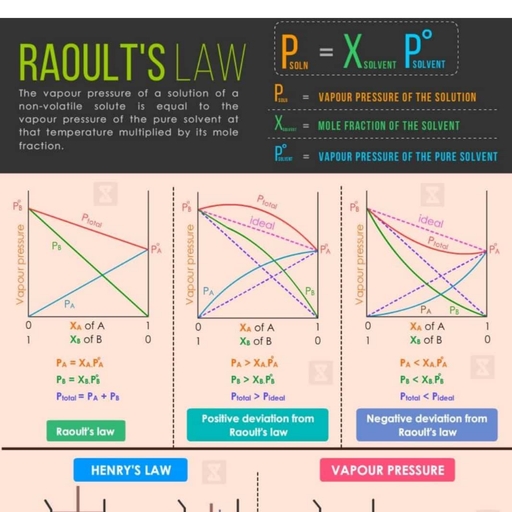
Page 1 :
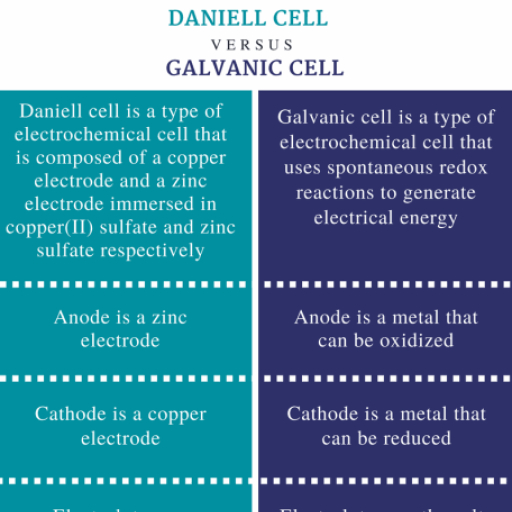
CO-ORDINATION COMPOUNDS, , COORDINATION COMPOUNDS, , 1. INTRODUCTION, , 2.2 Coordination Compounds, , Co-ordination compounds play a vital role. The importance, can be realised that life would not have been possible, without the existence of chlorophyll (Mg - complex) in plants, and haemoglobin (Fe- complex) in the blood of human, beings. The study of these compounds will enlarge our, understanding of chemical bonding, physical properties, such as magnetic properties of co-ordination compounds, , A coordination compound is a molecular compound that, results from the combination of two or more simple molecular, compounds and retains its identity in the solid as well as in, dissolved state, Example, , 2. MOLECULAR OR ADDITION COMPOUNDS, When solution containing two or more simple stable, compounds in molecular proportions are allowed to, evaporate, crystals of new substances called molecular or, addition compounds are obtained., Example, KCl + MgCl2 + 6H2O, , KCl.MgCl 2 .6H 2 O, ( Carnallite ), , CuSO4 + 4 NH3, , K4 [Fe(CN)6], , 4K+ + [Fe (CN)6]4–, , 3. COORDINATION COMPOUNDS, A Co-ordination compound consists of a ligand, central, atom, complex ion, a cation or an anion. The complex ion is, generally written in a square box and the ion (cation or anion), is written outside complex ion., eg : [Co (NH3)6] Cl3, , [Cu(NH 3 ) 4 ]SO 4, (Tetrammin e copper (II), sulphate), , Fe(CN)2 + 4KCN, , [Cu (NH3)4]2+ + SO 24�, , [Cu (NH3)4]SO4, , K 4 [Fe(CN )6 ], ( Potassium ferrocyanide), , [Complex ion] anion, eg : K4 [Fe (CN)6], cation [Complex ion], General formula : Ax [MLn]/[MLn]By, where : M is the central metal atom/ion, L is the ligand, A is the cation, B is the anion, Some Important Terms pertaining to Coordination Compounds, , 2.1 Types of Molecular compounds, , 3.1 Coordination entity, , 2.1.1 Double Salt, A double salt is a substance obtained by the combination, of two different salts which crystallize together as a single, substance but ionise as two distinct salts when dissolved, in water. These salts lose their identity in solution i.e. when, dissolved in water they give test of all the ions present in, the salt. eg. Potash alum, Mohr’s salt, FeSO4. (NH4)2 SO4.6H2O o Fe2+ (aq) + 6H2O + 2NH4+ (aq), (Mohr’s salt), , + 2 SO42– (aq), , K2SO4. Al2 (SO4)3 . 24 H2O o�2K+ (aq) + 2Al3+ (aq) +, (Potash alum), , 4SO43– (aq) + 24H2O, , It is the central metal atom or ion which is bonded to a, definite number of ions or molecules which is fixed. For, example, in [Co(NH3)6]Cl3, a coordination entity, six ammonia, molecules are surrounded by three chloride ions., 3.2 Central atom/ion, It is the central cation that is surrounded and coordinately, bonded to one or more neutral molecules or negatively, charged ions in a definite geometrical arrangement. For, example, in the complex [Co(NH3)6]Cl3, Co3+ represents the, central metal ion which is positively charged and is, coordinately bonded to six neutral NH3 molecules within, the coordination sphere. The central metal/ion is also, referred to as Lewis acid.
