Page 1 :
4. CHEMICAL, KINETICS, HAIZEL G. ROY, H.S.S.T. (HG) CHEMISTRY, GOVT. H.S.S. KALAMASSERY, ERNAKULAM
Page 2 :
CHEMICAL KINETICS, , Branch of chemistry which deals with the study of the rates of chemical, reactions, the factors which influence rates of chemical reactions, the, mechanism by which the chemical reaction proceeds., The word kinetics is derived from the Greek word ‘Kinesis’ meaning movement.
Page 3 :
INSTANTANEOUS REACTIONS, , Reactions which takes place very fast are called Instantaneous Reactions.
Page 4 :
SLOW REACTIONS, , These are chemical reactions which take place at very slow rate., These reactions can take days, months or even years to complete., , EXAMPLES, Rusting of Iron, Fermentation of Sugar, Transformation of carbon into diamond., Weathering of rocks
Page 5 :
EXAMPLES OF REACTIONS STUDIED UNDER CHEMICAL KINETICS
Page 6 :
RATE OF A CHEMICAL REACTION, , The rate of a reaction is defined as the change in concentration of the reactants or, products per unit time.
Page 9 :
Consider a reaction, Here one mole of A reacts with one mole of B to form 2 moles of C., This means that the rate of disappearance of both A and B are same., But the rate of formation of C is twice the rate of disappearance of A and B., For obtaining the rate expression, we must divide the rate expression by the, stoichiometric coefficient of the corresponding substance in the balanced, equation.
Page 11 :
INSTANTANEOUS RATE OF A REACTION, , The rate of a reaction at a particular instant is known as instantaneous rate of, a reaction., Instantaneous rate is the average rate of reaction at which the time interval ∆t, is as small as possible so that the rate of reaction remains almost constant at, this time interval.
Page 12 :
where d[A] and d[B] are small changes in the concentrations of A and B in a, small time ‘dt’., In order to determine the instantaneous rate of a reaction at any time ‘t’, A, graph is plotted between concentration of reactant [R] or product [P] and time., A tangent is drawn to the curve at the point corresponding to time ‘t’., The slope of this tangent gives the instantaneous rate of the reaction at the, time ‘t’.
Page 15 :
AVERAGE RATE OF A REACTION, , The rate measured over a long time interval is called average rate.
Page 16 :
RATE LAW OR RATE EQUATION, , The law states that at constant temperature, the rate of a reaction is directly, proportional to the product of the molar concentrations of the reactants with, each concentration term raised to the power equal to the numerical coefficient, of the species.
Page 17 :
where k is called rate constant. When [A] = [B] = 1, r = k., Rate constant of a reaction is the rate of the reaction when the molar, concentration of each of the reactant is unity.
Page 18 :
DIFFERENCE BETWEEN RATE OF A REACTION, AND RATE CONSTANT OF A REACTION
Page 19 :
ORDER OF A REACTION, , Order of a reaction is defined as the sum of the powers of the concentration, terms which is to express the rate of a reaction., For the reaction, aA +bB, , Products, , Rate = K[A]a [B]b, Then the order of the reaction = (a+b), Order of a reaction may be of different types.
Page 20 :
ZERO ORDER REACTION
Page 21 :
FIRST ORDER REACTION
Page 22 :
SECOND ORDER REACTION
Page 23 :
THIRD ORDER REACTION
Page 24 :
ELEMENTARY REACTIONS, , Chemical reactions which take place in a single step are called elementary, reactions., All the molecules of the reactants undergo simultaneous collision, as in the, balanced chemical equation.
