Page 1 :
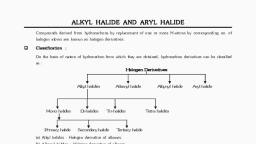
ALKYL HALIDE, , , , , , , , 1. INTRODUCTI, , The alkyl halides or haloalkanes are a group of chemical, compounds, derived from alkanes containing one or more, halogens. They are used commonly as flame retardants, fire, extinguishers, refrigerants, propellants, solvents and, pharmaceuticals. The alkyl! halides are classified broadly into three, categories based on type of carbon atom to which the halogen, atom is attached., , , , H, , |, R—C—X 1° Primary, , R—C—xX 2°, , Secondary, , R—C—X 3° Tertiary, , , , , , , , X may be F, Cl, Bror I., , 2. REACTIONS IN ORGANIC CHEMISTRY, , There are various types of reactions possible in organic, compounds depending on reaction conditions and attacking, reagent. Reactions in organic chemistry are classified into three, categories :, , 2.1 Addition Reaction >, , This reaction involves addition of groups to a x bond., , , , Noa +x ny —+ -|-\x ¥, , , , 2.2 Substitution Reaction >, , This reaction involves the replacement of an atom or a group of, , atoms by another atom or group of atoms., , -} Zeya Y+Z, , [23 Elimination Reaction >, , This reaction involves the loss of atoms or groups of atoms to, form an unsaturated compound., , reagent ‘\, , Lf sem, nc’, | | N, Y, , , , +X—Y, 7, , x, , RO 0) eli Ore SE EROS End ion een O i), , | |, “2 +Nu? —> =e +x?, , ‘The replacement of halogen atom (leaving group) by the attacking, nucleophile is called nucleophilic substitution reaction at sp’, carbon. This reaction was studied in great detail and two extreme, mechanisms have been outlined to explain the course of the reaction., , 3.1 Substitution Nucleophilic Bimolecular-S.2_>, , , , , , CC. | HO CBr | —> Cw yy * BE, Hy “pr " Hom ‘ x, cH, H.C H CH;, , Transition State
Page 2 :
3.2 Substitution Nucleophilic Unimolecular -S.1_ > [4.1 Elimination Bimolecular-E2 >, Step 1:, , , , , , , , , Gals, , eager C:, nore, C,H,, , SLOW, , Br HAC;, Step 2:, , CH,, , Aeon 2051, , H,Cy CH;, , 4.2 Elimination Unimolecular —E1, , Step 1:, ie te P P, RF R, Stow R, fs +x, H X, R,—-C—C—R,, | | z S Ze 1 e 4, —c—c— Se CC + HX, ri BS Pe H, B, Hx [esse, The removal of halogen from the carbon to which it is attached, along with the removal of hydrogen from adjacent carbon is called R R, R R,, 1 1, , @, B-elimination or simply elimination, Three mechanisms have, been outlined for elimination reactions.
Page 3 :
Nucleophilic nature, LON, , Nucleophilicity vs Basicity >, , (a) Nucleophilicity & basicity will be parallel if the comparing, nucleophile have same attacking atom e.g., , , , ll, CH,0° > OH® > CH,—C—OP, , (b) Negatively charged nucleophiles are stronger than neutral, nucleophiles. e.g., , , , OH®>H,O or NHS >NH,, , 4.3 Elimination Unimolecular via Conjugate Base- E1cB > (c) Electrons on larger atoms are less tightly bound by the, , nucleus and are more polarisable and more readily available, to carbon & will be better nucleophile. But they will be, Step 1: weaker base as their bond with smaller H-atom will be weaker, & their conjugate acids will be more reactive. If the attacking, , | | one atoms are of same size, the stronger bases are better, , |_|, — av a, “1 +BH nucleophile. (In a period basicity of anions decreases) e.g., x a x Acidic Strength :, , , , , , BP CH, < NH, < H,O< HF, Step 2: Basic Strength and Nucleophilicity :, , cH, >°NH, > oH> Fe, , | | stow, \_$/ .y8, , _- —— pos +X (d) If the attacking atoms are different in size, the nucleophilicity, dq depends on the solvents. However, in gaseous phase, x ec sg, , nucleophilicity parallels basicity., , (e) Nucleophilicity is inversely proportional to stability of anion., , R—C—O® is a weaker nucleophile as it is resonance, stabilized,, (f) Steric factor limits nucleophilicity, , CH,, , , , CH,CH,—O°> CH,—C—0O® Nucleophilicity, , 5. SUBSTITUTION AND ELIMINATION CH;, , Any species that acts as a base can also act as a nucleophile. To CH, , understand how elimination and substitution compete with each 2, , other, we compare the nucleophilic behaviour with the basic a > CH,CH,—O” Basicity, behaviour,, , CH,
