Page 1 :
E, D, , K, A, K, L, HALOGENUDERIVATIVES, F, A, PR, Career Point Katol
Page 2 :
Introduction, • The parent family of organic compounds is, hydrocarbon., • Replacement of hydrogen atom/s in aliphatic, or aromatic hydrocarbons by halogen atom/s, results in the formation of halogen, derivatives of hydrocarbons., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 3 :
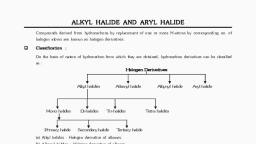
Classification of halogen derivatives :, Halogen derivatives of hydrocarbons are, classified mainly in two ways., 1. On the basis of hydrocarbon skeleton to, which halogen atom is bonded., 2. On the basis of number of halogen atoms., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 4 :
Classification of halogen derivatives :, a. On the basis of hydrocarbon skeleton to which, halogen atom is bonded, the halogen, derivatives are classified as haloalkanes,, haloalkenes, haloalkynes and haloarenes., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 5 :
b. On the basis of number of halogen atoms,, halogen derivatives are classified as mono, di,, tri or poly halogen compounds., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 6 :
Classification of monohalogen compounds :, Monohalogen compounds are further classified, on the basis of position of halogen atom and the, type of hybridization of carbon to which halogen, is attached., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 7 :
a. Alkyl halides or haloalkanes :, • In alkyl halides or haloalkanes the halogen, atom is bonded to sp3 hybridized carbon which, is a part of saturated carbon skeleton., • Alkyl halides may be primary, secondary or, tertiary depending on the substitution state of, the carbon to which halogen is attached, , P, , A, R, , K, L, FU, , K, A, , E, D
Page 9 :
c. Benzylic halide :, In benzylic halides halogen atom is bonded to a, sp3 hybridized carbon atom which is further, bonded to an aromatic ring., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 11 :
e. Haloalkyne :, When a halogen atom is bonded to a sp, hybridized carbon atom it is a haloalkyne., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 13 :
Nomenclature of halogen derivatives:, • Common names of alkyl halides are derived by, , E, D, , naming the alkyl group followed by the name of, , K, L, U, F, For example, A, methyl iodide, tert-butyl chloride., R, P, According to IUPAC system of nomenclature, halogen as halide., , •, •, , K, A, , alkyl halides are named as haloalkanes.
Page 14 :
Nomenclature of halogen derivatives:, • Aryl halides are named as haloarenes in, •, , E, D, common as well as IUPACKsystem., A, K, For dihalogen derivative, of an arene, prefix o-,, L, U, F, m-, p- are used, in common name system but in, A, R, P, IUPAC system the numerals 1,2 ; 1,3 and 1,4, respectively are used.
Page 15 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 16 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 17 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 18 :
Methods of preparation of alkyl halides:, From alcohol :, • The most widely used method of preparation, of alkyl halide is replacement of hydroxyl, group of an alcohol by halogen atom., • The hydroxyl group may be replaced by, halogen atom using, (a) halogen acid,, (b) phosphorous halide or, (c) thionyl chloride., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 19 :
a. By using halogen acid or hydrogen halide, (HX) :, • The conditions for reaction of alcohol with, halogen acid (HX) depend on the structure of, the alcohol and particular halogen acid used., • The order of reactivity of alcohols with a, given haloacid is 30>20>10., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 20 :
• Hydrogen chloride is used with zinc chloride, (Grooves' process) for primary and, secondary alcohols., • Tertiary, alcohols, readily, react, with, concentrated hydrochloric acid in absence of, zinc chloride., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 21 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 23 :
• Good yield of alkyl iodides may be obtained by, heating alcohols with sodium or potassium, iodide in 95 % phosphoric acid., • Here HI is generated in situ., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 24 :
E, D, , K, A, Phosphoric acid is used, instead of sulphuric, K, L, acid because sulphuric, acid oxidizes iodide, U, F, A, ions to iodine, and produces hardly any, R, hydrogen P, iodide.