Page 2 :
CO-ORDINATION COMPOUNDS, , 3.3 Ligands, , Example, , The ions or molecules bound to the central atom/ion in the, coordination entity are called ligands. These may be simple, ions such as Cl–, small molecules such as H2O or NH3, larger, molecules such as H2NCH2CH2NH2., 3.4 Co-ordination Number (C.N), The number of atoms of the ligands that directly bound to, the central metal atom or ion by co-ordinate bonds is known, as the co-ordination number of the metal atom or ion. It is, also equal to the secondary valency., Complex, , Co-ordination numbers, , K4 [Fe (CN)6], –, , [Ag (CN)2], , 6, 2, , [Pt (NH3)2 Cl2], , 4, , 2–, , 6, , [Ca (EDTA)], , 3.5 Coordination sphere, The central metal atom or ion and the ligands that are directly, attached to it are enclosed in a square bracket. This had, been called coordination sphere or first sphere of attraction., It behaves as a single unit because the ligands present in, the coordination sphere are held tightly by the metal ion., , 4–, , 3.8 Homoleptic and Hetroleptic Complexes, Complexes in which central atom is coordinated with only, one kind of ligands are called homoleptic complexes, eg., 3+, [Co(NH 3 ) 6] . Complexes in which central atom is, coordinated with more than one kind of ligands are called, +, hetroleptic complexes, eg. [Co (NH3)4 Cl2] ., , 4. NOMENCLATURE OF COORDINATION, COMPOUNDS, 4.1 Nomenclature, Following rules are adopted for naming a complex ion;, (a) Cations are named before anions, (b) Oxidation state (O.S.) of the central metal ion is denoted, by Roman numeral., Compound, , Cation, , O.S., , anion, , CuCl, CuCl2, , Copper, Copper, , (I), (II), , chloride, chloride, , FeCl2, , Iron, , (II), , chloride, , FeCl3, , Iron, , (III), , chloride, , The names of ligands are given first followed by the, name of the central metal ion., , (d) The names of ligands that are anions and ending with, ‘ide’ are changed to ‘o’, , A coordination polyhedron is the spatial arrangement of, the ligand atoms that are directly attached to the central, atom/ion. For example, [Co(NH3)6]3+ is octahedral, [Ni(CO)4], is tetrahedral and [PtCl4]2 is square planar., , ‘ite’ are changed to ‘ito’, ‘ate’ are changed to ‘ato’, (e), , 3.7 Oxidation Number of Central Metal Atom, It is defined as the charge that the central metal ion would, carry if all the ligands are removed along with electron pairs., It is calculate as follows :, , +, , K4 [Fe (CN)6] o 4 K + [Fe (CN)6], Charge on complex ion = – 4, Let charge on Fe = x,, –, Now charge on cyanide ion (CN ) = –1, x + 6 × (–1) = – 4, x=+ 2, Hence oxidation no of Fe = + 2 (or II), , (c), 3.6 Co-ordination Polyhedron, , K4 [Fe (CN)6], , Many ligands that are molecules carry the unmodified, name, , (f), , Positive groups end in – ium, hydrazinium.
Page 3 :
CO-ORDINATION COMPOUNDS, (g) When there are several ligands of the same kind, we, normally use the prefixes di, tri, tetra, penta and hexa, to show the number of ligands of that type. An, exception occurs when the name of the ligands includes, a number, for example as in ethylenediamine (en). To, avoid confusion in such cases, bis, tris and tetrakis, are used instead of di, tri and tetra, and the name of the, EXAMPLE : Write the name of the following compounds ?, , ligand is placed in brackets., e.g. bis (ethylenediamine), , (a) [Fe(NH3)6]Cl3, , (h) If anion is a complex, then metal ends with ‘ate’, , (i), , [ Ni(CN) 4 ]2� : tetracyanonickelate (II) ion, , (c) [CoSO4 (NH3)4] NO3, , lead, , –, , plumbate, , (d) [Pt (Py)4] [PtCl4], , gold, , –, , aurate, , zinc, , –, , zincate, , tin, , –, , stannate, , silver, , –, , argentate, , cobalt, , –, , cobaltate, , iron, , –, , ferrate, , aluminium, , –, , aluminate, , manganese, , –, , manganate, , copper, , –, , cuprate, , chromium, , –, , chromate, , platinum, , –, , platinate, , If the complex contains two or more metal atoms, it is, termed polynuclear. The bridging ligands which link, the two metal atoms together are indicated by the prefix, P–, , (j), , 2+, , (b) [CoCl(NH3)5], , (e) [Cr (en)3] Cl3, , (f), , Sol. (a) Hexaammineiron (III) Chloride, (b) Pentaamminechloridocobalt (III) ion, (c) Tetraamminesulphatocobalt (III) nitrate, (d) Tetrapyridineplatinum (II) tetrachloridoplatinate (II), (e) Tris (ethylenediamine) chromium (III) chloride, (f) Tetrakis (ethylenediamine)–P-hydroxo-P-imidodicobalt, (III) ion., , 5. WERNER’S THEORY, Werner explained the nature of bonding in complexes and, he concluded that in complexes the metal shows two different, types of valency., 5.1 Primary Valency, , Ambidentate ligands may be attached through, different atoms, M m NO2, , (NO2 joined to metal M through, N; it is nitrito-N), , M m ONO, , (NO2 joined to metal M through, O; it is nitrito-O), , Similarly, the SCN group may bond M – SCN, (thiocyanato) or M – NCS (isothiocyanato)., (k) When writing (not naming) the formula of the complex :, *, , Complex ion should be enclosed by square, brackets and, , *, , Ligands are placed after metal in the alphabetical, order but first negative ligands, then neutral, then, positive., , The primary valency is equal to the oxidation state of the, central metal atom or ion. These are non-directional., EXAMPLE : What are the primary valency of [Co (NH3)6] Cl3, & [Co (NH3)6]3+ ?, Sol. K4 [Fe (CN)6] : Primary valency is 2., [Cu (NH3)4] SO4 : Primary valency is 2., 5.2 Secondary Valency, The number of ligand atoms co-ordinated to the central metal, atom is called secondary valency. These are directional and, so a complex ion has a particular shape.
