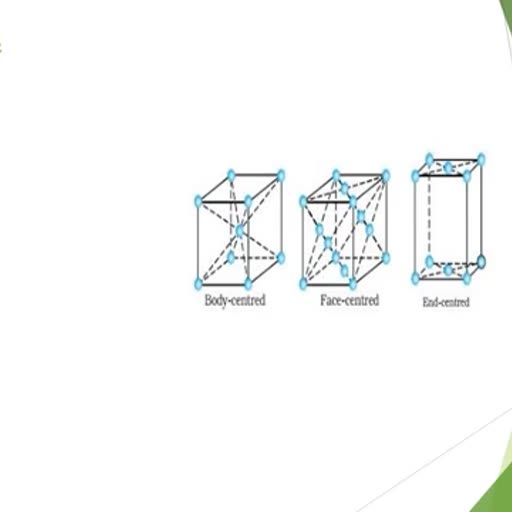
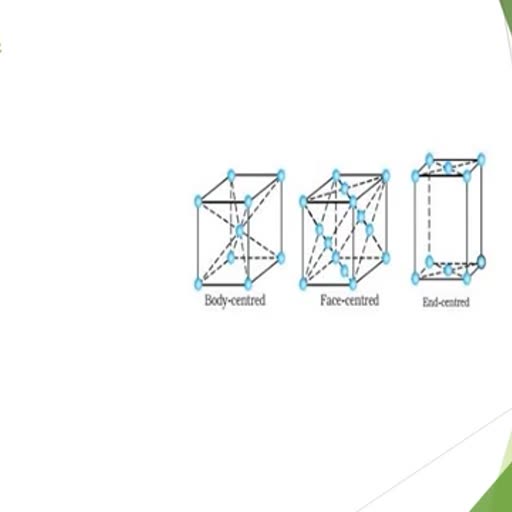
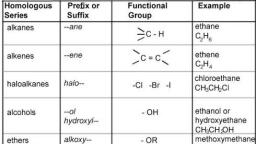
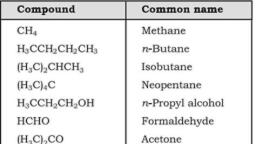
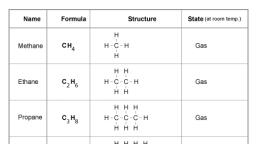
Page 2 :
www.chemzblog.wordpress.com Zaid Mansu9/1/2013, , , , © Kinetic studies help us to determine:, |. The speed or rate of a chemical reaction, , 2. Conditions by which the reaction rates can he altered, In this Unit, we shall be dealing with:, , |. Average and Instantaneous rate of reaction and the factors affecting these., 2 Some elementary ideas about the collision theory of reaction rates., , Hi, , , , , , , , , , Very fast reactions:, Eg: AGNDy.g + NoChay) > AuCly + NoNOy,., , Very slow reactions:, , Eg: rusting of iron in the presence of air and moisture., , Moderately fast reactions:, Eg: inversion of cane sugar, hydrolysis of starch, , , , Papi cin
Page 3 :
Zaid Mansu@/1/2013, , www.chemzblog.wordpress.com, , , , , , “Rate of chemical reaction can be defined as the change in, , concentration of a reactant or product in unit time.”, Or more specifically:, , “The rate of decrease in concentration of any one of the, Reactants”, OR, , “The rate of increase in concentration of any one of the Products.”, , Siac Anan Cc, , , , , , , , “Average rate of a reaction is defined as the rate of change of concentration, per unit time”, , It can be determined by meusuring the concentration of any one of the reactunts or, products after a definite interval of fime, , Rate of disappearance of RE =DCcrcase in concentration of R _ _ AIR], eens - Time taken "At, , (-ve sign to make if positive}, OR, , _ Increase in concentration of P__, A[P], , Rate of appearance of P. =——————_______ = +, PE Time taken At, , tunel einealenoi ase cin