Page 1 :
CC ————, , ee Oe, ”, , (v) Ethanol h, , as higher boiling Point, , because ite molecul, , #88 SAY 2010), , A compound A reacts with thi, , Ho:, to give compound B, B atin, magnesium in ether medium to fom, Grignard reagent which is treated ith, acetone and the product on hydrolysis, OH, = CH, -CH,, CH,, Identify A and B. Write down the chemical, equations for the reactions involved,, , : A—+CH,CH,OH, , eg Cli ~, gives, , B—CH,CH,Cl, CH,CH,Cl + Mg —Str_ CH,CH,Mgcl, , ll, CH, - CH,MgCl + CH, - C-CH,—>, , OMgBr, Gi Gm, + CH, na,, CH, on, CH, -6-ci, ~CH,, CH,, (HSE SAY 2011), , 3. Mixture of conc. HCland anhydrous ZnCl, is, an important reagent which helps to, distinguish between 1°, 2° and 3° alcohols., , (a) Give the name of the above reagent., , (by Give one example each for 1°, 2° and, 3° alcohols., , (c) Explain how the above reagent helps, to distinguish above three types of, alcohols., , Ans;(a) Lucas reagent, (\) CH,—CH,OH : ethanol(1*),, CH, ~ CH - CH; ; propan-2-0l{2°), , OH, , , , 3, CH, -¢- CH, Q-methylpropan-2-ol (3°), , HH, with Lucas reagent 3°, ee immediate turbidity due, to the formation of 3° alkyl halide. Secondary alcohol gives turbidity in about, five minutes. Primary alcohol does not, give turbidity at room temperature., , (HEE MARCH 2012), 4. (a) Write the name or structure of the, compounds A and B in the following, , (c), , reactions., CHC, + NaOH NaOH CO,, 7 gee, , (b) Vapours of an alcohol ‘C’ on passing, over heated copper produce compound, ‘p’. ‘D’ on reaction with CH,MgCl, followed by hydrolysis produces, 2-methyl butan-2-ol. Write the name, or structure of compounds ‘C’ and ‘D’., , Ane: (8) CHO” ‘COOH, A: B:, , Salicyl aldehyde Salicylic acid, 2-hydroxybenzaldehyde a-hydroxybenzoic acid, {b) The final product alcohol is, CH,, -OH, H, - CH,, , CH, , This is formed by the reaction of, CH,MgClwith CH,COCH,CH,., , i.e., compound D is, , CH, - L - CH, - CH, (butanone), ., Compound Cis, AT, CH, - CH - CH, - CH, (butan-2-ol), , , , pita.
Page 2 :
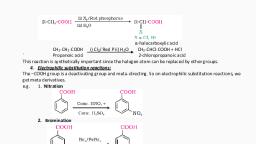
NRT ma ser 8B, (HSE BAY 2012), , 5. Methanol and ethanol are two commer, cially important alcohols., , (i) Write one method of preparation of, methanol and ethanol., , (ii) Name the products obtained when, ethanol is treated with CrO, in anhydrous medium. :, , (iii) The boiling point of ethanol is higher, than that of methoxy methane. Give, Treason., , Ans: (i) CH,Br + NaOH(aq)—»CH,OH + NaBr, CH, = CH, + H,O—"»CH, - CH,OH, {ii) Ethanal or acetaldehyde, (iii) Ethanol molecules are associated by, intermolecular hydrogen bonding., So the boiling point is high. There is, , no hydrogen bonding in methoxymethane (ether)., , (HSE MARCH 2013), 6. (a) Write the IUPAC names of all the, , possible isomers with molecular, formula C,H,O., , Primary, secondary and tertiary, alcohols can be distinguished by Lucas, test., , (b), , (i) What is Lucas reagent?, , (ii) Write the observations for primary, secondary the tertiary, alcohols in Lucas test., , Ans: (a) Isomers of C,H,O are, (i) CH,-CH,-CH, OH: Propan-1-ol, (ii) CH, -CH-CH, : Propan-2-ol, , OH, , (iii) CH,-O-CH,-CH, : Methoxy- |, , ethane, , (i) Lucas