O, Cl, , (c), , H2O, , OH, , OH + HCl, , Steric strain is decreased when Cl– dissociates to form the carbocation in the, rate–limiting step since the hybridization of the carbon atom changes from sp3 to sp2,, allowing the bond angle between the bulky groups to increase from 109.5° to 120°. This is, referred as steric acceleration., H O, , (d) (CH3)3CBr 2 (CH3)3COH + HBr, Because the reactants are neutral, the reaction will be faster in the more polar solvent., H2O is more polar than EtOH., 7. The all–cis isomer is more reactive in an SN2 reaction., In an SN2 reaction, the leaving group must be in an axial position in order to allow backside, attack to occur without steric hindrance from the cyclohexane ring. When the bromine is in, the axial position in the all–cis isomer, both methyl substituents are in equatorial positions, so, the reaction takes place easily as the nucleophile does not experience any crowding when it, approaches from 180° w.r.t. Br. In the other isomer, (all trans), the conformer has bromine in the axial position and both methyl substituents in, , Page number, , 66, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 71 :
HALO ALKANES AND HALOARENES, (d) trans-but-2-ene, 13. 2,4,6-Trinitrochlorobenzene on warming with water produces, (a) chlorobenzene, (b) picric acid, (c) phenol, (d) no compound since C – Cl bond is stable., , (UP CPM), , (AMU (Med.)), , 14. Which one of the following compounds cannot be used for the preparation of Gignard reagent?, (a) C2H5Br, (b) HO – CH2CH2Cl, (c) C6H5CH2Cl, (AIPMT (Mains)), (d) CH2 = CH – CH2Cl, 15. Assertion: 1,2-dichloroethane is optically active., Reason: Meso compound is optically active., (a) If both assertion & reason are true & reason is the correct explanation of assertion., (b) If both assertion & reason are true but reason is not the correct explanation of assertion., (c) If assertion is true but reason is false., (d) If both assertion & reason are false., (AIIMS), 16. An aromatic compound C7H6Cl2 (A), gives AgCl on boiling with alcoholic AgNO2 solution & yields, C7H7OCl on treatment with sodium hydroxide. (A) on oxidation gives monochlorobenzoic acid., The compound (A) is:, (a), CH2Cl, , Cl, CH2Cl, Cl, Cl, , (b), , Cl, CH2Cl, Cl, , (c), , Cl, (d), , CH2Cl, (AIIMS), , 17. A nucleophilic substitution reaction proceeds through SN1 mechanism. So, the reaction is, (a) unimolecular, (b) bimolecular, (c) termolecular, , Page number, , 71, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 74 :
HALO ALKANES AND HALOARENES, 28. If there is no rotation of plane polarised light by a compound in a specific solvent, though to be chiral,, it may mean that,, (a) the compound is certainly meso, (b) there is no compound in the solvent, (c) the compound may be a racemic mixture, (d) the compound is certainly a chiral, (AIPMT), 29. CH3 – CHCl – CH2 – CH3 has a chiral centre. Which one of the following represents its R-configuration?, (a), , C2H5, H – C – CH3, Cl, , (b), , C2H5, Cl – C – CH3, H, , (c), , CH3, H – C – Cl, C2H5, , (d), , C2H5, H3C – C – Cl, H, , (AIPMT), , 30. Pick out the correct statements., (i) If a compound has no asymmetric carbon atom it is always achiral., (ii) If a compound has just one asymmetric carbon atom, it is chiral., (iii) If a compound has more than one asymmetric carbon atoms, it may or may not be chiral., (a) (i), (ii)& (iii) are correct., (b) (i) & (ii) only are correct, (c) (ii) & (iii) only are correct., (d) only (ii) is correct., , Page number, , 74, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 75 :