Page 4 :
CO-ORDINATION COMPOUNDS, , EXAMPLE, , : What are the secondary valency of, [Co (NH3)6] Cl3 & K4 [Fe (CN)6] ?, , be tetrahedral or square planar. This postulate predicted, the existence of different types of isomerism in coordination, compounds., , Sol. In [Co (NH3)6] Cl3 the secondary valency is 6., K4 [Fe (CN)6] : six ligands are coordinated to Fe. Hence, secondary valency is 6., The primary valency is satisfied by ions attached to the, complex ions. It is shown by dotted lines. Primary valency, is also known as ionisable valency., The secondary valency is satisfied by the ligands, they, are non ionisable and are shown by a solid line [Co (NH3)6], Cl3 can be represented as, EXAMPLES :, , An anion present in co-ordination and ionization sphere, is shown by, Every element tends to satisfy both its primary and, secondary valencies. A negative ion when present in the, coordination sphere shows a dual behaviour. It may satisfy, both primary and secondary valencies., , Octahedral, , Square planar, , Tetrahedral, , (C.N = 6), , (C.N = 4), , (C.N. = 4), , [Cr(CH3)6]3+, , [Ni(CN)4]2–, , [Ni(CO)4], , [Co(NH3)6]3+; [Cr(H2O)6]3+, , [Ni(NH3)4] 2+, , [CuX4]2– ;[ZnCl4] 2–, , [Fe(CN)6]2–; [Fe(F6)]3–, , [Cu(NH3)4]2+, , [NiX4]2–, , [Pt(NH3)6]4+; [PtCl6] 2–, , X = Cl–, Br–, I–, , Familiar C.N.’s of some common metal ions., Univalent, Ag, Au, , +, +, , +, , Ti, , +, , Cu, , C.N., , Divalent, , 2, , V, , 2, 4, 2, 2, 4, , 2+, , Fe, , 2+, 2+, , Co, , 2+, , Ni, , 2+, , Cu, Zn, , 2+, , 2+, , Pd, , 2+, , Pt, , Ag, Trivalent, Sc, The ligand which satisfy the secondary valencies are, directed toward fixed positions in space. The geometry of, the complex ion depends on the coordination number. If the, metal has coodination number 6, the complex is octahedral,, i.e. six positions around the metal are occupied by six donor, atoms of the ligands octahedrally. On the other hand, if the, coordination number is 4, the geometry of the complex may, , 3+, , 3+, , Cr, , Fe, , 3+, , C.N., , Tetravalent, , 6, , Pt, , 6, 6, , 3, , Co +6, Os, Ir, , 3+, , 3+, , Au, , 3+, , 2+, , 6, 6, 4, , 4+, , Pd, , 4+, , C.N., 6, 6, 4, 6, 4,6, 4, 6, 4, 4, 4, 4, C.N., 6, 6
Page 5 :
CO-ORDINATION COMPOUNDS, inert gas in some cases. EAN is calculated by the following, relation :, , 6. EFFECTIVE ATOMIC NUMBER (EAN), Sidgwick proposed effective atomic number abbreviated as, EAN, which is defined as the resultant number of electrons, with the metal atom or ion after gaining electrons from the, donor atoms of the ligands. The effective atomic number, (EAN) generally coincides with the atomic number of next, , Complex, , Metal (Oxid. state), , EAN = Atomic number of the metal – number of electrons, lost in ion formation + number of electrons gained from, the donor atoms of the ligands. (2 × CN), The EAN values of various metals in their respective, complexes are tabulated below :, , At. No. of metal, , Coordination number, , K4 [Fe (CN)6], , +2, , 26, , 6, , (26 – 2) + (6 × 2) = 36 [Kr], , [Cu (NH3)4] SO4, , +2, , 29, , 4, , (29 – 2) + ( 4 × 2) = 35, , [Co (NH3)6] Cl3, , +3, , 27, , 6, , (27 – 3) + (6 × 2) = 36 [Kr], , Ni (CO)4, , 0, , 28, , 4, , (28 – 0) + (4 × 2) = 36 [Kr], , K2 [Ni(CN)4], , +2, , 28, , 4, , (28 – 2) + (4 × 2) = 34., , 7. VALENCE BOND THEORY, The bonding in coordination compounds can be explained, by Valence Bond Theory (VBT) since majority of the, complexes formed by the transition metals have their dorbitals incomplete. Valence bond takes into account the, hybridisation of orbitals since penultimate d-orbitals are near, in energy to s and p-orbitals of the outer most shell, various, kinds of hybridization is possible., , COMMON TYPES OF HYBRIDISATION, Coordination HybridiNumber, zation, , (i), , A number of empty orbitals are available on the central metal, ion which can accomodate electrons donated by the ligands., The number of empty d-orbitals is equal to the coordination, number of the metal ion for the particular complex., , (ii), , The metal orbitals and ligand orbitals overlap to form strong, bonds. Greater the extent of overlapping, more is the stability, of the complex. Different orbitals (s, p or d) hydridize to give, a set of equivalent hybridized orbital which take part in, bonding with the ligands., , (iii) Each ligand donates a pair of electrons to the central metal, ion/atom., , Shape, , Geometry, X —A— X, , 2, , sp, , Linear, , 4, , sp 3, , Tetrahedron, , 4, , dsp2, , Square planar, , 5, , sp 3d, , Trigonal, , or dsp3, , bipyramid, , d 2 sp 3, , Octahedral, , VBT makes the following assumption, , (iv) The non-bonding metal electrons present in the inner, orbitals do not take part in chemical bonding., (v), , Effective atomic number, , If the complex contains unpaired electrons, the complex is, paramagnetic. If it does not contain unpaired electron, the, complex is diamagnetic in nature., , (vi) Under the influence of strong ligand (CN, CO) the electrons, can be forced to pair up against the Hund’s rule of, multiplicity., , 6, , or sp3d2
Page 6 :
CO-ORDINATION COMPOUNDS, , NOTE :, , The lobes of the eg orbitals (d x 2 � y 2 and d z2 ) point along, , In d2sp3 hybridisation, the inner d-orbitals (3d orbital), , the x, y and z axes. the lobes of the t 2g orbitals, , has been used for bonding, such complexes are called, inner orbital complexes or low spin complexes., , (d xy , d xz and d yz ) point in between the axes. The approach, , In sp3d2 hybridisation the outer d-orbitals (4d orbital) has, been used for bonding, such complex are called outer, orbital complexes or high spin complexes., The magnetic moment is given by, , n ( n � 2) BM where, , n is the number of unpaired electrons., , of six ligands along the x, y, z, –x, – y and – z directions will, increase the energy of the d x 2 � y2 and d z2 orbitals (which, point along the axes) much more than it increases the energy, of the dxy, dxz and dyz orbitals (which point between the, axes). Thus under the influence of an octahedral ligand field, the d orbitals split into two groups of different energies., , 7.1 Limitations of VBT, 1., 2., 3., 4., , The change in the properties of the ligands and the metal, ions could not be explained., The valence bond theory does not explain why certain, complexes are more labile than other., The VBT does not provide satisfactory explanation for the, existence of inner orbital and outer orbital complexes., The VBT could not explain the colour of complexes, , 8. CRYSTAL FIELD THEORY, , 1., 2., 3., , The Crystal Field Theory is more widely accepted than the, valence bond theory. It assumes that the attraction between, the central metal and the ligands in a complex is purely, electrostatic. In the crystal field the following assumptions, are made., Ligands are treated as point charges., There is no interaction between metal orbitals and ligand, orbitals., The d orbitals on the metal all have the same energy (that is, degenerate) in the free atom. However, when a complex is, formed the ligands destroy the degeneracy of these orbitals,, i.e. the orbitals now have different energies., , Ligands which cause only a small degree of crystal field, splitting are termed weak field ligands. Ligands which cause, a large splitting are called strong field ligands. The common, ligands can be arranged in ascending order of crystal field, splitting '., Spectrochemical Series, I– < Br– <S2–< Cl– < NO3� <F– < OH– < EtOH< oxalate < H2O, (weak field ligands), , < EDTA < (NH3 = pyridine) < ethylenediamine < dipyridyl, < o- phenanthroline < NO �2 < CN– < CO, (strong field ligands), , A pattern of increasing V donation is followed :, Halide donors < O donors < N donors < C donors, , The total crystal field stabilization energy is given by, CFSE ( octahedral), , �0.4n ( t 2 g ) � 0.6n ( eg ), , where n ( t 2g ) and n ( eg ) are the number of electrons, 8.1 Octahedral complexes, In an octahedral complex, the metal is at the centre of the, octahedron, and the ligands are at the six corners. The, directions x, y and z point to three adjacent corners of the, octahedron as shown., , occupying the t2g and eg orbitals respectively. The CFSE is, zero for ions with d0 and d10 configurations in both strong, and weak ligand field. The CFSE is also zero for d 5, configurations in a weak field.
