Page 2 :
KLE College of Pharmacy, Belagavi., Identification of Unknown Organic Compound, , The analysis and identification of unknown organic compounds constitutes a very important, aspect of experimental organic chemistry. Often, a common first step in the identification of an, unknown substance is to determine what elements are present in the sample. Although it is often, possible to establish the structure of a compound on the basis of spectra alone (IR, NMR, etc.), the, spectra typically must be supplemented with other information about the compound: physical state and, properties (melting point, boiling point, solubility, odour, colour, etc.), elemental analysis, and, confirmatory tests for functional groups., In this experiment you will carry out several qualitative tests that will allow you to identify, functional groups in organic molecules. You will then apply what you have learned by characterizing, unknown organic compounds in terms of their functional group and solubility behavior., There is no definite set procedure that can be generally applied to organic qualitative analysis., Various books have different approaches, but a systematic approach based on the scheme given below, will give good results. Each functional group has a particular set of chemical properties that allow it to, be identified. Some of these properties can be demonstrated by observing solubility behavior, while, others can be seen in chemical reactions that are accompanied by color changes, precipitate formation,, or other visible effects., In carrying out identification of an organic compound following tests and observations are, carried out:, I., , Preliminary Tests and Physical Examination, , II., , Determination of Physical Constants (M.P/B.P), , III., , Detection of Elements, , IV., , Determination of Solubility Group, , V., , Detection of Functional Group, , VI., , Special Tests, if any.
Page 4 :
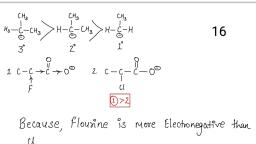
2., , Ignition Test, Flame Test:, Take a small quantity of compound, and put it on a metallic spatula or in a, porcelain dish and ignite it directly on, the, , Non-sooty Flame, , Aliphatic compounds, , Sooty Flame, , Aromatic compounds, , Ammonical odour, , Urea, thiourea, amides may be present, , Chars and swells without, melting, Melts and chars with a smell of, burnt sugar, Produces coughing upon, charring, , Sulphur containing compounds, , Chars without melting, , Sulphanilic acid, starch, uric acid may, be present, , Burns with green flame, , Urea, Chlorides, Bromides may be, present, , Burns without green flame, , Flourides may be present, , a. Action of KMnO4(Baeyers test):, Sub (solid/Liquid) + Sodium, carbonate solution + few drops of, 2% KMnO4 solution – Shake, vigorously, , Decolourization of KMnO4, , Unsaturated compounds may be, present, , No Decolourization of KMnO4, , Saturated compounds may be present, , b. Action of Bromine Water (for, freely or sparingly water soluble, compounds):, Sub (solid/Liquid) + Bromine water, dropwise – Shake vigorously, , Decolourization, , Unsaturated compounds may be, present, , No Decolourization, , Saturated compounds may be present, , c., , Decolourization, , Unsaturated compounds may be, present, , No Decolourization, , Saturated compounds may be present, , Beilstein’s Test:, Make one end of a copper wire in the, form of a loop and heat it on a burner, till flame is no longer coloured. Cool, the wire and dip the loop in little of, the sample and ignite the loop again, in flame., 3., , Carbohydrates may be present, Benzoic acid, salicylic acid etc may be, present, , Test for Unsaturation, , Action of Bromine Water (for, water insoluble compounds):, Sub (solid/Liquid) + 2ml CCl4 or, CHCL3 solution dropwise with shaking, + Bromine in CCl4 dropwise – Shake, vigorously.