Page 25 :
COMPLEX REACTIONS, A reaction which takes place in a sequence of number of elementary reactions., Reaction between H2 and Br2 takes place in a number of steps involving both Br, and H atoms., EXAMPLES
Page 26 :
RATE EXPRESSION FOR A ZERO ORDER REACTION
Page 28 :
RATE EXPRESSION FOR A FIRST ORDER REACTION
Page 31 :
PLOTS FOR INTEGRATED RATE EQUATIONS
Page 32 :
PLOTS FOR INTEGRATED RATE EQUATIONS
Page 33 :
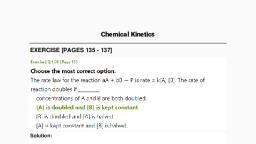
PROBLEMS
Page 34 :
PROBLEMS
Page 35 :
PROBLEMS
Page 36 :
UNITS OF RATE CONSTANT, , Rate constant has different units for reactions of different order., General rule for rate of reaction may be given as:, Mol 1-n Litre n-1 Time -1 where n = order of reaction., 1. For a zero order reaction, the unit of rate constant is mol L ―1 T―1 or( mol L ―1 S ―1 )., 2. For a first order reaction, unit of rate constant is Time ―1 or (S ―1 )., 3. For a second order reaction, unit of rate constant is Litre mol ―1 Time―1 or ( Litre mol ―1 S ―1 )., 4. For a third order reaction, the unit of rate constant is Litre 2mol―2 Time ―1 or (Litre2 mol―2 Time ―1 ).
Page 37 :
PROBLEMS
Page 38 :
PROBLEMS
Page 39 :
PROBLEMS
Page 40 :
HALF LIFE PERIOD, , The half life period of a reaction is the time required to complete half of the, reaction., Or The time required to reduce the concentration of a reactant to one half of its, initial value., It is generally denoted by t1/2 ., The shorter the half-life period, the faster is the reaction.
Page 41 :
HALF LIFE PERIOD OF A ZERO ORDER REACTION
Page 42 :
HALF LIFE PERIOD OF A FIRST ORDER REACTION
Page 43 :
PROBLEMS
Page 44 :
PROBLEMS
Page 45 :
PROBLEMS
Page 46 :
PSEUDO FIRST ORDER REACTION, , The Reactions which appear to be of higher order but actually follow first order, kinetics are called pseudo first order reactions., Eg: Acid hydrolysis of ethyl acetate into ethyl alcohol and acetic acid., Eg: Inversion of cane sugar producing glucose and fructose.
Page 47 :
EXAMPLE - 1, , Eg: Acid hydrolysis of ethyl acetate into ethyl alcohol and acetic acid., , The concentration of water in the reaction mixture is in large excess as compared to ester., Therefore the concentration does not decrease appreciably during the reaction., Therefore, the hydrolysis of ethyl acetate is pseudo first order reaction.
Page 48 :
EXAMPLE - 2, , Eg: The inversion of cane sugar producing glucose and fructose., , The concentration of water in the reaction mixture is in large excess as compared to cane, sugar., Therefore the concentration does not decrease appreciably during the reaction., Therefore, the inversion of cane sugar is a pseudo first order reaction.
Page 49 :
MOLECULARITY OF A REACTION, , Molecularity is the number of reacting species which collide simultaneously to, bring about a chemical reaction.
Page 50 :
UNIMOLECULAR REACTIONS, , If a reaction involves the decomposition of only a single species, the molecularity, is one or it is called a unimolecular reaction.
Page 51 :
BIMOLECULAR REACTIONS, , If a reaction involves the collision of two species, the molecularity is two or it is, called a bimolecular reaction.
Page 52 :
TRIMOLECULAR REACTIONS, , If a reaction involves the collision of three species, the molecularity is three or, it is called a trimolecular reaction.
Page 53 :
DIFFERENCE BETWEEN ORDER AND MOLECULARITY, OF A REACTION
Page 54 :
PROBLEMS
Page 55 :
TEMPERATURE DEPENDENCE OF RATE CONSTANT, ARRHENIUS EQUATION, When the temperature is increased, the kinetic energy of the reactant molecule, increases., This will increase the number of collisions., Ultimately the rate of reaction will be enhanced., Arrhenius, suggested an equation which describes rate constant, k as a function, of temperature on the basis of the observations from a large number of, experiments.
Page 57 :
EVALUATION OF ACTIVATION ENERGY
Page 58 :
GRAPHICAL REPRESENTATION
Page 59 :
EFFECT OF TEMPERATURE, ON REACTION RATE
Page 60 :
EFFECT OF TEMPERATURE ON REACTION RATE, , According to collision theory, a chemical reaction takes place when the reactant, molecules collide with one another., All collisions are however, not effective collisions., An effective collision which results into a chemical reaction., For effective collisions, the colliding molecules possess a certain minimum amount, of energy called threshold energy., Colliding molecules must have proper orientation.
