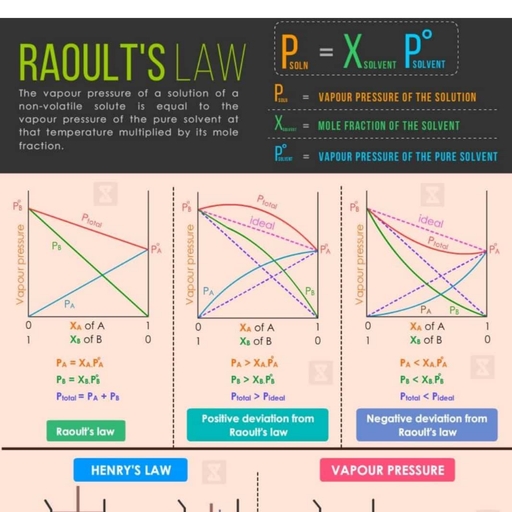
Page 1 :
Sample Question Paper - 22, Chemistry (043), Class- XII, Session: 2021-22, TERM II, Time allowed : 2 hours, , Maximum marks : 35, , General Instructions :, Read the following instructions carefully., 1. There are 12 questions in this question paper with internal choice., 2. SECTION A - Q. No. 1 to 3 are very short answer questions carrying 2 marks each., 3. SECTION B - Q. No. 4 to 11 are short answer questions carrying 3 marks each., 4. SECTION C - Q. No. 12 is case based question carrying 5 marks., 5. All questions are compulsory., 6. Use of log tables and calculators is not allowed., , SECTION - A, 1., , How will you bring about the following conversion? (any two), (a) Ethanal to but-2-enal , (b) Propanone to propene, (c) 2-Methylpropanol to 2-methylpropene, , 2., , Rate constant, k for a first order reaction has been found to be 2.54 × 10–3 sec–1. Calculate its 3/4th life., (log 4 = 0.6020), , 3., , Describe cross aldol condensation reaction., , SECTION - B, 4., , (a) �Why do transition metals and their compounds generally exhibit a paramagnetic behaviour., (b) What are the transition elements? Write two characteristics of the transition elements., OR, Give examples and suggest reasons for the following features of the transition metal chemistry :, (a) The lowest oxide of transition metal is basic, the highest is amphoteric/acidic., (b) A transition metal exhibits highest oxidation state in oxides and fluorides., (c) The highest oxidation state is exhibited in oxoanions of a metal., , 5., , Define lyophobic and lyophilic sol with a suitable example of each. Why is coagulation of lyophilic sol, difficult as compared to lyophobic sol?, , 6., , Write the IUPAC name of the following complexes :, (a) [Cr(NH3)3Cl3] (b) [Cr(NH3)4Cl2]+ (c) K3[Fe(C2O4)3], OR, What is spectrochemical series? Explain the difference between a weak field ligand and a strong field, ligand., , 7., , For the first order thermal decomposition reaction, the following data were obtained :, C2H5Cl(g), C2H4(g) + HCl(g)
Page 2 :
Time/sec, Total pressure/atm, 0, 0.30, 300, 0.50, Calculate the rate constant. (Given : log 3 = 0.4771), OR, For a first order reaction, show that time required for 99% completion is twice the time required for the, completion of 90% of reaction., 8., , Give reason :, (a) pH of reaction should be carefully controlled while preparing ammonia derivatives of carbonyl, compounds., (b) Aldehyde and ketone have lower boiling point than corresponding alcohol., , 9., , Arrange the following in increasing order of the properties indicated in the bracket., (a) C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2 (Basicity), (b) C2H5OH, (CH3)2NH, C2H5NH2 (Boiling Point), (c) C6H5NH2, (C2H5)2NH, C2H5NH2 (Solubility in water), , 10. Define CFSE. On the basis of CFT, write the electronic configuration of d5 in terms of t2g and eg in an, octahedral field when, (i) Do > P, (ii) Do < P, 11. Accomplish the following conversion., (a) Benzoic acid to aniline , , (b) Benzyl chloride to 2-phenylethanamine, OR, , Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical, equations of the reactions involved., , SECTION - C, 12. Read the passage given below and answer the questions that follow., Molar conductivity of ions are given as product of charge on ions to their ionic mobilities and Faraday’s, constant., lAn+ = nmAn+ F (here m is the ionic mobility of An+)., For electrolytes say AxBy, molar conductivity is given by, lm(AxBy) = xn mAn+ F + ymlAm–F, Ions Ionic mobility, K+ , 7.616 × 10–4, Ca2+ , 12.33 × 10–4, Br– , 8.09 × 10–4, 2–, SO4 , 16.58 × 10–4, (a) At infinite dilution, calculate the equivalent conductance of CaSO4., (b) �, What will be the equivalent conductance of CaSO4 if the degree of dissociation of CaSO4 solution is 10%?, OR, Write the correct order of equivalent conductance (at infinite dilution) of LiCl, NaCl, KCl., (c) What is the unit of equivalent conductivity?, (d) If the molar conductance value of Ca2+ and Cl– at infinite dilution are 118.88 × 10–4 m2 mho mol–1 and, 77.33 × 10–4 m2 mho mol–1 respectively then what will be the molar conductance of CaCl2?