Page 5 :
4., , Lassaigne’s Test (Elemental Analysis), Place about a pea size of freshly cut sodium metal into a sodium fusion tube and heat the tube gently to melt the, sodium to a shining globule. Add a small quantity of the sample (solid/liquid) into the fusion tube. Heat the tube, carefully at first and then as strongly as possible until the bottom of the tube is glowing red, holding the tube at, this heat for about 2 min. Quickly plunge the hot tube in a china dish containing about 8 ml of distilled water, and cover the china dish immediately with a wire gauge. The tube crumbles into pieces and the mass comes out, and dissolves in water. Boil the contents of the china dish thoroughly, filter the contents and collect the filtrate, (which is also called Stock solution) in a test tube. Divide the filtrate into 3-4 portions and test each portion for, the elements separately., Test, Observation, Inference, 1., , Test for Nitrogen:, , a. Prussian Blue test, Stock solution + FeSO4 solution – boil, and cool. Add conc. H2SO4, , Green or Blue colour, , Nitrogen present, , No Green or Blue colour, , Nitrogen absent, , a. Sodium nitroprusside test, Stock solution + Sodium nitroprusside, , Pink colour, No Pink colour, , Sulphur present, Sulphur absent, , b. Lead acetate Test, Stock solution + Acetic Acid + 2 ml, Lead acetate (5%), , Black Ppt, , Sulphur present, , No Black Ppt, , Sulphur absent, , White ppt, which freely, dissolves in 2 ml of ammonia, solution, , Chlorine present, , Pale yellow ppt, which is, difficult to dissolve in 2 ml of, ammonia solution, , Bromine present, , Yellow ppt, which is insoluble, in 2 ml of ammonia solution, No Ppt, , Iodine present, , 2. Test for Sulphur:, , 3. Test for Halogens:, a. Silver Nitrate test, Stock solution + dil. HNO3 – heat, boil, and reduce to half the volume + a few, drops of silver nitrate solution., , Test for Nitrogen:, 6 NaCN, + FeSO4, Sodium cyanide, FeSO4, , , , Na4[Fe(CN)6] + Na2SO4, Sod. Ferrocyanide, , 2Fe2(SO4)3, , Na4[Fe(CN)6], , + 2Fe2(SO4)3 Fe4[Fe(CN)6], Ferricferrocyanide, (Persian Blue), , + Na2SO4dil. H, , Halogens absent
Page 8 :
Test, , Observation, , Inference, , 6., , Group Detection:, , A, , Test for Group I: Low molecular weight compounds like Acids, Alcohols, Aldehydes, ketones, esters, phenols, etc, , a., , Test for Aldehydes:, Sub + 2,4-dinitrophenol, , Red ppt, , Aldehydes or Ketones present, , b., , Test for Alcohols:, Sub + Sodium metal in a dry test tube, , Effervescence, , Alcohol present, , c., , Test for Acids:, Sub + NaHCO3 solution, , Effervescence, , Acids present, , d., , Test for Esters:, Sub + NaOH solution + 1 drop of, phenolphthalein indicator, , Pink colour disappears, , Esters present, , e., , Test for Phenols:, Sub + Alcoholic FeCl3 solution, , Violet colour, greenish purple, colour, bluish purple colour, , Phenols present, , B, , Test for Group II: Sugars and Amides, Nitrogen absent-Sugars: Glucose- see aldehydes, under group V;, Nitrogen present-Urea/Thiourea,, , a., , Test for Sugars, , b., , i. Molisch Test:, Sub + Molisch’s reagent + 2 ml Conc., H2SO4 along sides of the test tube., , Violet ring at the junction of 2, layers, , Sugars present, , ii. Fehling’s test:, Sub + Fehling’s reagent A and B –, warm on water bath, , Brick Red colour, , Reducing Sugar present, , iii. Benedict’s Test:, Sub + Benedict’s reagent - warm on, water bath, , Orange colour, , Reducing Sugar present, , iv. Barfoed’s Test:, Sub + Barfoed’s reagent - warm on, water bath, , Red colour, , Monosaccharide present, , v. Rapid Furfural test:, Sub + rapid Furfural reagent + Conc., HCl – boil, , Violet Colour, , Glucose present, , i. Burn substance over an open flame, , Burns without soot and leaves, white residue, , Aliphatic compounds., Urea may be present., , ii. Sub + NaOH solutions (heat in a, dry test tube), , Smell of NH3 / Red litmus held, on top of test tube turns blue., , Amides (urea) may be present., , Test for Amides:
Page 9 :