Page 25 :
b. By using phosphorous halide :, • An alkyl halide may be prepared by action of, phosphorous halide on alcohol., • Phosphorous tribromide and triiodide are, usually generated in situ (produced in the, reaction mixture) by the action of red, phosphorous, on, bromine, and, iodine, respectively., • Phosphorous pentachloride reacts with, alcohol to give alkyl chloride., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 26 :
b. By using phosphorous halide :, • Phosphorous pentachloride, alcohol to give alkyl chloride., , P, , A, R, , K, L, FU, , K, A, , E, D, , reacts, , with
Page 27 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 28 :
c. By using thionyl chloride :, • Thionyl chloride reacts with straight chain, primary alcohols to give unrearranged alkyl, chloride., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 29 :
c. By using thionyl chloride :, • The byproducts obtained are gases., • There is no need to put extra efforts for its, separation., • Therefore this method is preferred for, preparation of alkyl chloride., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 30 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 32 :
Problem 10.1 : How will you obtain, 1-bromo-1-methylcyclohexane from alkene?, Write possible structures of alkene and the, reaction involved., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 33 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 34 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 35 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 36 :
Halogen exchange :, • Alkyl iodides are prepared conveniently by, treating alkyl chlorides or bromides with, sodium iodide in methanol or acetone, solution., • The sodium bromide or sodium chloride, precipitates from the solution and can be, separated by filtration., , P, , A, R, , K, L, FU, , E, D, , K, A, , The reaction is known as Finkelstein reaction.
Page 38 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 39 :
Electrophilic substitution:, • Aryl chlorides and bromides can be prepared, by direct halogenation of benzene and its, derivatives through electrophilic substitution., • It may be conveniently carried out in dark at, ordinary temperature in presence of suitable, Lewis acid catalyst like Fe, FeCl3 or, anhydrous AlCl3., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 40 :
• When toluene is brominated in presence of, iron, a mixture of ortho and para bromo, toluene is obtained., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 41 :
• Aromatic electrophilic substitution with iodine, is reversible., , •, , E, D 4 removes HI by, In this case use of HNOK, 3/HIO, A, K, oxidation to I2, equilibrium, is shifted to right, L, U, F, and iodo product, is formed., A, PR, , • F2 being highly reactive, fluoro compounds, are not prepared by this method.
Page 42 :
Sandmeyer's reaction :, • Aryl halides are most commonly prepared by, replacement of nitrogen of diazonium salt., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 43 :
Physical properties :, • Physical properties of alkyl halides are, considerably different from those of, corresponding alkanes., • The boiling point of alkyl halides is, determined by polarity of the C-X bond as, well as the size of halogen atoms., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 44 :
Nature of intermolecular forces:, • Halogens (X = F, Cl, Br and I) are more, electronegative than carbon., • Carbon atom that carries halogen develops a, partial positive charge while the halogen, carries a partial negative charge., • Thus carbon-halogen bond in alkyl halide is a, polar covalent bond., • Therefore alkyl halides are moderately polar, compounds., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 45 :
• Size of the halogen atom increases from, fluorine to iodine., • Hence the C-X bond length increases., • The C-X bond strength decreases with an, increase in size of halogen., • This is because as the size of p-orbital of, halogen increases the p-orbital becomes, more diffused and the extent of overlap with, orbital of carbon decreases., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 46 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 47 :
Boiling point :, • Boiling points of alkyl halides are considerably, higher than those of corresponding alkanes due, to higher polarity and higher molecular mass., • Within alkyl halides, for a given alkyl group, the, boiling point increases with increasing atomic, mass of halogen, because magnitude of van der, Waals force increases with increase in size and, mass of halogen., • Thus boiling point of alkyl halide decreases in, the order RI > RBr > RCl > RF, , P, , A, R, , K, L, FU, , K, A, , E, D
Page 49 :
For the given halogen, boiling point rises with, increasing carbon number., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 50 :
For isomeric alkyl halides, boiling point, decrease with increased branching as surface, area decreases on branching and van der Waals, forces decrease. For example :, , P, , A, R, , K, L, FU, , K, A, , E, D
Page 51 :
