Page 2 :
These are compounds containing halogen atoms attached to, an alkyl or aryl group. The general representation of, , haloalkanes is R-X and that of haloarenes is Ar-X, [where X = F, Cl, Br, I]., , Haloalkanes contain halogen atom(s) attached to the sp3, hybridised carbon atom of an alkyl group whereas, haloarenes contain halogen atom(s) attached to sp2, , hybridised carbon atom(s) of an aryl group.
Page 3 :
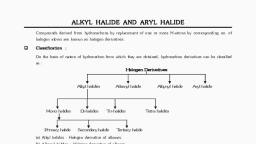
Classification, , I), , On the basis of number of halogen atoms:, , Based on this, haloalkanes and haloarenes are classified as mono, di, or polyhalogen compounds., -Monohalogen compounds contain only one halogen atom,, -di-halocompounds contain 2 halogen atoms and, , -polyhalogen compounds contain more than 2 halogen atoms., II) Compounds containing sp3 C-X bond:, They include, a) Alkyl halides or haloalkanes (R-X): Here the halogen atom is, directly bonded to an sp3 hybridized C atom of an alkyl group., They are further classified as primary, secondary or tertiary, according to the nature of carbon to which halogen atom is, attached.
Page 7 :
III) Compounds having sp2 C-X bond:, They include, a) Vinylic halides:, Here the halogen atom is directly bonded to an sp2 hybridized, carbon atom of a C=C bond., , E.g.: CH2=CH-X, b) Aryl halides:, Here the halogen atom is directly bonded to an sp2 hybridized, , carbon atom of an aromatic ring., E.g. : C6H5-X
Page 9 :
Nomenclature, Common name of alkyl halides is obtained by adding –yl halide to, the word root (i.e. word root + yl halide) and the IUPAC name is, obtained by adding the prefix ‘halo’ to the name of the parent, alkane (i.e. halo + alkane)., Some examples are:
Page 10 :
❑ Methods of Preparation of Haloalkanes, , 1. From Alcohols, •The hydroxyl group of an alcohol is replaced by halogen on reaction, with concentrated halogen acids, phosphorus halides or thionyl, chloride., •Thionyl chloride is preferred because in this reaction alkyl halide is, formed along with gases SO2 and HCl. The two gaseous products are, escapable, hence, the reaction gives pure alkyl halides., •The reactions of primary and secondary alcohols with HCl require, the presence of a catalyst, ZnCl2 ., •With tertiary alcohols, the reaction is conducted by simply shaking, the alcohol with concentrated HCl at room temperature., , The order of reactivity of alcohols with a given, haloacid is 3°>2°>1°.
Page 11 :
For the preparation of alkyl bromides and iodides, alcohols, are treated with bromine or iodine in presence of red, phosphorus, since PBr3 and PI3 are unstable.
Page 13 :
Preparation of Haloarenes :, , (i) From hydrocarbons by electrophilic substitution, Benzene or its derivatives when heated with Cl2 or Br2 in presence of, iron or Lewis acids like anhydrous FeCl3 (ferric chloride) or AlCl3,, we get aryl chlorides or bromides., , •The ortho and meta isomers can be easily separated due to their, large difference in melting point., •For the preparation of aryl iodides, arenes are treated with I2 in, presence of an oxidising agent like HNO3 or HIO4 (periodic acid), to oxidise the HI formed during the reaction.
Page 15 :
Note: If the cuprous halide is replaced by copper powder, the, reaction is called Gattermann’s reaction., •For the preparation of iodobenzene, the diazonium salt is, treated with potassium iodide (KI)
Page 16 :
Physical Properties, Melting and boiling points:, •In haloalkanes, the C-X bond is polar due to the greater, electronegativity of halogen atom., , •Due to greater polarity and higher molar mass, the inter molecular, forces of attraction (dipole-dipole and van der Waals forces) are, strong and so they have higher melting and boiling points than, hydrocarbons of comparable molar mass., •For the same alkyl group, the boiling points of alkyl halides, , decrease in the order:, RI> RBr> RCl> RF., , •This is because with the increase in size, and mass of halogen atom, the magnitude, of van der Waal forces increases..
Page 17 :
Solubility:, •The haloalkanes are only very slightly soluble in water., •This is because they cannot form hydrogen bonds with water (except, , alkyl fluorides)., REACTIONS OF HALOALKANES –, , Nucleophilic Substitution Reactions, These are reactions in which a weak nucleophile is replaced by a, strong nucleophile [Nucleophiles are electron rich species attacks at, electron deficient centre].
Page 18 :
•Nucleophiles are electron rich species. Therefore, they attack at that, part of the substrate molecule which is electron deficient., , •The reaction in which a nucleophile replaces already existing, nucleophile in a molecule is called nucleophilic substitution reaction., , •Haloalkanes are substrate in these reactions. In this type of, reaction, a nucleophile reacts with haloalkane (the substrate), having a partial positive charge on the carbon atom bonded to, , halogen., •A substitution reaction takes place and halogen atom, called leaving, group departs as halide ion. Since the substitution reaction is initiated, , by a nucleophile, it is called nucleophilic substitution reaction.
Page 23 :
Mechanism:, This reaction has been found to proceed by two different, mechanims which are described below:, , (a) Substitution nucleophilic bimolecular (SN2) :, Mechanism:, ➢Here the incoming nucleophile interacts with alkyl halide causing, , the carbon-halogen bond to break while forming a new carbon-OH, bond., ➢These two processes take place simultaneously in a single step and, , no intermediate is formed., ➢In the case of optically active alkyl halides, this mechanism, , proceeds through inversion of configuration.
Page 24 :
Since this mechanism requires the approach of the nucleophile to the, carbon bearing the leaving group, the presence of bulky substituents on, or near the carbon atom decreases the rate of this reaction., , Thus the order of reactivity of alkyl halides towards SN2 reaction is:, Primary halide > Secondary halide > Tertiary halide.
Page 25 :
b.Substitution nucleophilic unimolecular (SN1):, •SN1 reaction occurs in two steps. In the first step, the C—X bond, undergoes slow cleavage to produce a carbocation and a halide ion., •In the second step, the carbocation is attacked by the nucleophile to, , form the product., •Here first step is the slowest and reversible. So it is the rate, determining step. Since this step contains only one reactant, it, , follows first order kinetics., , E.g.: The reaction between tert-butyl bromide and, hydroxide ion to give tert-butyl alcohol.
Page 26 :
•This reaction occurs in two steps. In step I, the polarised C—Br bond, , undergoes slow cleavage to produce a carbocation and a bromide ion., •The carbocation thus formed is then attacked by nucleophile in step II, to form the product., , •Thus in SN1 reaction, there is an intermediate called carbocation., , •The greater the stability of the carbocation, the greater will be, the rate of the reaction.
Page 27 :
•The order of reactivity of alkyl halides towards S N1 reaction is:, 30 > 20 > 10 ., •In the case of optically active compounds, the S N1 reaction proceeds, through retention of configuration.
Page 33 :
Polyhalogen compounds, , Trichloromethane (Chloroform, CHCl3):, •It is used as a solvent for fats, alkaloids, iodine and other substances., •The major use of chloroform is in the production of the freon, refrigerant R-22., , •Chloroform is stored in closed dark coloured bottles filled up to the, neck in order to avoid air., •This is because chloroform is slowly oxidised by air in the presence, , of light to an extremely poisonous gas, carbonyl chloride (COCl2),, also known as phosgene.