Page 1 :
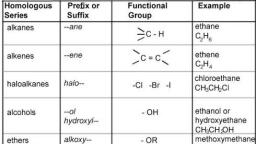
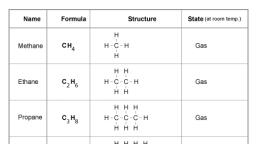
1|Page, , Chemistry Notes for class 12 Chapter 11, Alcohols, Phenols and Ethers, Alcohols and Phenols, Alcohols and phenols are formed when a hydrogen atom in hydrocarbon, aliphatic and, aromatic respectively, is replaced by hydroxyl group (-OR group)., Classification of Alcohols and Phenols, In alcohols, -OR group is attached to Sp3 hybridised carbon. These alcohols are usually, classified as primary, secondary and tertiary alcohols., , Alcohols may be, (i) monohydric-containing one – OR group,, (ii) dihydric-containing two – OR groups and, (iii) polyhydric-containing three or more -OR groups., In phenols, -OR group is attached to Sp2 hybridised carbon. These may also be monohydric,, dihydric, etc. The dihydric phenol further rosy be ortho, meta’ or para derivative., , In allylic alcohols, – OH group is attached to sp3 hybridised carbon but next to C=C bond., www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 2 :
2|Page, , e.g., CH2 = CH – CH2OH, Benzylic alcoho1(C6H5CH2OH), Structure of Alcohols and Phenols, The oxygen atom of alcohols is Sp3 hybridised and they have tetrahedral position of hybrid, atomic orbitals ., , The value of LROH bond angle depends upon the R group. For methyl alcohol, it is (∠C – O –, H) 108.9° due to repulsion of lone pairs., In phenols, the – OH group is attached to Sp2 hybridised carbon and thus, the C – O bond, acquires a partial double bond character., , Nomenclature of Alcohols and Phenol, In IUPAC, system, alcohol or alkanols are named by replacing the last word ‘e’ of the, corresponding alkane by ‘ol’. e.g.,, , Preparation of Alcohols, (i) From alkenes, (a) By acid catalysed hydration in accordance with Markownikoff’s rule., , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 4 :
4|Page, , (ii) From carbonyl compounds, (a) By reduction of aldehydes and ketones, , Aldehydes yield primary alcohols whereas ketones give secondary alcohols, when subjected to, reduction., (b) By reduction of carboxylic acids and ester, , Reduction of aldehyde, ketones and esters with No Alcohol is called Bouveault-blanc, reduction., , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 7 :
7|Page, , Physical Properties of Alcohols, 1. Lower alcohols are colourless liquids, members from C5 – C11 are oily liquids and higher, members are waxy solids., 2. The hydroxyl groups in alcohols can form H-bonds with water, so alcohols are miscible with, water. The solubility decreases with increase in molecular mass., , 3. Boiling points of alkanes are higher than expected because of the presence of intermolecular, hydrogen bonding in the polar molecules., [The boiling point decreases in the order 1° > 2° > 3° as the van der Waals’ forces of attraction, decreases], Physical Properties of Phenols, 1. These are colourless liquids or crystalline solids but become coloured due to slow oxidation, with air., 2. Phenol is also called carbolic acid., 3. Because of the presence of polar -OH bond, phenols form intermolecular H-bonding with, other phenol molecules and with water., Chemical Reactions of Alcohols and Phenols, (i) Reactions involving cleavage of O – H Bond, (a) Acidity of alcohols and phenols, , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 8 :
8|Page, , Alcohols are weaker acids than water due to +1 group present in alcohols, which decreases the, polarity of -O-H bond., Acid strength of alcohols, , Electron releasing group increases electron density on oxygen to decrease the polarity of – OH, bond., Order of acidity is, RCOOH > H2CO3 > C6H5OH > H2O > R – OH., Phenol is more acidic than alcohols due to stabilisation of phenoxide ion through resonance., Presence of electron withdrawing group increases the acidity of phenol by ,, stabilising phenoxide ion while presence of electron releasing group decreases the acidity of, phenol by destabilising phenoxide ion., Thus. increasing acidic strength is, o-cresol < p-cresol < m-cresol < phenol < o-nitrophenol < 2, 4. 6.trinitrophenol (picric acid), Higher Ka and lower pKa value corresponds to the stronger acid., (b) Esterification, , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 9 :
9|Page, , The reaction with R’COOH and (R’ CO)2O is reversible, so cone, H2SO4 is used to remove, water., The reaction with R’ COCI is carried out in the presence of pyridine so as to neutralise HCI, which is formed during the reaction., The introduction of acetyl (CH3CO-) group in phenols is known as acetylation., Acetylation of salicylic acid produces aspirin., , (ii) Reaction involving cleavage of C-O bond in alcohols In these reactions, the reactivity, order of different alcohols :, , Alkyl group due to +1 effect increases the electron density on the carbon and oxygen atom of, C-OH bond. As a result, the bond cleavage becomes easy. Greater the number of alkyl groups, present, more will be the reactivity of alcohol. Thus, the relative order of reactivity of the, alcohols is justified., , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 10 :
10 | P a g e, , (a) Reaction with halogen acids Alcohols can be converted into haloalkanes by the action of, halogen acids., R – OH + HX (HCI, HBr, HI) → R-X +H2O, For a given alcohol order of reactivity of HX is, H-1 > H-Br > H-Cl, For a given halogen acid order of reactivity of alcohols, Tertiary > Secondary > Primary, Lucas test, , (b) Reaction with phosphorus halides, , (c) Reaction with thionyl chloride, , d) Dehydration of alcohols It requires acid catalyst and the reaction proceeds via intermediate, carbonium ion. Acidic catalyst converts hydroxyl group into a good leaving group., Since, the rate determining step is the formation of carbocation, the ease of dehydration is, , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 11 :