Solubility :, • Though alkyl halides are moderately polar,, they are insoluble in water., • It is due to inability of alkyl halides to form, hydrogen bonds with water., • Attraction between alkyl halide molecules is, stronger than attraction between alkyl halide, and water., • Alkyl halides are soluble in non-polar organic, solvents., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 52 :
• Aryl halides are also insoluble in water but, soluble in organic solvents., • If aryl halides are not modified by presence of, any other functional group, they show, properties similar to corresponding alkyl, halides., • The isomeric dihalobenzenes have nearly the, same boiling points, but melting points of, these isomers show variation., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 53 :
• Melting point of para isomer is quite high, compared to that of ortho or meta isomer., • This is because of its symmetrical structure, which can easily pack closely in the crystal, lattice., • As a result intermolecular forces of, attraction are stronger and therefore greater, energy is required to overcome its lattice, energy., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 54 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 55 :
Problem 10.2 Arrange the following compounds, in order of increasing boiling points :, bromoform, chloromethane, dibromomethane,, bromomethane., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 56 :
Optical isomerism in halogen derivatives :, • Isomers, , having, , the, , same, , bond, , connectivity's, thatDE is, structural, K, A, formula are called stereoisomers, ., K, , P, , A, R, , L, FU
Page 57 :
Chiral atom and molecular chirality:, Let us, now, jot down the atoms/groups attached, to each carbon in 2 - chlorobutane., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 58 :
• Carbon atom in a molecule which carries four, different groups/atoms is called chiral carbon, atom., • Thus, the C-2 in 2-chlorobutane is a chiral, carbon., • Chiral atom in a molecule is marked with, asterisk (*)., • For example, CH3-*CHCl-CH2-CH3., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 59 :
• When a molecule contains one chiral atom, it, acquires a unique property., • Such a molecule can not superimpose, perfectly on its mirror image., • It is called chiral molecule., • A chiral molecule and its mirror image are, not identical, , P, , A, R, , K, L, FU, , K, A, , E, D
Page 60 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 61 :
• A chiral molecule and its mirror image both, have the same structural formula and, of, , E, D, , course, the same molecular formula., •, , K, A, The spatial arrangement, of the four different, K, L, groups aroundFU, the chiral atom, however, is, A, R, P, different., , • In other words, a chiral molecule and its mirror, image are stereoisomers of each other.
Page 62 :
• The relationship between a chiral molecule, and its mirror image is similar to the, •, , E, D right hands., relationship between left, and, K, A, K, Therefore it is called, handedness or chirality., L, U, F, (Origin : Greek, word : Cheir means hand), A, PR
Page 63 :
• The stereoisomerism in which the isomers, have different spatial arrangements of, groups/ atoms around a chiral atom is called, optical isomerism., • The optical isomers differ from each other in, terms of a measurable property called optical, activity., • To understand optical activity, we must know, what is plane polarized light., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 64 :
Plane polarized light :, • An ordinary light consists of electromagnetic, waves having oscillations of electric and, magnetic field in all possible planes, perpendicular to direction of propagation of, light., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 65 :
• When ordinary light is passed through Nicol's, prism, oscillations only in one plane emerge, out., • Light having oscillations only in one plane, perpendicular to direction of propagation of, light is known as plane polarized light., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 66 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 67 :
Optical activity :, • When an aqueous solution of certain organic, compounds like sugar, lactic acid is placed in, the path of plane polarized light, the, transmitted light has oscillations in a, different plane than the original., • In other words, the incident light undergoes, rotation of its plane of polarization., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 68 :
• The plane of polarization rotates either to the, right (clockwise) or to the left (anticlockwise)., , E, D, , • Property of a substance by which it rotates, plane of polarization of incident plane, polarized light is known as optical activity., , K, L, FU, , A, compounds, PR, , K, A, , • The, which rotate the plane of, plane polarized light are called optically, active compounds and those which do not, rotate it are optically inactive compounds.
Page 69 :
• Optical activity of a substance is expressed, numerically in terms of optical rotation., , E, D, , • The angle through which a substance rotates, the plane of plane polarized light on passing, through it is called optical rotation., , K, L, FU, , A, accordance, PR, , K, A, , • In, with the direction of optical, rotation an optically active substance is, either dextrorotatory or laevorotatory.