Page 7 :
CO-ORDINATION COMPOUNDS, , EFFECTS OF CRYSTAL FIELD SPLITTING, CFSE and electronic arrangements in octahedral complexes, Number, of d, electrons, , Arrangement in weak ligand field, t2g, , eg, , CFSE, 'o, , Arrangement in strong ligand field, , Spin only, , t2g, , magnetic moment, , eg, , CFSE, 'o, , Spin only, magnetic moment, , ����Ps (D), , Ps (D), , d1, , 1.73, , 1.73, , d2, , 2.83, , 2.83, , d3, , 3.87, , 3.87, , d4, , – 0.6, , 4.90, , 2.83, , d5, , – 0.0, , 5.92, , 1.73, , d6, , – 0.4, , 4.90, , 0.00, , d7, , – 0.8, , 3.87, , – 1.8, , 1.73, , d8, , – 1.2, , 2.83, , – 1.2, , 2.83, , d9, , – 0.6, , 1.73, , – 0.6, , 1.73, , d10, , 0.0, , 0.00, , 0.0, , 0.00, , 8.2 Tetrahedral Complexes, , Thus the t2 orbitals are nearer to the direction of the ligands, than the e orbitals. The approach of the ligands raises the, energy of both sets of orbitals. The energy of the t2 orbitals, is raised most because they are closest to the ligands. The, crystal field splitting is the opposite way round to that in, octahedral complexes, , A regular tetrahedron is related to a cube. One atom is at the, centre of the cube, and four of the eight corners of the cube, are occupied by ligands as shown., , The t2 orbitals are 0.4't above weighted average energy of, the two groups (the Bari centre) and the e orbitals are 0.6't, below the average., The magnitude of the crystal field splitting 't in tetrahedral, complexes is considerably less than in octahedral fields., There are two reasons for this :, , The directions x, y and z point to the centres of the faces of, the cube. The e orbitals point along x, y and z axes (that is to, the centres of the faces). The t2 orbitals point between x, y, and z axes (that is towards the centres of the edges of the, cube). The direction of approach of the ligands does not, coincide exactly with either the e or the t2 orbitals., , 1., , There are only four ligands instead of six, so the ligand field, is only two third the size ; hence the ligand field splitting is, also two third the size., , 2., , The direction of the orbitals does not coincide with the, direction of the ligands. This reduces the crystal field, splitting by roughly a further two third.
Page 8 :
CO-ORDINATION COMPOUNDS, Thus the tetrahedral crystal field splitting 't is roughly, 2/3 × 2/3 = 4/9 of the octahedral crystal field splitting 'o., , The number of carbon atoms bound to the metal in these, compounds is indicated by the Greek letter ‘K’ (eta) with a, 2, 5, 6, number. The prefixes K , K and K indicate that 2, 5 and 6, carbon atoms are bound to the metal in the compound., 9.3 �V–, �V and S–bonded organometallic compounds, , 9. ORGANOMETALLIC COMPOUNDS, Compounds that contain at least one carbon–metal bond, are called organometallic compounds., Grignard reagent, RMgX is a familiar example of, organometallic compounds where R is an alkyl group. Diethyl, zinc [Zn(C2H5)2], lead tetraethyl [Pb(C2H5)4], ferrocene, [Fe(C 5H5) 2], dibenzene chromium [Cr(C6H6 )2], metal, carbonyls are other examples of organometallic compounds., , Metal carbonyls, compounds formed between metal and, carbon monoxide belong to this class. These compounds, posses both V– and S– bonding. The oxidation state of, metal atoms in these compounds is zero. Carbonyls may be, monomeric, bridged or polynuclear., , Organometallic compounds may be classified in three classes :, 1., , Sigma (V) bonded complexes., , 2., , Pi (S) bonded complexes,, , 3., , Complexes containing both V– and S–bonding, characteristics., 9.1 Sigma bonded complexes, In these complexes, the metal atom and carbon atom of the, ligand are joined together with a sigma bond, i.e., the ligand, contributes one electron and is, therefore, called one electron, donor. Examples are :, , (i), , Grignard reagent, R–Mg–X where R is an alkyl or aryl group, and X is halogen., , (ii), , Zinc compounds of the formula R2Zn such as (C2H5)2Zn., This was first isolated by Frankland in 1849. Other similar, compounds are (CH3)4Sn, (C2H5)4Pb, Al2(CH3)6, Al2(C2H5)6, and Pb(CH3)4, etc., , In a metal carbonyl, the metal–carbon bond possesses both, the V– and S–character. A V–bond between metal and carbon, atom is formed when a vacant hybrid orbitals of the metal, atom overlaps with an orbital on C atom of carbon monoxide, containing a lone pair of electrons., –, , +, , Formation of S–bond is caused when a filled orbital of the, metal atom overlaps with a vacant antibonding S* orbital of, C atom of carbon monoxide. This overlap is also called back, donation of electrons by metal atom to carbon. It has been, shown below :, , 9.2 �S–bonded, organometallic compounds, �S, These are the compounds of metals with alkenes, alkynes,, benzene and other ring compounds. In these complexes,, the metal and ligand form a bond that involves the S electrons, of the ligand. Three common examples are Zeise’s salt,, ferrocene and dibenzene chromium. These are shown here :, , The S–overlap is perpendicular to the nodal plane of V–bond., In olefinic complexes, the bonding S–orbital electrons are, donated to the empty orbital of the metal atom and at the, same time back bonding occurs from filled orbital of the, metal atom to the antibonding S–orbital of the olefin.