HALO ALKANES AND HALOARENES, (e) only (i) is correct., 31. Which of the following is optically inactive?, (a), , (Kerala PMT), , H, H3C, Cl, , Cl, CH3, H, H, , (b), Cl, H3C, , CH3, Cl, H, H, , (c), H3C, H3C, , Cl, Cl, , H, (d) None of these, 32. What happens when chloroform is left open in air in the presence of sunlight?, , (AIIMS), , (a) Explosion takes place., (b) Phosgene, a poisonous gas is formed., (c) Polymerisation takes place., (d)No reaction takes place., 33. Assertion: Chloroform is stored in dark coloured bottles., Reason: Chronic chloroform exposure may cause damage to liver & kidneys., , (AIIMS), , (a) If both assertion & reason are true & reason is the correct explanation of assertion., (b) If both assertion & reason are true but reason is not the correct explanation of assertion., (c) If assertion is true but reason is false., (d) If both assertion & reason are false., 34. A sample of chloroform before being used as an anaesthetic agent is tested by, (a) Fehling’s solution, (b) ammoniacal cuprous chloride, (c) silver nitrate solution in the cold, (d) silver nitrate solution after boiling with alcoholic KOH., 35. The organic halogen compound used as refrigerant in refrigerators & air-conditioners is, (a) DDT, (b) Freon, (c) BHC, (d) BFC, (e) CCl4, , Page number, , (AIIMS), , (AFMC), , (Kerala PMT), , 75, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 76 :
HALO ALKANES AND HALOARENES, , 36. Chemical formula of phosgene is, (a) COCl2, (b) CaOCl2, (c) CaCO3, (d) COCl, (AFMC), 37. Chronic chloroform exposure may cause damage to liver & kidney, due to the formation of, (a) phosgene, (b) methylene chloride, (c) methyl chloride, (d) carbon tetrachloride, (e) phosphoryl chloride, 38. Which of the compounds is synthesised by chloral?, , (Kerala PMT), , (a) DDT, (b) BHC, (c) Chloroform, (d) Michlers ketone, (AIIMS), 39. CCl4 is well known fire exhauster. However after using it to extinguish fire, the room should be well, ventilated. This is because, (a) it is flammable aat higher temperatures, (b) it is toxic, (c) it produces phosgene by reaction with water vapour at higher temperatures, (d) it is corrosive, (e) it is anaesthetic, 40. Assertion: Chloral reacts with phenyl chloride to form DDT., Reason: It is an electrophilic addition reaction., , (Kerala PMT), , (a) If both assertion & reason are true & reason is the correct explanation of assertion., (b) If both assertion & reason are true but reason is not the correct explanation of assertion., (c) If assertion is true but reason is false., (d) If both assertion & reason are false., (AIIMS), 41. In solvents like DMSO, acetonitrile, F ion of dissolved NaF is more reactive than in methyl alcohol, explains, (a) CH3OH is more poar than DMSO & CH3CN, (b) CH3OH is less polar than DMSO & CH3CN, (c) Unsolvated F- ion in DMSO or CH3CN acts more effectively as nucleophile, (d) –OH group is a better leaving group than F- ion, 42. Arrange the given compounds in decreasing order of boiling points, CH3, , Page number, , 76, , For any queries, , ACTIVE SITE EDUTECH:, , (AIIMS), , Contact No. : 9844532971
Page 77 :
HALO ALKANES AND HALOARENES, CH3 CH2 CH2 CH2 Br, , CH3 – C – Br, , CH3 – CH2 – CH - Br, , CH3, (I), , (II), , CH3, (III), , (a) I > III > II, (b) II > I > III, (c) I > II > III, (d) III > I > II, , (AIIMS), , 43. Assertion: Chlorobenzene is more reactive than benzene towards the electrophilic substitution, reaction., Reason: Resonance destabilises the carbocation., (a) If both assertion & reason are true & reason is the correct explanation of assertion., (b) If both assertion & reason are true but reason is not the correct explanation of