Page 70 :
• A compound which rotates the plane of plane, polarized light towards right is called, dextrorotatory and designated by symbol dor by (+) sign., , K, L, FU, , K, A, , E, D, , • A compound which rotates plane of plane, polarized light towards left is called, laevorotatory and designated by symbol l-or, by (-) sign., , P, , A, R
Page 71 :
• Isomerism in which isomeric compounds have, different optical activity is known as optical, isomerism., , K, A, , E, D, , • French scientist Louis Pasteur first recognized, that optical activity is associated with certain, type of 3-dimensional structure of molecules., , P, , A, R, , K, L, FU, , • Pasteur introduced the term enantiomers for, the optical isomers having equal and opposite, optical rotation.
Page 72 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 73 :
Remember..., • Optical activity is an experimentally observable, property of compounds. Chirality is a description of, molecular structure., Optical activity is the consequence of chirality., • Molecules which contain one chiral atom are chiral,, that is, they are nonsuperimposable on their mirror, image., • The two non-superimposable mirror image structures, are called pair of enantiomers., • Enantiomers have equal and opposite optical rotation., Thus, enantiomers are a kind of optical isomers., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 74 :
Enantiomers :, • The optical isomers which are nonsuperimposable mirror image of each other, are called enantiomers or enantiomorphs or, optical antipodes., • For example, 2 - chlorobutane exists as a, pair of enantiomers, , P, , A, R, , K, L, FU, , K, A, , E, D
Page 75 :
• Enantiomers have identical physical properties, (Such as melting point, boiling points,, densities, refractive index) except the sign of, optical rotation., • The magnitude of their optical rotation is equal, but the sign of optical rotation is opposite., • They have identical chemical properties except, towards optically active reagent., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 77 :
Representation of configuration of molecules :, , P, , A, R, , K, L, FU, , K, A, , E, D
Page 78 :
a. Fischer projection formula (cross formula) :, Two representations are used to represent, configuration of chiral carbon and the 3dimensional structure of optical isomers on, plane paper., These are, (a) Wedge formula and, (b) Fischer projection formula, , P, , A, R, , K, L, FU, , K, A, , E, D
Page 79 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 80 :
a. Wedge formula :, • When a tetrahedral carbon is imagined to be, present in the plane of paper all the four bonds at, this carbon cannot lie in the same plane., • The bonds in the plane of paper are represented by, normal lines,, • The bonds projecting above the plane of paper are, represented by solid wedges (or simply by bold, lines), • While bonds going below the plane of paper are, represented by broken wedges (or simply by, broken lines)., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 81 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 82 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 83 :
Chemical properties :, Laboratory test of haloalkanes :, • Haloalkanes are of neutral type in aqueous, medium., • On warming with aqueous sodium or, potassium hydroxide the covalently bonded, halogen in haloalkane is converted to halide, ion., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 84 :
• When this reaction mixture is acidified by, adding dilute nitric acid and silver nitrate, solution is added a precipitate of silver halide, is formed which confirms presence of, halogen in the original organic compound., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 85 :
Nucleophilic, substitution, reactions, of, haloalkanes :, • When a group bonded to a carbon in a, substrate is replaced by another group to get, a product with no change in state of, hybridization of that carbon the reaction is, called substitution reaction., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 86 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 87 :
• The C-X bond in alkyl halides is a polar, covalent bond and the carbon in C-X bond is, positively polarized., • The C-X carbon is an electrophilic centre and, it has a tendency to react with a nucleophile., • Alkyl halides react with a variety of, nucleophiles to give nucleophilic substitution, reactions (SN)., • The reaction is represented in general form, as shown below., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 88 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 89 :
• When a substrate reacts fast it is said to be, reactive., • The reactivity of alkyl halides in SN reaction, depends upon two factors, namely, the, substitution state (10, 20 or 30) of the carbon, and the nature of the halogen., • The order of reactivity influenced by these, two factors is as shown below., Tertiary alkyl halide (30) > secondary alkyl halide, (20) >primary alkyl halide (10), , P, , A, R, , K, L, FU, , E, D, , K, A, , R - I > R - Br > R - Cl
