Page 2 :
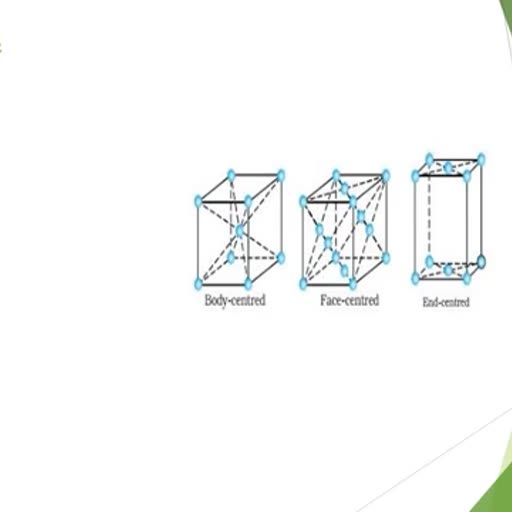
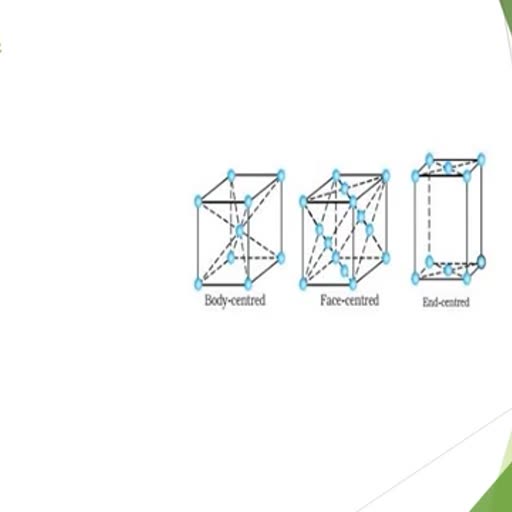
, , MONO HALIDES : These are classified on the basis of nature of C-atom carrying the halogen atom ., (A), Primary halide or 1 0 alkyl halides : Halogen atom attached with a primary or 1 0 C-atom., Example : C H 3 — X, Halo methane or methylhalide, C H 3— C H 2— X, Halo ethane or ethyl halide, C H 3— C H 2— C H 2— X, 1-Halo propane or n-propyl halide, (B), Secondary or 2 0 alkyl halides : Halogen atom linked with 2 0 C-atom., Example : CH3 CH CH3, , 2-halo propane, or, Iso propyl halide, , X, , 2-halo butane, or, Sec. butyl halide, , CH3 CH CH2 CH3, X, (C), , Ter tiar y halide or 3 0 alkyl halide : halogen atom linked with 3 0 C-atom., , R, Example : R, , (tert.alkyl halide), , C X, R, , , , Isomerism : Alkyl halides shows position and chain isomerism Example : C 3 H 7 Cl, , CH3CHCH, 2, 2Cl, Position isomers, , CH3CHCH3, Cl, Example : C 4 H 9 Cl(a), , CH 3 CH 2 CH 2 CH 2 Cl, , 1 - chloro butane, , CH3, (b), , 1-chloro-2-methyl propane, , CH CH2Cl, CH3, , (c), , CH3 CH2 CH CH3, , 2 - chloro butane, , Cl, , CH3, (d), , CH3 C Cl, , 2-chloro-2-methyl propane, , CH3, a, b Chain isomers, a, d Chain and position isomers, b, d Position isomers, , a, c Position isomers, b,c Chain and position iosmers, c, d Chain isomers, , Reactivity order : The order of reactivity of alkyl halides is -, , RI > RBr > RCl > RF, , Bond energy values : C–I ( 57. 4), C–Br (65.9), C–Cl (78.5) and C–F (105.4) K.cal/mole, C–I bond is most reactive because lower energy is required to break the bond. On the basis of nature of alkyl, group the reactivity order of alkyl halide is -, , tert > Sec. > Primary, , Since alkyl groups, are electron repelling or electron releasing, larger no. of alkyl groups on C-atom of C–X, greater is the electron density on C-atom hence ease in release of, CH3, , CH3 C X , CH3, , CH3, , X atom as X – ion (+ I effect of alkyl group), CH3, +, , –, , C+ X, CH3
Page 3 :
Alkyl halides are generally more reactive than the corresponding alkanes due to the presence of polar covalent, , , (, , C X ) bond. So alkyl halides ( R–X) undergo nucleophilic substitution reaction., , , +, , C atom, , The centre for attacking Nu is, , 1., , Genreal Met ho d of Preparat ion of Monohalide s :, By direct halogenat ion of alka ne s :, R—H + Cl2, , 2., , U.V.light, , R—Cl + HCl, , (excess), By the addition of H—X on alkenes :, R—CH, CHR + HX , CH2 CH2 + HX, , CH 3–CH, , CH 2 + HX, , R CH 2 — C H X R, CH 3 —CH 2 X, , , , CH CH3, , CH3, , X, Isopropyl halide, 3., , By Alcohols :, (a), By t he act ion of hydrogen halide s :, Example : R—CH 2 —OH, , H –X, , ZnCl2 R C H 2 — X, , Mechanism :, , R CH2, , .., OH, , H+, (H—X), , R CH2, , , , –H2O, , , , X, , O H R CH2 R CH2 X, , H, (unstable), , (Product), , In this reaction intermidiate carbocation is formed so rearrangement ( H – shifting or CH 3– shifting) can takee, place., ZnCl2 act as dehydrating agent and absorbs H2O from the reaction so good yield of halide is obtained. Also it, generates H + from HCl., HCl + ZnCl 2, , , , , ZnCl , 3 + H, , Reactivity order for alcohol :, , Reactivity, , stability of intermediate carbocation , so reactivity order : Tert. alc. > Sec. alc. > Pri. alc., , Reactivity order of H—X is :, HI > HBr > HCl, HI is maximum reactive so it reacts readily with 1°, 2° and 3° alcohols., R—OH + HI, , R—I + H 2 O, HCl and also 1° alcohol are less reactive so ZnCl 2 or some amount of H 2 SO 4 is needed to increase the, reactivity., Example : CH 3 —CH 2 —OH + HCl, , ZnCl, , 2, , , , CH 3 —CH 2 —Cl, , At normal condition :, CH 3—CH 2 —OH + HCl × (no reaction), Note : HCl + ZnCl 2 is called as lucas reagent, alchol gives turbidity with lucas reagent., Reactivity towards lucas reagent (difference in 1°, 2° and 3° alcohol)., Time to, give turbidity, , 1° alcohol, in 30 min., , 2° alcohol, in 5 min., , 3° alcohol, in 1 min.