Page 9 :
CO-ORDINATION COMPOUNDS, , 10.1.4 Linkage Isomerism : This type of isomerism occurs, in complex compounds which contain ambidentate ligands, , 10. ISOMERISM, The compounds having same molecular formula but different, structural formula are called isomers., , like NO�2 , SCN � , CN � , S2O32� and CO., For example, [Co(NH3)5NO2] Cl2 and [Co(NH3)5ONO] Cl2, are linkage isomers as NO �2 is linked through N or through, O., 10.1.5 Ligand Isomerism : Some ligands themselves are, capable of existing as isomers, e.g., diamino propane can, exist both as 1, 2-diamino propane (pn) and 1, 3-diamino, propane, also called trimethylene diamine (tn)., , 10.1 Structural Isomerism, 10.1.1 Ionisation Isomerism : This type of isomerism arises, when the coordination compounds give different ions in, solution. For example, there are two isomers of the formula, Co (NH3)5 BrSO4., [Co ( NH 3 )5 Br] SO 4, ( Violet ), , [Co(NH 3 ) 5 Br]2 � � SO 24 �, Pentaammin ebromido, � cobalt (III) ion, , This isomer gives a white percipitate of BaSO4 with BaCl2, solution., [Co( NH 3 )5 SO 4 ]Br, (Red), , When these ligands (i.e., pn and tn) are associated into, complexes the complexes are isomers of each other. One, example of isomeric complexes having this ligand is :, [Co(pn)2 Cl2]+ and [Co(tn)2 Cl2]+ ions., 10.1.6 Coordination position isomerism : This type of, isomerism is exhibited by polynuclear complexes by, changing the position of ligands with respect to different, metal atoms present in the complex. For example,, , [Co(NH 3 ) 5 SO 4 ]� � Br �, Pentaammin esulphato, � cobalt (III) ion, , Above isomer gives light yellow precipitate with AgNO3 solution., 10.1.2 Hydrate Isomerism : This type of isomerism arises, when different number of water molecules are present inside, and outside the coordination sphere. This isomerism is best, illustrated by the three isomers that have the formula, CrCl3.6H2O., [Cr(H2O)6]Cl3 , [Cr(H2O)5Cl]Cl2.H2O, and [Cr(H2O)4Cl2], , and, , 10.2 Stereo Isomerism, , Cl.2H2O are its Hydrate Isomers., 10.1.3 Cordination Isomerism : This type of isomerism is, observed in the coordination compounds having both, cationic and anionic complex ions. The ligands are, interchanged in both the cationic and anionic ions to form, isomers. An examples is :, , [Pt ( NH3 ) 4 ] [CuCl 4 ] and [Cu ( NH3 ) 4 ] [PtCl 4 ], Tetraammineplatinum (II), tetrachloridocuprate (II), , Tetraamminecopper (II), tetrachloridoplatinate (II), , Stereo isomerism is exhibited by those compounds which, have the same position of atoms or groups but these atoms, or groups have different arrangement around the central atom., 10.2.1 Geometrical Isomerism, The complex compounds which have the same ligands in, the co-ordination sphere but the relative position of the, ligands around the central metal atom is different are called
Page 10 :
CO-ORDINATION COMPOUNDS, , geometrical isomers and the phenomenon is called, geometrical isomerism., , 3., , Mabcd, , 10.2.1.1 Geometrical Isomerism in square planar complexes, A square planar complexe having similar ligands at adjacent, positions (90º a part) is called cis - isomer while a square, planar complex having two similar ligands at opposite, positions (180º a part) is called trans-isomer., 1., , Ma2b2, , Example - 1, , Example - 3, , Draw the geometrical isomers of [PtClBrpy (NH3)], , Draw the geometrical isomers of [PtCl2(NH3)2], Sol., , Sol., , 4., 2., , Ma2bc, , M (AB)2, , Example - 4, , Draw the geometrical isomers of [Pt(gly)2], Sol., , Example - 2, , Draw the geometrical isomers of [PtCl2(NH3)py], Sol.
Page 11 :
CO-ORDINATION COMPOUNDS, 10.2.1.2 Geomertical Isomerism in octahedral complexes, 1., , Ma4b2, , Example - 7, , Draw the geometrical isomers of [Cr(gly)3], Sol., , Example - 5, +, , Draw the geometrical isomers of [CrCl2(NH3)4], Sol., , 5., , 2., , M(AA)2b2, , Ma3b3, , Example - 8, , Draw the geometrical isomers of [CoCl2(en)2]+, Sol., Example - 6, , Draw the geometrical isomers of [RhCl3(py)3], Sol., , Cis and trans-isomers of [CoIII (en)2 Cl2]+ ion., (a) Cis-isomer, (b) trans-isomer, , 3., 4., , Mabcdef : They form 15 isomers, M (AB)3
Page 13 :
CO-ORDINATION COMPOUNDS, 2., , M (AA)3, , 5., , cis Ma2b2c2, , Example - 13, Draw the optical isomers of [Co(en)3]3+, Sol., , Example - 16, , Draw the optical isomers of [PtCl2Br2(NH3)2], The two optical isomeric forms of the complex [Co(en)3], 3., , M (AB)3, , 3+, , Sol., , 6., Example - 14, , Draw the optical isomers of [Cr(gly)3], , cis M(AA)b2c2, , Example - 17, , Draw the optical isomers of [CoCl2 (en) (NH3)2]+, , Sol., Sol., , 4., , cis M (AA)2b2, , 7., Example - 15, Draw the optical isomers of RhCl2(en)2]+, , cis M(AA)2bc, , Example - 18, , Draw the optical isomers of [CoCl (en)2 Br]2+, , Sol., , Optical active isomers of cis [RhCl2(en)2]+
Page 14 :