Page 90 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 91 :
Do you know ?, Cyanide ion is capable of attacking through more, than one site (atom)., , K, A, , E, D, , • Such nucleophiles are called ambident nucleophiles., • KCN is predominantly ionic (K⊕C- ≡ N) and provides, cyanide ions., • Both carbon and nitrogen are capable of donating, electron pair., • C-C Bond being stronger than C-N bond, attack occurs, through carbon atom of cyanide group forming alkyl, cyanides as major product., , P, , A, R, , K, L, FU
Page 92 :
Do you know ?, • However AgCN (Ag-C ≡ N) is mainly covalent, compound and nitrogen is free to donate pair, of electron., • Hence attack occurs through nitrogen, resulting in formation of isocyanide., • Another ambident nucleophile is nitrite ion,, which can attack through ‘O’ or ‘N’., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 93 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 94 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 95 :
Mechanism of SN reaction :, • The halogen atom of alkyl halide is, therefore, called, ‘leaving group’ in the context of this reaction., • Leaving group is the group which leaves the carbon, by taking away the bond pair of electrons., • The substrate undergoes two changes during a SN, reaction., • The original C-X bond undergoes heterolysis and a, new bond is formed between the carbon and the, nucleophile using two electrons of the nucleophile., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 96 :
• These changes may occur in one or more, steps., • The description regarding the sequence and, the way in which these two changes take, place in SN reaction is called mechanism of, SN reaction., • The mechanism is deduced from the results, of study of kinetics of SN reactions., • Two mechanisms are observed in various SN, reactions. These are denoted as SN1 and SN2, mechanisms., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 97 :
a. SN2 Mechanism :, • The reaction between methyl bromide and, hydroxide ion to give methanol follows a, second order kinetics., • The rate of this reaction depends on, concentration of two reacting species,, namely, methyl bromide and hydroxide., • Hence it is called subtitution nucleophilic, bimolecular, SN2., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 98 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 99 :
•, •, •, •, , Rate of a chemical reaction is influenced by the, chemical species taking part in the slowest step, of its mechanism., In the above reaction only two reactants are, present and both are found to influence the rate, of the reaction., This means that the reaction is a single step, reaction which can also be called the slow step., This further implies that the two changes,, namely, bond breaking and bond forming at the, carbon take place simultaneously., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 100 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 101 :
Salient features of SN2 mechanism :, i. Single step mechanism with simultaneous, bond breaking and bond forming., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 102 :
ii. Backside attack of nucleophile :, • The, nucleophile, attacks, the, carbon, undergoing substitution from the side, opposite to that of the leaving group., • This is to avoid steric repulsion (repulsion, due to bulkyness of the groups) and, electrostatic repulsion between the incoming, nucleophile and the leaving group., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 103 :
iii. In the transition, and leaving groups, with partial bonds, charge. (Thus, the, diffused.), , K, L, FU, , A, R, , P, , state (T.S.) the nucleophile, are bonded to the carbon, and carry partial negative, total negative charge is, , K, A, , E, D
Page 104 :
iv. The T.S. contains pentacoordinate carbon, having three σ (sigma) bonds in one plane, making bond angles of 1200 with each other and, two partial covalent bonds along a line, perpendicular to this plane., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 105 :
v. When SN2 reaction is brought about at chiral, carbon (in an optically active substrate), the, product is found to have opposite configuration, compared to that of the substrate., • In other words, SN2 reaction is found to, proceed with inversion of configuration., • This is like flipping of an umbrella., • It is known as Walden inversion., • The inversion in configuration is the result of, backside attack of the nucleophile., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 106 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 107 :
b. SN1 Mechanism :, • The reaction between tert-butyl bromide and, hydroxide ion to give tert-butyl alcohol, follows a first-order kinetics, • The rate of this reaction depends on, concentration of only one species, which is, the substrate molecule, tert-butyl bromide., • Hence it is called substitution nucleophilic, unimolecular, SN1., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 108 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 109 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 110 :
