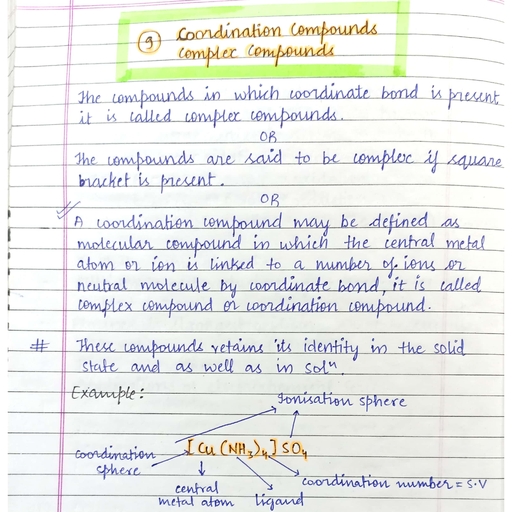
Page 1 :
Unit-4, CHEMICAL KINETICS, Chemical Kinetice deals with the grate, Chemi cal reactions, factong afecting the, rates o, mechanism by which reaction proceed., # following, of, Chemical geaction? and, are, the main aspects, related to Chemical -tm, 1) whether the reaction ig feasibleor not, 2) To what extent a greaction will, 3) Speed of reaction., ) To Study the factorg which offect, the rate, proceed., reaction., oreaction, Con Centration, 5 To deteomine the order, by studying the ffect of, ', on the rate g reactions., G) To explain the, at the molecular level by, theosy., Types chemical Reaction, mechanism 9 reaction, Collision, 1. Instantaneous or, Such reactions take place ing tanten eouely, and it is difficult to determine the, rate of $uch reaction $ by Common, laboratory method., # Ieric reactieng take place, eg Agt an) t Cr Cag) = Agcl(s), Very tast reaction, Thetantaneoul, y
Page 2 :
2: Very, Such" reactiong, slow reactiens ;-, are Completel in days, O montths., g suiting of, Reactions having, iron., moderate rates, ., Such reaction&, neither very, fact, are, U have, Very slow. Such reactions, moderate Speeds., Reasons for the Difference in Rates g, Reactions., nor, The ionic reactions are instantane ous, do not involve any breaking, g the bonds Other rea ctions involve, bond s. The, they, because, breaking and formation, eneregy involved in the boreaking and, making f diferent band a via l different, more over activation energy' is different, for ditferent reactions Therefore, rates, of different reactions, Rate of a chemi cal reaction :-, are different, Rate, a Chemical reaction i8 equal, of, to the change in Concentration of any, reactant o pooduct per Unit time.
Page 3 :
Rate of a chemical reaction:-, Rate, change in Comentration, Poroduct per Unit ime, (3), Chemi cal reaction i8 equal to the, of any reactant or, a, A As the, reaction proceeds the Con centration, of, the reactant decreases and those g, Poroductz increas es ., R., At= t2-ts, A [R] = [Ro]=[RJ, AP = [B]-LP], g -A[R], At, disappearance of R =, E, ーの, Rate, Rate of appearance of p= tA[P], -ACR], st, Rate, f reaction =, +ALP], # The rate, e reaction is always +ve, Average, rate, of, * reaction, A [P], Ati, Unit og Rate of Reaction,, a A [R], Il mole L sec!, OR mole L7min-!, atm sec! OR, atm min!
Page 5 :
Rate, reaction =, -A [pcls] _ +APOL] +A[CL], At, For a general reaction, 5), At B, >C+D, -A LA], At, -A [B], +A[c] _ +A [D], Rate, reaction=, st, reactions having Different stoichio-, metric Cofficient q the Reactants and, Products., let's Consider reaction, Rate, of, 2 N,05 (9) 4 NO2 CG.) t Oz (g), Rate g decomposi tion of, NLO5, - A[N,O5], Rate, formation, og No, =+ A [NG], At, Rate og formation og co = tA [O,], 2F, At, Rate g reaCtion, At, 4 At, 4 A [o,]