CO-ORDINATION COMPOUNDS, , (b), , Volumetric analysis : Hardness of water can be estimated, by titration with EDTA. The metal ions causing hardness,, that is Ca2+ and Mg2+, form stable complexes with EDTA., , 2., , Metal extraction and purification : Extraction of metals, such, as silver and gold, is carried out by forming their water soluble, cyanide complexes with the ore. Pure gold can then be, obtained from the solution by addition of zinc. Similarly,, metals can be purified by formation and then decomposition, of their coordination compounds. For example, impure nickel, obtained after extraction may be converted into pure nickel, by first converting it to nickel carbonyl and then, decomposing., , 3., , Catalysis : Coordination compounds are used as catalysts, in important commercial processes. For example,, , (a), , The Zeigler-Natta catalyst (TiCl4 and trialkyl aluminium) is, used as a catalyst in the formation of polyethene., , (b), , The Wilkinson catalyst - RhCl(PPh3 )3 is used in the, hydrogenation of alkenes., , (c), , In the Monsanto acetic acid process, various rhodium, complexes, such as [Rh(CO)2I2], [Rh(Cl)(CO)(PPh3)2] or, [Rh(Cl)(CO)2]2, are used as catalyst in the presence of CH3I,, I2 or HI as activator., , 4., , Electroplating : Coordination compounds of gold, silver, and copper are used as components in the baths used for, electroplating articles of other metals with these metals. For, example, in silver plating, K[Ag(CN)2] is used as an, electrolyte; in gold plating, K[Au(CN)2] is used as an, electrolyte; and in copper plating, K3[Cu(CN)4] is used as, an electrolyte., , 5., , Biological importance : Some important biological, compounds are coordination complexes. For example,, chlorophyll is a complex of Mg2+. This green pigment plays, a vital role in photosynthesis in plants. Similarly,, haemoglobin, the red pigment present in blood, is a, coordination complex of Fe2+ and vitamin B12, an essential, nutrient, is a complex compound of Co3+., , 6., , Medicinal uses : Complexing or chelating agents are used, in treating metal poisoning, wherein, the coordination, complex is formed between toxic metal in excess metal and, the complexing agent. For example, EDTA is used in lead, poisoning. EDTA, when injected intravenously into the, bloodstream, traps lead forming a compound that is flushed, out of the body with the urine. Other heavy metal poisonings, that can be treated similarly with chelation therapy are, mercury, arsenic, aluminium, chromium, cobalt, manganese,, nickel, selenium, zinc, tin and thallium. Similarly, chelating, ligands D-penicillamine and desferrioxime B are used for, removal of excess copper and iron, respectively. New potent, drugs are being created using various derivatives of, metallocene. A platinum complex [PtCl2(NH)32] called cisplatin is used in treatment of cancer., , 11. STABILITY OF COORDINATION COMPOUNDS, The stability of a complex in solution refers to the degree of, association between the two species involved in the state, of equilibrium., If we have a reaction of the type :, ZZX ML, M + 4L YZZ, 4, , then the larger the stability constant, the higher the, proportion of ML4 that exists in solution. Free metal ions, rarely exist in the solution so that M will usually be, surrounded by solvent molecules which will compete with, the ligand molecules, L, and be successively replaced by, them. For simplicity, we generally ignore these solvent, molecules and write four stability constants as follows :, ZZX, YZZ, , ML, , K1 = [ML]/[M][L], , L, , ZZX, YZZ, , ML2, , K2 = [ML2]/[ML][L], , ML2 +, , L, , ZZX, YZZ, , ML3, , K3 = [ML3]/[ML2][L], , ML3 +, , L, , ZZX, YZZ, , ML4, , K4 = [ML4]/[ML3][L], , M, , +, , L, , ML, , +, , where K1, K2, etc., are referred to as stepwise stability, constants. Alternatively, we can write the overall stability, constant thus :, M, , +, , 4L YZZ, ZZX, , ML4, , E4 = [ML4]/[M][L]4, , The stepwise and overall stability constant are therefore, related as follows :, E4 = K1 × K2 × K3 × K4 or more generally,, En = K1 × K2 × K3 × K4 ................. Kn, The instability constant or the dissociation constant of, coordination compounds is defined as the reciprocal of the, formation constant., , 12. IMPORTANCE AND APPLICATIONS OF, COORDINATION COMPOUNDS, 1., (a), , Analytical chemistry : The analytical applications of, coordination chemistry are in, Qualitative and quantitative analysis : Metals ions form, colored coordination compounds on reaction with a number, of ligands. These reactions are used for detection of the, metal ions. The colored complexes formed can be used for, the estimation of metals by classical or instrumental methods, such as gravimetry or colorimetry. Some examples are given, as follows :, The presence of iron ions (Fe3+) can be detected by the, addition potassium ferrocyanide solution, which results in, formation of Prussian blue complex., Fe2+ + K3Fe(CN)6 o KFe[Fe(CN)6] + 2K+
Page 15 :