Page 4 :
(g) A strong base can be made a good leaving agent e.g. Oxygen, containing group like OH® can be made a weak base in, acidic medium by protonation & become a better leaving, , @, agent —OH,., , (h) Protic solvent : These solvents have a hydrogen atom, attached to an atom of a strongly electronegative element, (e.g. oxygen). Molecules of protic solvent can, therefore, form hydrogen bonds to nucleophiles as :, , , , , , , , , , , , A small nucleophile which is having high charge density than the, larger nucleophile is strongly solvated and this solvation hinders, the direct approach to the nucleophilic centre. Hence the smaller, nucleophile doesn’t act as a good nucleophile as the larger one., Hence in protic solvent nucleophilicity is reverse of basicity., , Fe ce BP IP, increasing basicity, increasing nucleophilicity, , (@ Aprotic solvents : These are the polar solvents that don’t, have H atom, capable of forming H-bonds e.g., , CH, | oes oO, H—C—N, ll, CH, CH,—S—CH,, N, N-Dimethyl formamide Dimethyl] sulphoxide, (DMF) (DMSO), oO, CH., | 7?, CH CN, CH,, Dimethyl acetamide, , (DMA), , These solvents dissolve ionic compounds and solvate the cations., , , , , , , , , , , , Now the naked anions are highly reactive as nucleophile and now, nucleophilicity follows the basicity e.g., , F_ cf Be 1°, , increasing basicity, , increasing nucleophilicity in aprotic solvents, , 5.2 Saytzeff vs. Hofmann Rule >, , When alkene is formed by elimination of alkyl halides, the, orientation of the double bond formed is governed by two rules., , 5.2.1 Saytzeff’s Rule/Zaitsevy Rule >, , This rule suggests the formation of more stable alkene and, therefore more substituted double bond. Reactions following this, rule are said to be thermodynamically controlled., , [5.2.2 Hofmann Rule >, , This rule suggests the formation of less stable alkene and therefore, less substituted double bond. In such cases, the more acidic Bhydrogen is abstracted to produce alkene. Such reactions are, said to be kinetically controlled., , 5.3 Effect of Temperature >, , High temperature favours elimination while low temperature, favours substitution reaction., , 6. STEREOCHEMISTRY, , 6.1 Regioselect, , , , , , , , , , , , , , ity, , It is the preference of one direction of chemical bond making or, breaking over all other possible directions.
Page 5 :
ee ene, , aay, Br, alc KOH, , CH,=CH—CH,—CH, + CH,—CH=CH—CH,, , But-l-ene But-2-ene, , [662 Stereoselectivity >, , Stereoselective reactions give one predominant product because, the reaction pathway has a choice. Either the pathway of lower, activation energy (kinetic control) is preferred or the more stable, product (thermodynamic control)., , , , OH, , Ph’, , Ph’, Major, , [os Stereospecificity >, , Stereospecific reactions lead to the production of a single isomer, as a direct result of the mechanism of the reaction and the, stereochemistry of the starting material. There is no choice. The, reaction gives a different diastereomer of the product from each, stereoisomer of the starting material., , en eee}, , H., Lr i 7, — c=, 7 Br 7 \, , H CH,, , , , HC, , HC oH, Ly a, — cc, Br oe X, H.C H H H, , [64 Chemoselectivity >, , When there are two or more functional groups in a molecule, a, given reagent may react preferentially with one rather than the, other. Such reactions are called chemoselective., , | Preparation of Alkyl Halides >, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , Alkane,, Free radical, Halogenation} | Alkenes, Alcohol 1, Addition of X,, (HX, PX,, PX, 2. Addition of HX, SOCI,) 3. a-Halogenation, RX, - - Alkynes, : a | ™ 1. Addition of X,, SS 2. Addition of HX, Alkyl Halides, Halide Exchange, 1, Finkelstein Reaction|, 2. Swartz Reaction, 1. Alkanes, Ch, RH———>RCI+ HCI, , This method gives a mixture of mono, di & trihalides., 2. Alkenes, , cc, (i) R—CH=CH, + X, —> —, x Xx, , (ii) R—CH=CH, + H—X ——> R—CH—CH,, , x, x, (iii) (a) RCH CH=CH, posh, (b) R—CH;—CH=CH, “88+ R—CH—CH=CH,, be, 3. Alkynes, ex, () R—C=sC—H — es, , xX X