• Concentration of only substrate appears in, the rate equation; concentration of the, nucleophile does not influence the reaction, rate., • In other words, tert-butyl bromide reacts, with hydroxide by a two step mechanism., • In the slow step C-X bond in the substrate, undergoes heterolysis and in the subsequent, fast step the nucleophile uses its electron, pair to form a new bond with the carbon, undergoing change., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 111 :
Salient features of SN1 mechanism :, i. Two step mechanism., ii. Heterolyis of C-X bond in the slow and, reversible first step to form planar, carbocation intermediate., iii. Attack of the nucleophile on the carbocation, intermediate in the fast second step to form, the product., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 112 :
iv. When SN1 reaction is carried out at chiral carbon, in an optically active substrate, the product formed, is nearly racemic., • This indicates that SN1 reaction proceeds mainly, with racemization., • This means both the enantiomers of product are, formed in almost equal amount., • Racemization in SN1 reaction is the result of, formation of planar carbocation intermediate, • Nucleophile can attack planar carbocation from, either side which results in formation of both the, enantiomers of the product., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 113 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 116 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 118 :
• Tertiary, halides, undergo, nucleophilic, substitution by SN1 mechanism while primary, halides follow SN2 mechanism., , K, A, , E, D, , • Secondary halides react by either of the, mechanism or by mixed mechanism depending, upon the exact conditions., , P, , A, R, , K, L, FU
Page 120 :
b. Nucleophilicity of the reagent :, • A nucleophile is a species that uses its, electron pair to form a bond with carbon., , K, A, , E, D, , • Nucleophilic character of any species is, expressed in its electron releasing tendency,, which can be corelated to its strength as, Lewis base., , P, , A, R, , K, L, FU
Page 121 :
• A more powerful nucleophile attacks the, substrate faster and favours SN2 mechanism., , •, •, , •, , E, D is independent of, The rate of SN1 mechanism, K, A, K, the nature of nucleophile., L, U, F, Nucleophile does, not react in slow step of SN1., A, R, P, It waits till the carbocation intermediate is, formed, and reacts fast with it.
Page 122 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 123 :
c. Solvent polarity :, • SN1 mechanism proceeds via formation of, carbocation intermediate., • A good ionizing solvent, polar solvent,, stabilizes the ions by solvation., • Solvation of carbocation is relatively poor and, solvation of anion is particularly important., • Anions are solvated by hydrogen bonding, solvents, that is, protic solvents., • Thus SN1 reaction proceeds more rapidly in, polar protic solvents than in aprotic solvents., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 124 :
• Polar protic solvents usually decrease the, rate of SN2 reaction., • In the rate determining step of SN2, mechanism substrate as well as nucleophile, is involved., • A polar solvent stabilizes nucleophile (one of, the reactant) by solvation., • Thus solvent deactivates the nucleophile by, stabilizing it., • Hence aprotic solvents or solvents of low, polarity will favour SN2 mechanism., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 125 :
Problem 10.5 : Which of the following two compounds, would react faster by SN2 mechanism and Why ?, , E, D, , Solution :, In SN2 mechanism, a pentacoordinate T.S. is involved., The order of reactivity of alkyl halides towards SN2, mechanism is, Primary > Secondary > Tertiary, (due to, increasing crowding in T.S. from primary to tertiary, halides., 1-Chlorobutane being primary halide will react faster by, SN2 mechanism, than the secondary halide 2chlorobutane., , P, , A, R, , K, L, FU, , K, A
Page 126 :
Elimination reaction :, Dehydrohalogenation, • When alkyl halide having at least one, β-hydrogen is boiled with alcoholic solution of, potassium hydroxide, it undergoes elimination, of hydrogen atom from β-carbon and halogen, atom from α - carbon resulting in the, formation of an alkene., • This reaction is called β-elimination (or 1,2 elimination) reaction as it involves elimination, of halogen and a β – hydrogen atom., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 127 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 128 :
• As hydrogen and halogen is removed in this, reaction, it, is, also, known, as, dehydrohalogenation reaction., • If there are two or more non-equivalent, β-hydrogen atoms in a halide, then this, reaction gives a mixture of products., • Thus, 2-bromobutane on heating with, alcoholic KOH gives mixture of but-1-ene and, but-2- ene., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 129 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 130 :