CO-ORDINATION COMPOUNDS, , 14.2 Ligands, , 13. COORDINATION COMPOUNDS AND, COMPLEX IONS, (a), , An atom, ion or molecule which can donate alteast a pair of, electrons to the central atom to form a coordinate bond (or, dative linkage) is called as a ligand or a coordinating group., Further in a ligand, the particular atom which actually donates, the electron pair is called the donor atom., , Co-ordination compounds are the compounds in which the, central metal atom is linked to a number of ligands (ions or, neutral molecules) by co-ordinate bonds i.e. by donation of, lone pairs of electrons by these ligands to the central metal, atom ion., , The ligands act as Lewis bases as they donate one or more, electron pair to the central metal atoms or ions witch act as, Lewis acids by accepting electrons., , If a such a compound carries positive or negative charge, it, 4–, , 2+, , is called a complex ion, e.g. [Fe(CN)6] , [Cu(NH3)4] ., Hence Co-ordination compounds may also be defined as, those compounds which contain complex ions, e.g.,, , Example, , K4[Fe(CN)6], [Cu(NH3)4]SO4, etc. In general, a complex ion, is represented as [MLn], , r, , x, , . Where M is the metal ion, L, , represents ligands, n is the coordination number of metal, ion and x is the net charge on the complex., (b), , There are four types of complexes :, , (i), , Cation as complex ion, (carrying a net positive charge) e.g.,, , 14.2.1 Types of Ligands : Ligands can be classified on the, number of lone pair electrons they donate to the central, metal atom or ion., (a), , 3+, , [Cr (NH3)6] in [Cr(NH3)6]Cl3., (ii), , Monodentate or unidentate ligands : They have one donor, atom that donates only one electron pair to central metal, –, , –, , –, , –, , atom. eg : F , Cl , Br� , H2O, NH3, CN , NO �2 , OH , NH �2 ,, , Anion as complex ion, (carrying a net negative charge) e.g.,, , –, , –, , 3–, , –, , –, , –, , , I , SH , RCOO , RS ,, , CO, R–OH, SCN ,, , [Fe(CN)6] in K3 [Fe(CN)6]., (iii) Cation and anion both as complex ion. Carrying both +ive, and –ive change. For e.g., [Pt(Py)4] [PtCl4]., , (Dimethyl sulfoxide), NH2 NH2 NO �2 ,, , (iv) Neutral complex (A complex carrying no net charge) e.g.,, , NO3� , S2–, O2– , pyridine., , [Ni(CO)4] etc., , (b), , 14. TERMINOLOGY OF COORDINATION, COMPOUNDS, , Bidentate ligands : Ligands which have two donor atoms, and have the ability to link with central metal at two positions, are called bidentate ligands, , Example, , 14.1 Centre of Corrdination, (Central atom/ion or Acceptor atom/ion) :, The cation or neutral atom to which one or more ligands, (neutral molecules or anions) are attached or coordinated is, the centre of coordination., The central atom/ion must have empty orbitals as it acts as, an acceptor and thus has to accommodate electron pairs, donated by the donor atom of the ligand. This explains why, the transition metal having empty d-orbitals, form, coordination compounds very readily., For example in the complexes [Ni(NH3)6], Ni, , 2+, , and Fe, , 3+, , 2+, , 3–, , and [(CN)6] ,, , respectively are the central ions., , (1), , (2), , (3), , (4)
Page 17 :
CO-ORDINATION COMPOUNDS, , 14.2.3 Ambidentate ligands :, The ligands which have two donor atoms but in forming, complexes only one donor atom is attached to the metal atom, at a given time. Such ligands are called ambidentate ligands., , 16. STABILITY OF COORDINATION COMPOUNDS, IN SOLUTIONS, 16.1 In general, higher the charge density on the central ion, the, greater is the stability of its complexes, i.e., the higher value, , charge, of radius of the ion , the greater is the stability of its, , Example :, , complexes. Electronegativity of the central ion influences, the stability. The higher the electronegativity of the central, ion, the greater is the stability of its complexes., 16.2. The higher the oxidation state of the metal, the more stable, is the complex. The charge density of Co, Co, , 2+, , ion and thus, [Co (NH3)6], 2+, , 3+, , 15. COORDINATION NUMBER (C.N), The number of atoms of the ligands that directly bound to, the central metal atom or ion by co-ordinate bonds is known, as the co-ordination number of the metal atom or ion. It is, also equal to the secondary valency., Complex, , Co-ordination numbers, , K [Fe (CN)6], –, , [Ag (CN)2], , 6, 2, , [Pt (NH3)2 Cl2], [Ca (EDTA)]2–, , 6, , ion is more than, , is more stable than [Co, , 3–, , (NH3)6] . Similarly, [Fe (CN)6], , 3+, , is more stable than [Fe, , 4–, , (CN)6] ., The cyano and ammine complexes are far more stable than, those formed by halide ions. This is due to the fact that NH3, –, , and CN are strong Lewis bases., 2+, , The complexes of bivalent cations (M ) of 3d-series shown, the following order of stability :, Cation, , Mn, , 2+, , Ionic size 0.91, , Fe, , 2+, , 0.83, , Co, , 2+, , 0.82, , 2+, , 2+, , Ni, , Cu, , 0.78, , 0.69, , Stability of, , increases, , the complex, , (Irving William order), , decreases, , Chelating ligands form more stable complexes as compared, to monodentate ligands. Greater is the chelation, more is the, stability of complex.