11 | P a g e, , Mechanism, Step I Formation of protonated alcohol, , Step II Formation of carbocation, , Step III Formation of ethene by elimination of a proton, , In dehydration reaction, highly substituted alkene is the major product and if the major product, is capable of showing cis-trans isomerism, trans-product is the major product. (Saytzeff’s rule)., (iii) Oxidation reactions Oxidising reagents used for the oxidation of alcohols are neutral,, acidic or alkaline KMnO4 and acidified K2Cr2O7., , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 12 :
12 | P a g e, , A common reagent that selectively oxidises a primary alcohol to an aldehyde (and no further), is pyridinium chlorochromate (pCC)., , (iv) Dehydrogenation, , Distinction among 1°,2° and 3° Alcohols, 1°, 2° and 3° alcohols are distinguished by Lucas test, oxidation and reduced copper., Victor Meyer’s test is also used to distinguish them., In this test, primary (1°) alcohols give red colour, secondary (2°) alcohols give blue colour and, tertiary (3°) alcohols give no colouration., , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 14 :
14 | P a g e, , (b) SuLphonation, , (c) Nitration, , The ortho and para isomers can be separated by steam distillation. This is because onitrophenol is steam volatile due to intramolecular hydrogen bonding while p nitrophenol is, less volatile due to intermolecular hydrogen bonding which causes the association of, molecules., , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 16 :
16 | P a g e, , (iii) Reaction with zinc dust, , Terms Related to Alcohols, www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 17 :
17 | P a g e, , (a) Rectified spirit It contains 95% ethyl alcohol and 45% water. It is an azeotrope (constant, boiling mixture) and boils at 74°(., (b) Absolute alcohol Alcohol containing no water, i.e; 100% C2H5OH is known as absolute, alcohol. It is prepared as follows., (i) Quick lime process, (ii) Azeotropic method, (c) Methylated spirit The rectified spirit rendered poisonous by addition of 4-5% methyl, alcohol, traces of pyridine and some copper sulphate and is known as methylated spirit or, denatured alcohol., (d) Power alcohol Alcohol mixed with petrol or fuel and used In internal combustion engines, Is known as power alcohol., (e) Wood spirit Methyl alcohol (CH3OH) is also called wood spirit. It is obtained by, destructive distillation of wood. Pyroligneous add, the product of destructive distillation of, wood, contains acetic acid (10%), methyl alcohol (25%) and acetone (05%). Drinking of, methanol causes blindness., (f) Grain alcohol Ethyl alcohol C2H5OH is also called grain alcohol. It is used In the, preparation of various beverages containing different percentages., Dihydric Alcohols, These are generally called glycols because of their sweet taste. Ethylene glycol (CH2OH –, CH2OH) is the first and most important member of dihydric alcohol series., Methods of Preparation, , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 18 :
18 | P a g e, , Physical Properties, 1. It is a colourless, syrupy liquid with sweet taste., 2. Because of its tendency of formation of H-bonds, it is miscible with H2O and ethanol but not, with ether., Chemical Properties, It gives all the general reactions of -OH group., , The per-iodic acid cleavage of 1,2-g1ycols is sometimes called Malaprade reaction., Trihydric Alcohols, Glycerol or glycerine, CH2OH – CH(OH)- CH2OH is the first member of this group. Its, IUPAC name is propane-l,2,3-triol., Method of Preparation, , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 19 :
19 | P a g e, , It is obtained as a by product in saponification reaction., , Physical Properties, 1. It is a colourless, odourless, viscous and hygroscopic liquid., 2. It is sweet in taste and steam volatile., 3. It is soluble in water but insoluble in ether., 4. Due to excessive H-bonding, it is highly viscous and has high boiling point., Chemical Properties, It gives all the general reactions given by -OR group but 2° OR is less reactive as compared to, 1° ., Some of its specific reactions are :, , Glyceryl trinitrate or tri nitroglycerine, when adsorbed on Kieselguhr is known as dynamite., Mixture of TNG and cellulose trinitrate is called blasting gelatin., www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 20 :
20 | P a g e, , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 22 :
22 | P a g e, , (ii) Williamson’s synthesis Only primary alkyl halides when react with sodium alkoxide give, ether while tertiary alkyl halides give alkene due to steric hindrance., , Physical Properties of Ethers, Ethers are polar but insoluble inH20 and have low boiling point than alcohols of comparable, molecular masses because ethers do not form hydrogen bonds with water., www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 23 :
23 | P a g e, , Structure of Ether, The hybridisation of 0 atom in ethers is sp3 (tetrahedral) and its shape is V-shape., , Chemical Reactions of Ether, (i) Reaction with HX, , Ethers with two different alkyl groups are also cleaved in the same manner and results in the, formation of a primary halide (or smaller and less complex alkyl halide) by S N2 mechanism., R-O-R’ + HX → RX + R’OR, The order of reactivity of hydrogen halides is as follows, HI > HBr > HCl, In ethers if one of the alkyl groups is a tertiary group, the halide formed is a tertiary halide by, SN1mechanism., , (ii) Halogenation, , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)
Page 25 :
25 | P a g e, , Ethyl phenyl ester C6H5OC2H5 is also, known as phenetole., Uses of Ethers, 1. Dimethyl ether is used as refrigerant and as a solvent at low temperature., 2. Diethyl Ether is used as an anaesthesia in surgery ., , www.ncerthelp.com (Visit for all ncert solutions in text and videos, CBSE syllabus, note and many more)