c., , iii. Nitrous acid test, Dissolve substance in dil. HCl and add, to solution of NaNO2 – Boil off, nitrogen and cool test tube., , Effervescence due to evolution, of Nitrogen, , Amides present, , Clear solution, , Aliphatic amides present, , White ppt, , Aromatic amides present, , iv. Biuret test, Place 0.2 g of Urea in a dry test tube, and heat until it melts and all NH3 has, evolved – and it resolidifies. Dissolve, the residue in few ml of water + 1 ml, of dil. NaOH solution and finally add, CuSO4 solution dropwise., , Pink, Violet or Blue colour, , Urea confirmed., , Green colour changing to blue, , Thiourea present, , i. Sub + Acetyl chloride or Benzoyl, chloride (in dry test tube), , Vigorous reaction (solid, separates), , 10 and 20 Amines, , ii. Diazotization Test:, Sub + dil. HCl till soluble – cool in ice, water. Add ice cold solution of NaNO2, in water., , a. Clear solution/Brown oil, obtained – further add ice, cold -naphthol in NaOH, solution - Orange Dye, , 10 Amines, , b. Yellow oil separates, , 20 Amines, , Test for Thiourea, Sub + Potassium ferricyanide + dil., Acetic acid – warm slightly, , C, , Test for Group III: 10, 20 and 30 Amines, , c. Red/Brown oil - further add 30 Amines, NaOH solution - green solid, separates, iii. Carbylamine or Isocyanide reaction:, Sub + CHCl3 + NaOH solution – Boil, continuously, D, , 10 amine (Aliphatic / Aromatic), , No Carbylamine smell, , 20 Amines, , Compound dissolves with, Effervescence of CO2, , Carboxylic Acids may be present, , No Effervescence, , Phenols present, , Test for Group IV: Acids and Phenols, Sub + NaHCO3 solution, , a., , Carbylamine smell, , Test for Acids: Salicylic acid, Cinnamic acid, Benzoic acid, Confirmatory test for Acids: (Esterification reaction), Sub + 1 ml of Alcohol + Conc. H2SO4, (few drops) – heat for some time on, water bath, cool and pour in a china, dish containing water, , Fruity odour due to ester, formation, , Acid confirmed, , i. Sub + Alcoholic FeCl3, , Purple-Violet colour, , Phenolic acids – Salicylic acid present, , No colour, but ppt, , Salicylic acid absent., Benzoic acid/Cinnamic acid may be, present
Page 11 :
i. Tollen’s Test:, Sub + Tollen’s reagent (ammonical, AgNO3 soln) – warm on a water bath, , No Silver mirror, , Ketones present, , ii. Sub + Fehling’s solution (A+B) –, warm on a water bath, , No Red ppt, , Ketone present, , iii. Sub + Schiff’s reagent (shake well), , No Violet-purple/Magenta, colour, Pink/Red colouration, , Ketone present, , Surface of Sodium appears, greenish-blue, , Benzophenone confirmed, , ii. Sub + 2,4-dinitrophenylhydrazine, solution (excess) – warm if necessary, , No Red Precipitate, , Aldehyde/Ketone absent, Alcohol may be present, , iii. Sub + freshly cut shiny Sodium, metal (in a dry test tube), , Vigorous reaction (brisk, effervescence of hydrogen gas), , Alcohol present, , - after complete dissolution of sodium, add little ether, , - Formation of hazy solution /, solid salt after addition of ether, Fruity Odour, , iv. Sub + freshly prepared Sodium, nitroprusside solution – shake well, + dil. NaOH solution in excess, v. Sub + 0.2 g naphthalene (in a dry, test tube) – heat until molten +, small dry piece of Sodium – heat., c., , Test for Alcohols, , iv. Esterification reaction:, Sub + Benzoic acid + Conc. H2SO4 (in, a dry test tube) boil for some time, and pour in a china dish containing, water or Na2CO3 soln., v. Sub + Conc. H2SO4 (in a dry test, tube), d., , Ketone with methyl group(-CO-CH3), (Acetophenone/Acetone), , Alcohol confirmed, , Tube becomes hot and a white, gelatinous product is obtained, , Benzyl alcohol confirmed., , i. Sub + 2,4-dinitrophenylhydrazine, solution (excess) – warm if, necessary, , No Red Precipitate, , Aldehyde/Ketone absent, Alcohol/Esters may be present, , ii. Sub + Sodium metal (in a dry test, tube), , No Reaction, , iii. Sub + dil. NaOH Solution + drop of, phenolphthalein – pink colour –, shake continuously for a few, minutes/heat on flame, iv. Hydroxamic acid test:, Sub + 1 ml of methanolic, hydroxylamine hydrochloride +, methanolic KOH/NaOH – boil on a, water bath – cool and acidify with dil., HCl + FeCl3 solution, , Pink colour disappears, , Alcohol absent, Esters may be present, Ester present, , Pink colour, , Ester present, , Test for Esters, , F, , Test for Group VI: Hydrocarbons, Halogen hydrocarbons, , a., , Test for hydrocarbons