Page 3 :
Solution, CHEMISTRY - 043, Class 12 - Chemistry, O, , 1., , 1. NaOH, , OH–, 2CH3 C H Aldol, Ethanal, condensation, , (i), , 2. Heat, , CH3 CH, , OH, , CH3 CH CH2 CHO, , CH, , CHO, , But-2-enal, From two molecules of ethanal, , + CH3CH2 CH, , � H+, , Simple or self aldol products, , O, , CH3 CH, , Propene, , CH3 CH, , 2-Propanol, , CH3, , Dehydration, , CH3, , (iii) CH3 CH, , CH3 CH, , OH, , conc. H2SO4, 443 K, , CH2, , CH2OH, , C, , CHO + CH3CH2 CH CHCHO, , CH3, 2-Methylbut-2-enal, Pent-2-enal, From one molecule of ethanal and one molecule of propanal, Cross aldol products, , Ketones can also be used as one component in the, cross aldol reactions, , SOCl2, , CHO +, , C, , 2-Methylpropanol, , CH3, CH3 C CH2, , alc. KOH, , O, , CH3, CH3 CH, , CH2 Cl, , 2. The integrated rate equation for first order, reaction is, , ⇒ k=, ⇒ k=, , 2.303, a , log , a − x , t, 2.303, a , log, , 3 , t, a − a , 4, a, 2.303, log, t, 0.25a, , t3/ 4 =, , 2.303, , 2.54 × 10−3 sec, , OH –, , CH3 293 K, , O, , 2-Methylpropene, , k=, , CHO, , 2-Methylpent-2-enal, From two molecules of propanal, , But-2-enal, , NaBH4, CH3OH, CH3 C CH3 Reduction, Propanone, , C, , CH3, , CH3 CH CH CHO, , (ii) Propanone to propene, , CH3CHO, +, CH3CH2CHO, , × log, −1, , 1, = 546 s, 0.25, , Therefore, the 3/4th life of the reaction is 546 seconds., 3. Cross-aldol condensation : Aldol condensation, is the reaction that takes place, when aldehydes, or ketones with at least one a-H atom react in the, presence of dilute alkali to produce b-hydroxy, aldehydes or ketones. When two different aldehydes, or ketones are taken, it gives a mixture of products., Such a reaction is called cross-aldol condensation., , CH CH, , C, , 1,3-Diphenylprop-2-ene-1-one, (Benzalacetophenone) (Major product), , 4. (a) Transition metals and most of their, compounds contain unpaired electrons in the (n – 1)d, orbitals hence show paramagnetic behaviour., (b) Elements which have incompletely filled d-orbitals, in their ground state or in any one of their oxidation, states are called transition elements., Characteristics of transition elements :, (i) They show variable oxidation states., (ii) They exhibit catalytic properties., OR, (i) Lowest oxidation compounds of transition metals, are basic due to their ability to get oxidised to higher, oxidation states. Whereas the higher oxidation state of, metal and compounds gets reduced to lower ones and, hence acts as acidic in nature., e.g. MnO is basic whereas Mn2O7 is acidic., (ii) Due to high electronegativities of oxygen and, fluorine, the oxides and fluorides of transition metals, exhibit highest oxidation state., e.g., OsF6, V2O5
Page 4 :