Page 62 :
Threshold energy may be defined as the minimum amount of energy which the, colliding molecules must possess to make an effective collision., Collision of molecules which possess threshold energy or more, result in breaking, up of bonds and it leads to chemical reaction., At lower temperature, there are only a few molecules which possess threshold, energy., As the temperature increases, the kinetic energy of molecules increases and hence, more and more molecules possess threshold energy., Thus rate of reaction increases sharply with increase of temperature.
Page 63 :
COLLISION FREQUENCY (Z), , The number of collisions per second per unit volume of the reaction mixture is, known as collision frequency (Z).
Page 65 :
As the temperature is increased from ‘T’ Kelvin to (T+10) Kelvin, the curve shifts as, shown in the figure., The shaded area beyond ET now becomes double., This shows that the fraction of molecules possessing energy equal to or greater, than the threshold energy becomes almost double., Hence the number of effective collisions increases and hence the rate of the, reaction becomes double.
Page 66 :
TEMPERATURE COEFFICIENT, , Temperature co-efficient is the ratio of the rate constants of a reaction at two, temperatures differing by 100 ., , For every 100C rise in temperature, the rate of the reaction is nearly doubled.
Page 67 :
ARRHENIUS EQUATION FOR THE REACTION, AT TWO DIFFERENT TEMPERATURES, When the reaction is carried out at two different temperatures T1 and T2 with rate, constants K1 and K2 , then the Arrhenius Equation can be written as
Page 68 :
PROBLEMS
Page 69 :
PROBLEMS
Page 70 :
PROBLEMS
Page 71 :
PROBLEMS
Page 72 :
PROBLEMS
Page 73 :
TRANSITION STATE THEORY, , In a chemical reaction, the reactant molecules must come together to form an, activated complex., Its energy is higher than the reactant molecules, i.e., the reactant molecules have to acquire a certain amount of energy before, they react to form the products., The minimum extra energy which must be supplied to enable them to cross over, the energy barrier between reactants and products is called Activation Energy., Activation Energy = Threshold energy ― Average PE of the reactants
Page 75 :
ENERGY BARRIER, There is an energy barrier for each reaction., The reacting species must be provided with sufficient energy to cross the energy barrier., The minimum amount of energy required by reactant molecules to participate in a reaction, is called activation energy., Activation energy = threshold energy – average kinetic energy of reacting molecules, Threshold energy = initial potential energy of reactant molecules + activation energy.
Page 76 :
A collision between high energy molecules overcomes the forces of repulsion and, brings the formation of an unstable molecule cluster, called the activated complex., The life span of an activated complex is very small., Thus, the activated complex breaks either into reactants again or new substances, i.e.,, products., The activation energy (Ea) depends upon the nature of chemical bonds undergoing, rupture and is independent of enthalpies of reactants and products., The energy changes during exothermic and endothermic reactions versus the progress, of the reaction are shown in the figure.
Page 78 :
Thus, every chemical reaction whether exothermic or endothermic has an energy barrier., This has to be overcome before reactants can be transformed into products., If the reactant molecules have sufficient energy, they can reach the peak of the energy, barrier after collision, Then they can go to the right side of the slope and consequently change into products., If the activation energy for a reaction is low, the fraction of effective collisions will be, large and the reaction will be fast., If the activation energy is high, then fraction of effective collisions will be small and the, reaction will be slow.
Page 79 :
EFFECT OF CATALYST ON REACTION RATE, , A catalyst is a substance which alters the rate of a chemical reaction without itself, undergoing any permanent chemical change., The action of a catalyst can be explained by the intermediate complex formation, theory., According to this theory, a catalyst participates in a chemical reaction by forming, temporary bonds with the reactants resulting in the formation of an intermediate, complex., This has a transitory existence and decomposes to yield products and the catalyst.
Page 81 :
A Catalyst provides an alternate pathway or reaction mechanism by reducing the, activation energy between reactants and products., It lowers the potential energy barrier., From Arrhenius equation, it is seen that lower the value of activation energy faster, will be the rate of a reaction., This is done by catalyst.
Page 82 :
A small amount of the catalyst can catalyse a large amount of reactants., A catalyst does not alter Gibbs energy, ∆G of a reaction., It catalyses the spontaneous reactions but does not catalyze non spontaneous, reactions., A catalyst does not change the equilibrium constant of a reaction.