Page 12 :
i. Sub (In a dry test tube) + Conc., H2SO4 – heat till a clear solution is, obtained – cool and pour in a, beaker of water, ii. Formalin test (Le Rosen test for, Aromatic compounds), In a porcelain dish take 0.6 g of Sub, in CHCl3 + 4-5 drops of formaldehydesulphuric acid reagent., [Reagent: 5-6 drops of 37% HCHO +, 5 ml of Conc. H2SO4. It should be, prepared freshly], iii. Chloroform-Aluminium Test:, Heat strongly 0.2 g of AlCl3 in a test, tube so that it sublimes and sticks on, the walls of the test tube., Run down 2 ml of Sub in CHCl3 on, the sides of the test tube – note the, colour produced by contact of solution, of AlCl3, b., , G, a., , Hydrocarbons present, (sulphonation takes place), , Different colours like Red,, Green etc., , Toluene, Benzene, Naphthalene, present, , Appearance of red, orange, blue Aromatic hydrocarbons confirmed, or green colour due to the, formation of triphenylmethane, dyes., , Test for halogen hydrocarbons, i. Elemental Analysis (Lassaigne’s test) Halogen present., Chlorine/Bromine, ii. Sub + alcoholic AgNO3 solution –, White ppt, Warm, No white ppt, , Halogen hydrocarbon, , iii. Test for Alkyl/Aryl halides, Sub + 2 ml Conc. HNO3 + 2 ml Conc., H2SO4 – warm gently for 3 mins and, pour in a beaker of water, , Indicates arylhalides, , Yellow ppt, , Halogen present in side chain, Halogen present in nucleus, , Test for Group VII: Amides, Anilides, Nitro compounds, , Test for Amides, i. Sub + NaOH solution – heat, , ii. Nitrous acid test:, - Place and dissolve little of, compound in dil. HCl and add to, solution of NaNO2, - Boil off nitrogen and cool the test, tube, b., , Clear solution, (no oil layer/solid separates), , Evolution of NH3 detected by, smell or red litmus turned blue, No smell of NH3, , Amide present, , Effervescence due to evolution, of nitrogen, Clear solution, , Amides present, , White ppt, , Aromatic amine present, , Smell of carbylamine, , Anilide Present, , No Smell of carbylamines, , Anilide absent, , Amides absent, , Aliphatic amide present, , Test for Anilides, i. Sub + NaOH + CHCl3 – boil for, some time
Page 13 :
ii. Sub + Conc. HCl – boil for some, time till clear solution is obtained., Dilute with water – cool + ice cold, NaNO2 solution. Finally add ice cold, -naphthol in NaOH in solution, dropwise, c., , Red/Orange dye, , Anilide confirmed, , i. Sub + NaOH solution – boil, , No smell of NH3, , Amides absent, , ii. Sub + NaOH solution + CHCl3 –, boil continuously for some time, iii. Sub + Conc. HCl + Tin pieces –, wait till reaction ceases – then boil, till layer disappears – cool, filter,, dilute and add ice cold NaNO2, solution, , No smell of carbylamines, , Anilide absent, , Clear solution, - Further add ice cold naphthol in NaOH in, solution dropwise., - Red/Orange dye, obtained, Grey/Black ppt, (NO2 group present), , Nitro compound present, , Test for Nitro Compounds, , iv. Neutral Reduction:, Dissolve 0.1 g of sample in 2 ml, ethanol + 5 drops of CaCl2 solution +, pinch of Zinc dust and boil the, contents for 5 mins. Filter the solution, in a test tube containing 1 ml of, Tollen’s reagent, v. Test for Aromatic nitro compounds: Pink colour, Sub + acetone + NaOH solution, No pink colour, , Nitro compound present, , Dinitro compound, Mononitro compound
Page 14 :
Derivative Preparation, Derivatizations are the synthetic procedures regarding ‘conversions’ of one simple common, organic compound into another compound that can be thoroughly characterized. After performing the, preliminary and elemental analysis, solubility tests, functional groups test and determination of, melting/boiling point, the student can propose a list of possible compounds for the given unknown, sample. These possible compounds may contain a number of structural differences; therefore the final, confirmation for the identity of the given unknown sample can be accomplished by preparation of a, suitable derivative of the given sample and noting its melting/boiling point., Sr., No., , 1., , Procedure, , Urea Nitrate (For Urea), To a saturated solution in water add Conc. Nitric acid – Shake well. Crystals of Urea nitrate separate., , 2., , Benzoate Derivative (For