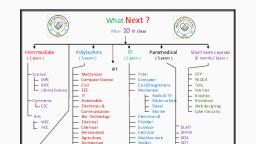
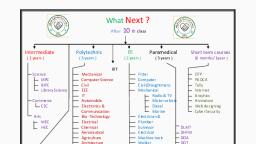
Page 1 :
Final Syllabus for NEET-UG, , CORE SYLLABUS, Physics, Chemistry, Biology, (Higher Secondary Stage), , For National Eligibility-cum-Entrance, Test (NEET) for admission to MBBS, courses across the country, Medical Council of India, The Medical Council of India (MCI) recommends the following syllabus for National, Eligibility-cum-Entrance Test for admission to MBBS courses across the country (NEETUG) after review of various State syllabi as well as those prepared by CBSE, NCERT and, COBSE. This is to establish a uniformity across the country keeping in view the, relevance of different areas in medical education., , 1
Page 2 :
Final Syllabus for NEET-UG, , S.No., 1, 2., 3., 4., 5., 6., 7, 8., 9, 10, , Physics syllabus of class 11th, Topics, Physical world and measurement, Kinematics, Laws of Motion, Work, Energy and Power, Motion of System of Particles and Rigid Body, Gravitation, Properties of Bulk Matter, Thermodynamics, Behaviour of Perfect Gas and Kinetic Theory, Oscillations and Waves, , Page No., 5, 5, 5, 6, 6, 6, 6-7, 7, 7, 7, , Physics syllabus of Class 12th, 1., 2., 3., 4., 5., 6., 7., 8., 9., , Electrostatics, Current Electricity, Magnetic Effects of Current and Magnetism, Electromagnetic Induction and Alternating Currents, Electromagnetic Waves, Optics, Dual Nature of Matter and Radiation, Atoms and Nuclei, Electronic Devices, , 8, 8, 9, 9, 9-10, 10, 10, 11, 11, , Chemistry syllabus of class 11th, 1, , Some Basic Concepts of Chemistry, , 13, , 2., , Structure of Atom, , 13, , 3., , Classification of Elements and Periodicity in Properties, , 13, , 4., , Chemical Bonding and Molecular Structure, , 13, , 5., , States of Matter: Gases and Liquids, , 14, , 6, , Thermodynamics, , 14, , 7, , Equilibrium, , 14, , 8, , Redox Reactions, , 14, , 9., , Hydrogen, , 15, , 10., , s-Block Element (Alkali and Alkaline earth metals), , 15, , 11., , Some p-Block Elements, , 15, , 12., , Organic Chemistry- Some Basic Principles and Techniques, , 16, , 13., , Hydrocarbons, , 16, , 14., , Environmental Chemistry, , 16, , 2
Page 3 :
Final Syllabus for NEET-UG, Chemistry syllabus of class 12th, S.No., , Topics, , Page No., , 1., , Solid State, , 17, , 2., , Solutions, , 17, , 3., , Electrochemistry, , 17, , 4., , Chemical Kinetics, , 17, , 5., , Surface Chemistry, , 18, , 6., , General Principles and Processes of Isolation of Elements, , 18, , 7., , p- Block Elements, , 18, , 8., , d and f Block Elements, , 19, , 9., , Coordination Compounds, , 19, , 10., , Haloalkanes and Haloarenes, , 19, , 11., , Alcohols, Phenols and Ethers, , 19-20, , 12., , Aldehydes, Ketones and Carboxylic Acids, , 20, , 13., , Organic Compounds Containing Nitrogen, , 20, , 14., , Biomolecules, , 20, , 15., , Polymers, , 21, , 16., , Chemistry in Everyday Life, , 21, Biology syllabus of class 11th, , 1, , Diversity in Living World, , 23, , 2., , Structural Organisation in Animals and Plants, , 23, , 3., , Cell Structure and Function, , 4., , Plant Physiology, , 24, , 5, , Human physiology, , 25, , 23-24, , Biology syllabus of class 12th, 1., , Reproduction, , 26, , 2., , Genetics and Evolution, , 3., , Biology and Human Welfare, , 27, , 4., , Biotechnology and Its Applications, , 27, , 5., , Ecology and environment, , 28, , 26-27, , 3
Page 5 :
Final Syllabus for NEET-UG, CONTENTS CLASS XI SYLLABUS, UNIT I: Physical World and Measurement, Details:• Physics: Scope and excitement; nature of physical laws; Physics, technology and, society., •, , Need for measurement: Units of measurement; systems of units; SI units,, fundamental and derived units. Length, mass and time measurements; accuracy, and precision of measuring instruments; errors in measurement; significant, figures., , •, , Dimensions of physical quantities, dimensional analysis and its applications., , UNIT II: Kinematics, Details:• Frame of reference, Motion in a straight line; Position-time graph, speed and, velocity. Uniform and non-uniform motion, average speed and instantaneous, velocity. Uniformly accelerated motion, velocity-time and position-time graphs,, for uniformly accelerated motion (graphical treatment)., •, , Elementary