Page 4 :
(b), , By the action of phosphorus halides ( S N1 mechanism) :, R—OH + PCl 5, , , , R—Cl + POCl3 + HCl, , 3R—OH + PCl 3, , , , 3RCl + H3PO3, , PBr3 and PI3 are less stable, thus for bromides and Iodides, ( P + Br2) Or ( P + I2) mixture is used., (c), , By react ion w ith thionyl chloride - (Darzen's procedure) ( S N i and S N 2 mechanism) :, R—OH, , +, , Pyridine, , (1 m ole ), , SOCl2, , One mole, , R—Cl + SO2 + HCl, , One mole, , Because of less stability of SOBr2 and SOI2, R—Br and RI does not obtained by this method., 4., , Boro di ne – Hunsdicker's react ion :, R—COOAg, Silver salt of, a fatty acid, , 5., , CCl 4, X2 , , , +, , R—X + CO2 + AgX, , (Cl2 or Br2), , By halide exchange :, R–Cl, , or, , Acetone, R—Br + KI , R–I, , + KCl, , or, , KBr (Conant finkelstein reaction), , 2CH 3Cl + HgF 2 2CH 3 –F + HgCl 2 (Swar t reaction), R– I and R—F can be prepared by this method only., 6., , By react ion of alka ne s, R—H, , , , +, , w it h sulphur yl chloride (SO 2 Cl 2 ) :, , SO2 Cl2, , light, , organic peroxide R—Cl + HCl, , + SO2, , Physical Proper t ie s :, (a), , The lower members CH3F, CH3Cl, CH3Br , C2H5Cl and C2H5F are gases at room temp., CH3I and members upto C18 are colourless sweet smelling liquids., , (b), , Higher B.P. than parent alkanes., Decreasing order of B.P. is :, , R – I, , >, , R—Br, , >, , R—Cl, , among isomeric R—X decreasing order of B.P. is : Primary, (c), , R—F, R—Br, , and, , R—Cl, , >, , >, , R—F, , Secondary, , >, , tertiary, , lighter than water, , and R—I heavier than water, , Decreasing order of density is : R—I, , >, , R—Br, , >, , R—Cl, , >, , R—F, , (d), , R—X are polar co-valent compounds but insoluble i n water because the y can not form, H–bonds. They dissolve in organic solvents., , (e), , R—X burns with a green flame due to interaction of X with Cu wire.(Beilstein test), , (f), , The stability order is :, , R—F >, , R—Cl, , >, , R—Br >, , R—I, , R—I is least stable and darken in light due to photodecomposition., 2R—I, , h, , R—R +, , I2, , , , Chemical Proper t ie s :, , A., , Nucleophilic substitution reaction (S N ) : Due to electronegativity difference the, , , polarised bond., , C X, , C, , X bond is highly
Page 5 :
, , , , Thus the C-atom of the C X bond becomes centre to attack by a nucleophile (Nu) ., , , X ion from R—X molecule is substituted by a Nu . i.e.SN reaction are the most common reactions in R—X., , , R—X, , + Nu, , X, , +, , R—Nu, , These may be takes place by two ways (a) S N1 mechanism, , (b) S N 2, , mechanism, , R2, R1, , C X, , Reactivity order is :, , >, , R1 CH X, , R3, , >, , R1CH2 X, , R2, SN1and SN2, (secondary), , SN1, (Tertiary), , SN2, (secondary), , , , Mechenism of S N 1 a n d S N 2 :, , , , S N 1 Mechanism : SN1 stands for uni molecular nucleophilic substitution. The mechanism involves two steps., Consider the hydrolysis of tert. butyl bromide with aqueous NaOH., Step 1: The alkyl halide ionises to give a planar corbonium ion. The corbonium ion is planar because