• The different products of elimination do not, form in equal proportion., • After studying a number of elimination, reactions,, Russian, chemist, Saytzeff, formulated an empirical rule given below., • In, dehydrohalogenation, reaction,, the, preferred product is that alkene which has, greater number of alkyl groups attached to, doubly bonded carbon atoms., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 132 :
Do you know ?, Elimination versus substitution:, • Alkyl halides undergo substitution as well as, elimination reaction., • Both reactions are brought about by basic, reagent, hence there is always a competition, between these two reactions., • The reaction which actually predominates, depends upon following factors., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 134 :
b. Strength and size of nucleophile :, • Bulkier electron rich species prefers to act as, base by abstracting proton, thus favours, elimination., • Substitution is favoured in the case of, comparatively weaker bases, which prefer to, act as nucleophile, , P, , A, R, , K, L, FU, , K, A, , E, D
Page 135 :
c. Reaction conditions :, • Less polar solvent, high temperature fovours, elimination where as low tempertaure, polar, solvent favours substitution reaction., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 136 :
Reaction with active metals, • Active metals like sodium, magnesium, cadmium readily combine with alkyl, chlorides, bromides and iodides to form, compounds containing carbon-metal bonds., • These are known as organometallic, compounds., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 137 :
a. Reaction with magnesium :, • When alkyl halide is treated with magnesium, in dry ether as solvent, it gives alkyl, magnesium halide., • It is known as Grignard reagent., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 138 :
• Grignard, reagents, are, very, reactive, compounds., • They react with water or compounds containing, hydrogen attached to electronegative element., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 139 :
b. Wurtz reaction :, • Alkyl halides react with metallic sodium in, dry ether as solvent, and form higher alkanes, containing double the number of carbon, atoms present in alkyl halide., • This reaction is called Wurtz reaction., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 140 :
When a mixture of two different alkyl halides is, used, all the three possible alkanes are formed., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 142 :
E, D, , K, A, In case only aryl halide, takes part in, K, L, reaction, the product, is biphenyl and, U, F, A, reaction is known, as Fittig reaction., R, P, , the, the
Page 144 :
i., , One of the lone pairs of electrons on halogen, atom is in conjugation with π -electrons of, the ring., For example the following different resonance, structures can be written for chlorobenzene., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 145 :
•, •, , Resonance structures II, III and IV show double bond, character to carbon-chlorine bond., Thus carbon-chlorine bond in chlorobenzene is, stronger and shorter than chloroalkane molecule, CCl bond length in chlorobenzene is 169 pm as, compared to C-Cl bond length in alkyl chloride 178, pm. Hence it is difficult to break., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 146 :
•, •, , Phenyl cation produced due to self-ionization of, haloarene will not be stabilized by resonance, which, rules out possibility of SN1 mechanism., Back side attack of nucleophile is blocked by the, aromatic ring, which rules out SN2 mechanism., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 147 :
• Thus, nuclophilic, substitution, reaction, involving cleavage of C-X bond in haloarene, proceeds with difficulty., • However, the presence of certain groups at, certain positions of the ring, markedly, activate the halogen of aryl halides towards, substitutuion., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 148 :
• For, example,, presence, of, electron, withdrawing group at ortho and/or para, postion greatly increases the reactivity of, haloarenes towards subsitution of halogen, atom., • Greater the number of electron withdrawing, groups at o/p position, greater is the, reactivity., • Electron withdrawing group at meta position, has practically no effect on reactivity., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 149 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 150 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 151 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 152 :
c. Electrophilic substitution (SE) in arylhalides:, • Aryl halides undergo electrophilic substitution, reaction slowly as compared to benzene., • In resonance structures of chlorobenzene elelctron, density is relatively more at ortho and para position., • Therefore incoming electrophilic group is more likely, to attack at these positions., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 153 :