concepts of differentiation and integration for describing motion., Scalar and vector quantities: Position and displacement vectors, general vectors,, general vectors and notation, equality of vectors, multiplication of vectors by a, real number; addition and subtraction of vectors. Relative velocity., , •, , Unit vectors. Resolution of a vector in a plane-rectangular components., , •, , Scalar and Vector products of Vectors. Motion in a plane. Cases of uniform, velocity and uniform acceleration- projectile motion. Uniform circular motion., , UNIT III: Laws of Motion, Details:• Intuitive concept of force. Inertia, Newton’s first law of motion; momentum and, Newton’s second law of motion; impulse; Newton’s third law of motion. Law of, conservation of linear momentum and its applications., •, , Equilibrium of concurrent forces. Static and Kinetic friction, laws of friction,, rolling friction, lubrication., , •, , Dynamics of uniform circular motion. Centripetal force, examples of circular, motion (vehicle on level circular road, vehicle on banked road)., , 5
Page 6 :
Final Syllabus for NEET-UG, UNIT IV: Work, Energy and Power, Details:• Work done by a constant force and variable force; kinetic energy, work-energy, theorem, power., •, , Notion of potential energy, potential energy of a spring, conservative forces;, conservation of mechanical energy (kinetic and potential energies); nonconservative forces; motion in a vertical circle, elastic and inelastic collisions in, one and two dimensions., , UNIT V: Motion of System of Particles and Rigid Body, Details:• Centre of mass of a two-particle system, momentum conservation and centre of, mass motion. Centre of mass of a rigid body; centre of mass of uniform rod., •, , Moment of a force,-torque, angular momentum, conservation of angular, momentum with some examples., , •, , Equilibrium of rigid bodies, rigid body rotation and equation of rotational motion,, comparison of linear and rotational motions; moment of inertia, radius of, gyration. Values of M.I. for simple geometrical objects (no derivation)., Statement of parallel and perpendicular axes theorems and their applications., , UNIT VI: Gravitation, Details:• Kepler’s laws of planetary motion. The universal law of gravitation., Acceleration due to gravity and its variation with altitude and depth., •, , Gravitational potential energy; gravitational potential. Escape velocity, orbital, velocity of a satellite. Geostationary satellites., , UNIT VII: Properties of Bulk Matter, Details:• Elastic behavior, Stress-strain relationship. Hooke’s law, Young’s modulus, bulk, modulus, shear, modulus of rigidity, poisson’s ratio; elastic energy., •, , Viscosity, Stokes’ law, terminal velocity, Reynold’s number, streamline and, turbulent flow. Critical velocity, Bernoulli’s theorem and its applications., , •, , Surface energy and surface tension, angle of contact, excess of pressure,, application of surface tension ideas to drops, bubbles and capillary rise., , 6
Page 7 :
Final Syllabus for NEET-UG, •, , Heat, temperature, thermal expansion; thermal expansion of solids, liquids, and, gases. Anomalous expansion. Specific heat capacity: Cp, Cv- calorimetry;, change of state – latent heat., , •, , Heat transfer- conduction and thermal conductivity, convection and radiation., Qualitative ideas of Black Body Radiation, Wein’s displacement law, and Green, House effect., , •, , Newton’s law of cooling and Stefan’s law., , UNIT VIII: Thermodynamics, Details:• Thermal equilibrium and definition of temperature (zeroth law of, Thermodynamics). Heat, work and internal energy. First law of, thermodynamics. Isothermal and adiabatic processes., •, , Second law of the thermodynamics: Reversible and irreversible processes. Heat, engines and refrigerators., , UNIT IX: Behaviour of Perfect Gas and Kinetic Theory, Details:• Equation of state of a perfect gas, work done on compressing a gas., •, , Kinetic theory of gases: Assumptions, concept of pressure. Kinetic energy and, temperature; degrees of freedom, law of equipartition of energy (statement only), and application to specific heat capacities of gases; concept of mean free path., , UNIT X: Oscillations and