the, central positively charged carbon is sp2 hybridized., , R, , R, , R, C, , Slow, , + Br, , , , Br , , C, , R, , R, , R, t–alkyl bromide, Planar, Step-2 : The nucleophile can attack the planar carbonium ion from either side to give the product., , R, , R, Fast, , C, , R, , R, , , R, , C, , OH + OH, , R, , R, , R, , R, t–alkyl alcohol, , OH–, , C, , t–alkyl alcohol, , (i), , Ionisation is the rate determining step because it is the slow step. In other words, the rate at which, alcohol is formed should depend upon the concentration of tertiary alkyl halide alone., , , , Rate = K[R 3 C—Br], It is obvious that the reaction follows first order kinetics, therefore reaction is called S N1 ., , (ii), , The reactivity order for S N1 reaction, , , , reactivity order of halides ( S N1 ) varies as follows :, Benzyl halide, , (iii), , >, , Allylhalide, , >, , stability of carbocations formed by halides., 3°halide, , >, , 2° halide, , Remember that in case alkyl halide is optically active,, , SN1, , >, , 1°, , halide, , >, , methyl halide., , reactions lead to racemisation.
Page 16 :
T he Elimi nat ion-Addit ion Mecha nism of Nucleoph i lic Aromat ic Subst itut ion (Benz yne) :, (i) Very stong base such as sodium or potassium amide react with aryl halide, even those without electron, withdrawing substituents to give products corresponding to nucleophilic substitution of halide by the base., , Cl, , NH2, , KNH2, NH3, , +, , –33°C, , NH2, 14, , 14, , Chlorobenzyne-1- C, , 14, , Aniline - 1- C, , Aniline-2- C, , Mechanism, Step-1 : Elimination stage ; Amide ion is a very strong base and brings about the dehydrohalogenation of, chlorobenzene by abstracting a proton from the carbon adjacent to the one that bears the leaving group. The, product of this step is an unstable intermediate called benzyne., , H, , H, , .., , .., Cl, .., , H, , H, +, H, , H NH, .. 2, , .., , H, , .. –, :NH3 + :Cl, .. :, , H, , H, , Chlorobenzene, , Benzyne, , Step-2 : Beginning of addition phase ; Amide ion acts as a nucleophile and adds to one of the carbons of the, triple bond. The product of this step is a carbanion., H, , H, , H, , H, .., :NH, .. 2, , H, , .., NH2, , H, , H, , H, , Benzyne, Aryl anion, Step-3 : Completion of addition phase ; The aryl anion abstracts a proton from the ammonia used as the, solvent in the reaction., , H, H, , H, .., , H–NH, .. 2, .., NH2, , H, H, Aryl anion, , H, , H, +, , H, , NH2, H, Aniline, , –, , NH2
Page 21 :
SOLVED EXA MPLES, Ex.1, , Which of the following undergoes Hydrolysis most easily :, , Cl, Cl, , Cl, , (A), , (B), , Cl, NO2, , NO2, , (C), , NO2, , NO2, , (D), , NO2, , NO2, Ans. (D), , Sol., Ex.2, , If there is more m-directing group then there will be more nuclephilic substitution reaction., The product in the following reaction is :, Ph – Cl + Fe / Br2 Product, (A) o– bromo-chloro benzene, , (B) p– bromo-chloro benzene, , (C) (A) and (B) both, , (D) 2, 4, 6-tribromo chloro benzene, , Ans. (C), , Sol., , Since – Cl group is deactivating and o/p directing group so only o– and p– products are formed., , Ex.3, , The most reactive towards SN1 is :, (A) PhCH 2Cl, , (B) Ph–Cl, , (C) CH 3CHCl(CH 3 ), , (D) p–NO 2 —Ph—CH 2 —Cl, Ans. (A), , Sol., , S N1 the intermediate carbocation is formed., , , C6H5—CH2Cl C 6 H 5CH 2 is maximum stable due to resonance., Ex.4, , Which of the following is used as insecticide :, (1) D.D.T., , (2) Chloritone, , (3) Chloropicrin, , (4) (A) and (C) both Ans. (D)