c. Electrophilic substitution (SE) in arylhalides:, • But due to steric hinderance at ortho position, para, product usually predominates., • In haloarenes, halogen atom has strong electron, withdrawing inductive effect (-I)., • This deactivates the ring and electrophilic substitution, reaction occurs slowly., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 154 :
i., , Halogenation : It is carried out by reacting haloarene, with halogen in presence of ferric salt as Lewis acid, catalyst., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 156 :
iii. Sulfonation : It is carried out by heating haloarene, with fuming H2SO4., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 157 :
iv. Friedel Craft’s reaction : It is carried out by, treating haloarene with alkyl chloride or acyl, chloride in presence of anhydrous AlCl3 as a, catalyst., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 158 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 159 :
Uses and Environmental effects of some, polyhalogen compounds, 1. Dichloromethane/ methylene chloride (CH2Cl2) :, • It is a colourless volatile liquid with moderately, sweet aroma., • It is used as a solvent, and used as a propellant, in aerosols., • Over exposure to dichloromethane causes, dizziness, fatigue, nausea, headaches, numbness,, weakness., • It is highly dangerous if it comes in direct contact, with eyes as it damages cornea., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 160 :
Chloroform / trichloromethane (CHCl3) :, • It is a colourless liquid with peculiar sweet, smell., • It is used to prepare chlorofluromethane, a, refrigerant R-22., • It is used as a solvent for extraction of natural, products like gums, fats, resins., • It is used as a source of dichlorocarbene., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 161 :
Chloroform / trichloromethane (CHCl3) :, • Chloroform causes depression of central, nervous system., • Inhaling chloroform for a short time causes, fatigue, dizziness and headache., • Long exposure to chloroform may affect liver., • Chloroform when exposed to air and light, forms a poisonous compound phosgene so it, is stored in dark coloured air tight bottles., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 162 :
Carbon tetrachloride /tetrachloromethane (CCl4) :, • It is a colourless liquid with sweet smell., • It is very useful solvent for oils, fats, resins., • It serves as a source of chlorine., • It is used as a cleaning agent., • It is highly toxic to liver., • Exposure to high concentration of CCl4 can, affect central nervous system and it is, suspected to be carcinogenic., • Prolonged exposure may cause death., • It is a green house gas., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 163 :
Idoform or triiodomethane (CHI3):, • It is a yellow crystalline substance with, disagreeable smell., • It is used in medicine as a healing agent and, antiseptic in dressing of wounds, however its, use is limited., • It causes irritation to skin and eyes., • It may cause respiratory irritation or, breathing difficulty, dizziness, nausea,, depression of central nervous system, visual, disturbance., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 164 :
Freons :, • These are organic compounds of chlorine and, fluorine, chlorofluorocarbons, CFC's are, commonly used as refrigerants., • The most common representative is, dichlorodifluromethane (Freon-12) others, include, chlorodifluromethane, (R-22),, trichlorofluromethane (R-11) and so on., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 165 :
Freons :, • They are used as refrigerants in fridge and, airconditioning, propellants in aerosol and, solvents., • They are used as blowing agents in making, foams and packing materials., • Chloroflurocarbons are responsible for, depletion of ozone in stratosphere., • Regular large inhalation of freons results in, breathing problems, organ damage, loss of, consciousness., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 166 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 167 :
Dichlorodiphenyltrichloroethane (DDT) :, • It is colourless, tasteless and odorless, crystalline compound having insecticidal, property., • It kills insects such as houseflies, mosquitoes, and body lice., • It was used for controlling maleria and, typhus., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 168 :
Dichlorodiphenyltrichloroethane (DDT) :, • Exposure to high doses of DDT may cause vomiting,, tremors or shakiness., • Laboratory animal studies showed adverse effect of, DDT on liver and reproduction., • DDT is a persistent organic pollutant, readily absorbed, in soils and tends to accumulate in the ecosystem., • When dissolved in oil or other lipid, it is readily, absorbed by the skin., • It is resistant to metabolism., • It accumulates in fatty tissues., • There is a ban on use of DDT due to all these adverse, effects ., , P, , A, R, , K, L, FU, , K, A, , E, D
Page 169 :
P, , A, R, , K, L, FU, , K, A, , E, D
Page 170 :
THE ENDE, D, K, THANK, YOU, A, K, L, UPOINT KATOL, F, CAREER, A, R, P