(iii) In oxoanions of metals, the metals form bonds with, oxygen and hence are present in their highest oxidation, states. For example : Cr forms CrO42– and Cr2O72–,, both contain chromium in +6 oxidation state., Permanganate ion, MnO4– contains Mn in its highest, oxidation state of +7., 5. A colloidal sol in which dispersed phase and, dispersion medium attract each other is called, lyophilic colloid. e.g., gum. A colloidal sol in which, dispersed phase and dispersion medium repel each, other is called lyophobic colloid. e.g., gold solution., In lyophobic sol, there is hardly any affinity between the, particles of dispersion medium and dispersed phase., Therefore, it is unstable and can be easily coagulated., Since there is strong affinity between the particles in, case of lyophilic sol, so coagulation is rather difficult., 6. (a) IUPAC name of the complex [Cr(NH3)3Cl3] is, triamminetrichloridochromium(III)., (b) IUPAC name of [Cr(NH3)4Cl2]+ is, Tetraamminedichloridochromium(III) ion, (c) IUPAC name of K3[Fe(C2O4)3] is potassium, trioxalotoferrate(III)., OR, The crystal field splitting, Do, depends upon the field, produced by the ligand and charge on the metal ion., Some ligands are able to produce strong fields in which,, the splitting will be large whereas others produce, weak fields and consequently result in small splitting, of d-orbitals. In general, ligands can be arranged in a, series in the order of increasing field strength as given, below :, I– < Br– < SCN– < Cl– < S2– < F– < OH– < C2O42–, < H2O < NCS– < edta4– < NH3 < en < CN– < CO, Such a series is termed as spectrochemical series., If Do < P, the fourth electron enters one of the eg, 3 1, orbitals giving the configuration t2g, eg . Ligands for, o, which D < P are known as weak field ligands and, form high spin complexes. If Do > P, it becomes more, energetically favourable for the fourth electron to, 4 0, occupy a t2g orbital with configuration t2g, eg . Ligands, which produce this effect are known as strong field, ligands and form low spin complexes., 7., , The given reaction is, C2H5Cl(g), , At time t = 0, , 0.30 atm, , At time t = 300 sec, , 0.30 – x, , C2H4(g) + HCl(g), 0, , 0, , x, , x, , Total pressure = 0.30 – x + x + x = 0.50, or 0.30 + x = 0.50, \ x = 0.50 – 0.30 = 0.20, \ Initial pressure, P0 = 0.30 atm, Pressure of C2H5Cl after 300 sec,, Pt = 0.30 – 0.20 = 0.10 atm, Using formula for first order reaction,, k=, , P , 2.303, log 0 , t, Pt , , k=, , 2.303, 0.30 , log , 0.10 , 300, , k=, , 2.303 × 0.4771, 2.303, log 3 =, 300, 300, , = 3.66 × 10–3 sec–1, OR, 99% completion means that x = 99% of [R]0, or, [R] = [R]0 – 0.99[R]0 = 0.01[R]0, For first order reaction, t =, ∴ t99% =, =, , [ R], 2.303, log 0, k, [ R], , [ R]0 , 2.303, log , k, 0.01[ R]0 , , 2.303, 2.303, log 10 2 = 2 ×, k, k, , 90% completion means that [R] = [R]0 – 0.90[R]0, = 0.1[R]0, [ R]0 2.303, 2.303, log 10 =, =, , R, k, k, 0, ., 1, [, ], , 0, , \ t90% = 2.303 log , k, , \, , t99% 2 × 2.303 , =, , k, t90% , , 2.303 , k = 2, , or, t99% = 2 × t90%, 8. (a) In strongly acidic medium, ammonia, derivatives being basic will react with acids and will, not react with carbonyl compound. In basic medium,, OH– will attack carbonyl group., Therefore, pH of the reaction should be carefully, controlled., (b) The boiling points of aldehydes and ketones are, lower than that of corresponding alcohols and acids, due to absence of intermolecular H–bonding in, aldehydes and ketones., 9., , (a) C6H5NH2 < C6H5N(CH3)2 < CH3NH2 <, (C2H5)2NH