Waves, Details:• Periodic motion-period, frequency, displacement as a function of time. Periodic, functions. Simple harmonic motion(SHM) and its equation; phase; oscillations of, a spring-restoring force and force constant; energy in SHM –Kinetic and potential, energies; simple pendulum-derivation of expression for its time period; free,, forced and damped oscillations (qualitative ideas only), resonance., •, , Wave motion. Longitudinal and transverse waves, speed of wave motion., Displacement relation for a progressive wave. Principle of superposition of, waves, reflection of waves, standing waves in strings and organ pipes,, fundamental mode and harmonics. Beats. Doppler effect., , 7
Page 8 :
Final Syllabus for NEET-UG, , CONTENTS OF CLASS XII SYLLABUS, UNIT I: Electrostatics, Details:• Electric charges and their conservation. Coulomb’s law-force between two point, charges, forces between multiple charges; superposition principle and continuous, charge distribution., •, , Electric field, electric field due to a point charge, electric field lines; electric, dipole, electric field due to a dipole; torque on a dipole in a uniform electric field., , •, , Electric flux, statement of Gauss’s theorem and its applications to find field due to, infinitely long straight wire, uniformly charged infinite plane sheet and uniformly, charged thin spherical shell (field inside and outside), , •, , Electric potential, potential difference, electric potential due to a point charge, a, dipole and system of charges: equipotential surfaces, electrical potential energy of, a system of two point charges and of electric diploes in an electrostatic field., , •, , Conductors and insulators, free charges and bound charges inside a conductor., Dielectrics and electric polarization, capacitors and capacitance, combination of, capacitors in series and in parallel, capacitance of a parallel plate capacitor with, and without dielectric medium between the plates, energy stored in a capacitor,, Van de Graaff generator., , UNIT II: Current Electricity, Details:• Electric current, flow of electric charges in a metallic conductor, drift velocity and, mobility, and their relation with electric current; Ohm’s law, electrical resistance,, V-I characteristics (liner and non-linear), electrical energy and power, electrical, resistivity and conductivity., •, , Carbon resistors, colour code for carbon resistors; series and parallel, combinations of resistors; temperature dependence of resistance., , •, , Internal resistance of a cell, potential difference and emf of a cell, combination of, cells in series and in parallel., , •, , Kirchhoff’s laws and simple applications. Wheatstone bridge, metre bridge., , •, , Potentiometer-principle and applications to measure potential difference, and for, comparing emf of two cells; measurement of internal resistance of a cell., , 8
Page 9 :
Final Syllabus for NEET-UG, UNIT III: Magnetic Effects of Current and Magnetism, Details:• Concept of magnetic field, Oersted’s experiment. Biot-Savart law and its, application to current carrying circular loop., •, , Ampere’s law and its applications to infinitely long straight wire, straight and, toroidal solenoids. Force on a moving charge in uniform magnetic and electric, fields. Cyclotron., , •, , Force on a current-carrying conductor in a uniform magnetic field. Force, between two parallel current-carrying conductors-definition of ampere. Torque, experienced by a current loop in a magnetic field; moving coil galvanometer-its, current sensitivity and conversion to ammeter and voltmeter., , •, , Current loop as a magnetic dipole and its magnetic dipole moment. Magnetic, dipole moment of a revolving electron. Magnetic field intensity due to a magnetic, dipole (bar magnet) along its axis and perpendicular to its axis. Torque on a, magnetic dipole (bar magnet) in a uniform magnetic field; bar magnet as an, equivalent solenoid, magnetic field lines; Earth’s magnetic field and magnetic, elements., , •, , Para-, dia-and ferro-magnetic substances, with examples., , •, , Electromagnetic and factors affecting their strengths. Permanent magnets., , UNIT IV: Electromagnetic Induction and Alternating Currents, Details:• Electromagnetic induction; Faraday’s law, induced emf and current; Lenz’s Law,, Eddy currents. Self and mutual inductance., •, , Alternating currents, peak and rms value of alternating current/ voltage; reactance, and impedance; LC oscillations (qualitative treatment only), LCR series circuit,, resonance; power in AC circuits, wattles current., , •, , AC generator and transformer., , UNIT V: Electromagnetic Waves, Details:• Need for displacement current., •, , Electromagnetic waves and their characteristics (qualitative ideas only)., Transverse nature of electromagnetic waves., , 9