reagent is a solution of, , anhydrous zinc chloride in, concentrated hydrochloric acid., , Tertiary alcohols give a turbidity, immediately at room temperature. Secondary alcohols develop, turbidity in a few minutes (about, 5 minutes) while primary alcohols, , (ii), , , , Focus Ares Topics 2022 ~ Chemistry ., , do not develop any turbidity a,, room temperature. The principle, of this test is based on the, formation of alkyl chloride from, the alcohol by the reaction with, con. HCl in the presence of 2nct,,, , ROH + Ho1—2- RCL + H,O, , (HSE MARCH 2014), , 7. (a) How will you prepare the following, compounds using Grignard reagent?, , (i) Primary alcohol, , (ii) Secondary alcohol, , How will you distinguish primary ang, secondary alcohols using Lucas test?, , Write the correct pair of reactants for, the preparation of t-butyl ethyl ether, by Williamson Synthesis., , (i) Primary alcohol: Grignard reagent, when treated with formaldehyde, followed by hydrolysis give primary, alcohol., , CH, - MgBr + HCHO—-+, CH, ~ CH, - OMgBr—%°5, CH, - CH, -OH, P alcohol, , (b), , (), , , , , Ans: (a), , (ii) Secondary alcohol: Grignard rea, gent when treated with aldehyde, (other than formaldehyde) followed by hydrolysis give secondary, alcohol,, , CH, - MgBr + CH, - CHO—>, CH, - CH - OMgBr —22_,, Hi,, , CH, ~ i - OH (2° alcohol), , CH, >, (b) Refer to answer HSE MARCH 2013 (6), {c) CH, - r -ONa and CSC Be, CH, ethyl bromide, sodium tert-butoxide, , , , eRe. Thrisur
Page 3 :
GAY 2014), fa)’ Write the n, : feliowing: ‘ame or formula of the, , () A simple ether, , (iv) A trihydric alcohol, , f@) (i) CH,-O-CH, : Dimethy! ether, (ii) C.H,-OCH, : Ethyl methyl ether, (iil) OR-CH,~CH.-oH, , CH, -QH, , (ii) CH-OH : Propane-1, 2, 3-triol, CH, -OH, MARCH 2015), , Alcohola are compounds with, eneral, formula R- OH ‘, , (a) Alcohols are soluble in water. What is, the reason?, , _( (i) Explain a method for manufacture of Ethanol., , {ii) How will you convert phenol tp, benzene?, , (a) Alcohols form hydrogen. bonds with, water. Therefore soluble in water., , & (i) Ethanol is manufactured by, the fermentation of molasses., Molasses is diluted with water., Yeast is added and temperature, maintained at 305 K. Fermentation takes place and a dilute, solution of ethanol is formed., , OHn%,+ H,0 #82, ‘eucrose, , CFOs + CgFl30e, , C,H,,0, 22+ 20, H,OH +200,, (ii) Phenol ie heated with zinc dust., , + an—» («m0, , : Ethane-1, 2-diol, , , , 9 Aleohats, Phenols and Ethene, , (H@e BAY 2018), 10. (a) Write a test to distinguish between, phenol and alcohol., (b) Write suitable reagent of reagents, used for the following conversions :, , (1) CH,-CH,-Cl — CH, -CH,-OH, , (it) CH,-CH,-OH —, CH,-CH,-O- CH,-CH,, , CHO, (iii) >, , With neutral ferric chloride solution,, phenol gives violet colour while alcohol, , will not give colour., (b) (i) Aqueous KOH, (ii) Con. H,SO,/413 K, , (iii), CHCl,/aq. NaOH,, Reimer-Tiemann reaction., , (HSE MARCH 2016), 11. (a) Complete the following:, , Ans: (a), , dul, Lg HNO, , Explain the following:, (i) Esterification, (ii) Williamson Synthesis, , Ans: (a), dil, +, 10,, ON NO,, 2 2O_,, , , , , , ware