Alcohols/Phenols), Place 0.5 g of sample in 2.5 ml of water in a test tube. To this add 2.5 ml of 10% NaOH, followed by by 0.3, ml of benzoyl chloride. Shake for several mins. (or boil on low heat). The odour of benzoyl chloride should, disappear. The mixture is poured into water and the product is recrystallized from alcohol., , (Caution: Benzoyl chloride is highly corrosive and irritating – Handle with care), , 3., , 2,4-Dinitrophenylhydrazone (For Aldehyde/Ketone), Dissolve 0.1 g of aldehyde/ketone in 5 ml of alcohol. To this add 2 ml of 2,4-DNP reagent. Shake vigorously, and allow the resulting mixture to stand for some time at room temperature. Crystallization of 2,4dinitrophenylhydrazone usually occurs within 5-10 mins. The ppt is recrystallized from alcohol., (2,4-DNP reagent: 0.4 g 2,4-DNP + 2 ml Conc.H2SO4 + 3 ml water – Shake well until 2,4-DNP is dissolved –, To this add 5 ml 95% alcohol)
Page 15 :
4., , Oxime Derivative (Aldehydes/Ketone), Dissolve 0.8 g of hydroxylamine hydrochloride in 5 ml of water. Add 4 ml of 10% NaOH solution and 0.4 g of, unknown aldehyde/ketone (If sample is insoluble in water, add sufficient ethanol to give a clear solution)., Warm the mixture on a water bath for 10-15 mins and allow the product to stand in an ice bath so that, crystalline product is obtained from water or dilute ethanol., , 5., , Benzoate Derivative – Schotten-Baumann reaction (For 10/20/30 Amines, Phenols, Sulphonamide), Dissolve 0.5 g of unknown compounds in 10 ml of 5% NaOH. Add 0.5 g of Benzoyl chloride with continuous, shaking for 20 mins – Allow to cool. The reaction mixture is again vigorously shaken for 15 mins – Ppt, obtained is filtered off and recrystallized in alcohol., , (Caution: Benzoyl chloride is highly corrosive and irritating – Handle with care), , 6., , Base Catalyzed Derivative (For Amides/Anilides/Imides/nitriles), Reflux a mixture of 0.5 g unknown sample and 15 ml of NaOH for 15-20 mins. The aqueous solution is, allowed to cool in ice bath. Add conc. HCl drop-wise until the mixture becomes acidic to litmus. Filter the, solid carboxylic acid ppt. (If no ppt obtained extract the mixture thrice with 8 ml diethyl ether and pass the, combined extract over anhydrous magnesium sulphate and evaporate the ether at room temp to leave the acid, residue.), , 7., 8., 9., 10.
Page 16 :
11., 12., 13., 14., 15., 16., 17., 18., 19., 20.
Page 17 :
Physical Constants of Compounds and their derivatives, Name of the, Compound, [M.P./B.P. (0C)], , Structure, , Derivative with, [M.P./B.P. (0C)], , Name of the, Compound, [M.P./B.P. (0C)], , Derivative with, [M.P./B.P. (0C)], , Structure, , Name of the, Compound, [M.P./B.P. (0C)], , Structure, , Derivative with, [M.P./B.P. (0C)], , Phenylhydrazine, [129], Amide [82], , Benzoic acid, M.P - 121, , Phenylhydrazine, [167], Amide [139], Anilide [162], , Cinnamic acid, M.P - 133, , Amide [153], Anilide [147], , Salicylic acid, M.P – 158, , Amide [135], Anilide [139], , Methyl acetate, B.P – 57, , Amide [82], 3,5Dinitrobenzoate, [108], , Ethyl acetate, B.P – 77, , Amide [82], 3,5Dinitrobenzoate, [93], , Methyl benzoate, B.P – 199, , Amide [130], 3,5Dinitrobenzoate, [108], , Ethyl benzoate, B.P – 212, , Amide [130], 3,5Dinitrobenzoate, [93], , Benzyl benzoate, B.P – 323, , Amide [130], 3,5Dinitrobenzoate, [113], , Methyl salicylate, B.P – 224, , Amide [142], 3,5Dinitrobenzoate, [108], , o-cresol, , p-Nitrobenzoate, [94], , m-cresol, , p-Nitrobenzoate, [90], , p-cresol, , p-Nitrobenzoate, [98], , Acetic acid, B.P – 118, , M.P – 30, B.P -191, , M.P – 12, B.P -202, , Phenol, M.P- 30, B,P – 182, , Benzoate [69], , Resorcinol, M.P – 110, , -naphthol, M.P – 123, , Benzoate [107], , Formaldehyde, B.P -, , Acetone, B.P – 56, , Benzoate [117], , Phthalic acid, M.P – 208, , Amide [219], Anilide [251], , Acetylsalicylic acid, M.P – 135, , Amide [135], Anilide [139], , M.P – 36, B.P -202, -naphthol, M.P – 94, , Benzoate [56], , -, , Benzaldehyde, B.P – 179, , 2,4-dinitrophenyl, hydrazone [237], , 2,4-dinitrophenyl, hydrazone [250], , Benzophenone, M.P – 49, B.P – 306, , 2,4-dinitrophenyl, hydrazone [238], , HCHO, , 2,4-dinitrophenyl, hydrazone [128], , Acetophenone, B.P – 202