assertion., (c) If assertion is true but reason is false., (d) If both assertion & reason are false., (AIIMS), 44. The products expected to be formed in the Wurtz reaction of a mixture of neopentyl bromide &, isobutyl bromide are, (i) 2,2,4-trimethylpentane, (ii) 2,2,5,5-tetramethylhexane, (iii) 2,2,4,4-tetramethylhexane, (iv) 2,5-dimethylhexane, (v) 2,2,5-trimethylhexane, (a) (ii), (iii) & (v), (b) (ii), (iv) & (v), (c) (i), (iv) & (v), (d) (i), (iii) & (v), (e) (i), (ii) & (iv), , (Kerala PMT), , 45. The correct sequence of reactions to be performed to convert benzene into m-bromoaniline is, (a) nitration, reduction, bromination, (b) bromination, nitration, reduction, (c) nitration, bromination, reduction, (d) reduction, nitration, bromination, (Kernataka CET), 46. Alkyl halide can be converted into alcohol in a single step reaction. It’s an example of ................., reaction., (a) electrophilic substitution, (b) nucleophilic substitution, , Page number, , 77, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 78 :
HALO ALKANES AND HALOARENES, (c) electrophilic addition, (d) free radical substitution, (OJEE), 47. Which of the following compounds undergoes nucleophilic substitution reaction most easily?, (a) Cl, , NO2, Cl, (b), , CH3, Cl, (c), , OCH3, (d), , Cl, (AIPMT (Mains)), , 48. Which of the following will give yellow precipitate on shaking with an aqueous solution of NaOH, followed by acidification with dil. HNO3 & addition of AgNO3 solution?, (a), , Cl, , Br, (b), , CH2 – Cl, , (c), , I, Br, CH3, , (d), , Page number, , Cl, , 78, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971
Page 83 :
HALO ALKANES AND HALOARENES, Toluene, , Benzyl chloride, , Benzyl alcohol, , 28. (a) Meso compound does not rotate plane polarised light. Compound which contains tetrahedral atoms with, four different froups but has a plane of symmetry & is optically inactive. One of the asymmetric carbon, atoms turns the plane of polarised light to the right & other to the left & to the same extent so that the, rotation due to upper half is compensated by the lower half, i.e., internally compensated, & finally there, is no rotation of plane polarised light., 29. (b), 30. (c), 31. (c) Due to internal compensation, this compound is strictly inactive., H, H3C, Cl, 1, Cl, H3C, 2, H, Meso, , 32. (b) CHCl3 + [O], , COCl2 + HCl, , phosgene, , 33. (b) Chloroform is slowly oxidised by air in the presence of light to an extremely poisonous gas ‘phosgene’., Therefore, it is stored in dark bottles, completely filled so that air is kept out., 34. (c) Chloroform is tested before use for the presence of extremely poisonous gas phosgene, that may be, formed, on storage. If CHCl3 sample contains phosgene then it will give a white ppt. when treated with AgNO3, solution in the cold. However, after boiling CHCl3 with alcoholic KOH, the resulting solution will always, give white ppt. with AgNO3 solution whether it contains COCl2 or not., , 35. (b) Chlorofluorocarbon’s (CFC’s) or feron are used as refrigerant in refrigerators & air-conditioners., 36. (a) Carbonyl chloride is commonly known as phosgene & its chemical formula is COCl2., 37. (a) Chronic chloroform exposure causes damage in liver & kidney as CHCl3 decomposes slowly into, phosgene & hydrogen chloride., CHCl3 + [O], , Light & air / CCl3OH, , COCl2 + HCl, , Chloroform, , Phosgene, , 38. (a), , Cl, 2C6H5Cl + Cl3CCHO H2SO4 Cl3C – H, Cl, DDT, , 39. (c) Carbon tetrachloride vapours react with steam above 5000C to form phosgene, a poisonous gas., , Page number, , 83, , For any queries, , ACTIVE SITE EDUTECH:, , Contact No. : 9844532971