Page 10 :
Final Syllabus for NEET-UG, •, , Electromagnetic spectrum (radio waves, microwaves, infrared, visible, ultraviolet,, x-rays, gamma rays) including elementary facts about their uses., , UNIT VI: Optics, Details:• Reflection of light, spherical mirrors, mirror formula. Refraction of light, total, internal reflection and its applications optical fibres, refraction at spherical, surfaces, lenses, thin lens formula, lens-maker’s formula. Magnification, power, of a lens, combination of thin lenses in contact combination of a lens and a mirror., Refraction and dispersion of light through a prism., •, , Scattering of light- blue colour of the sky and reddish appearance of the sun at, sunrise and sunset., , •, , Optical instruments: Human eye, image formation and accommodation,, correction of eye defects (myopia and hypermetropia) using lenses., , •, , Microscopes and astronomical telescopes (reflecting and refracting) and their, magnifying powers., , •, , Wave optics: Wavefront and Huygens’ principle, reflection and refraction of, plane wave at a plane surface using wavefronts., , •, , Proof of laws of reflection and refraction using Huygens’ principle., , •, , Interference, Young’s double hole experiment and expression for fringe width,, coherent sources and sustained interference of light., , •, , Diffraction due to a single slit, width of central maximum., , •, , Resolving power of microscopes and astronomical telescopes. Polarisation, plane, polarized light; Brewster’s law, uses of plane polarized light and Polaroids., , UNIT VII: Dual Nature of Matter and Radiation, Details:• Photoelectric effect, Hertz and Lenard’s observations; Einstein’s photoelectric, equation- particle nature of light., •, , Matter waves- wave nature of particles, de Broglie relation. Davisson-Germer, experiment (experimental details should be omitted; only conclusion should be, explained)., , 10
Page 11 :
Final Syllabus for NEET-UG, UNIT VIII: Atoms and Nuclei, Details:• Alpha- particle scattering experiments; Rutherford’s model of atom; Bohr model,, energy levels, hydrogen spectrum. Composition and size of nucleus, atomic, masses, isotopes, isobars; isotones., •, , Radioactivity- alpha, beta and gamma particles/ rays and their properties decay, law. Mass-energy relation, mass defect; binding energy per nucleon and its, variation with mass number, nuclear fission and fusion., , UNIT IX: Electronic Devices, Details:• Energy bands in solids (qualitative ideas only), conductors, insulators and, semiconductors; semiconductor diode- I-V characteristics in forward and reverse, bias, diode as a rectifier; I-V characteristics of LED, photodiode, solar cell, and, Zener diode; Zener diode as a voltage regulator. Junction transistor, transistor, action, characteristics of a transistor; transistor as an amplifier (common emitter, configuration) and oscillator. Logic gates (OR, AND, NOT, NAND and NOR)., Transistor as a switch ., , 11
Page 13 :
Final Syllabus for NEET-UG, , CONTENTS OF CLASS XI SYLLABUS, UNIT I: Some Basic Concepts of Chemistry, Details:• General Introduction: Important and scope of chemistry., •, , Laws of chemical combination, Dalton’s atomic theory: concept of elements,, atoms and molecules., , •, , Atomic and molecular masses. Mole concept and molar mass; percentage, composition and empirical and molecular formula; chemical reactions,, stoichiometry and calculations based on stoichiometry., , UNIT II: Structure of Atom, Details:• Atomic number, isotopes and isobars. Concept of shells and subshells, dual nature, of matter and light, de Broglie’s relationship, Heisenberg uncertainty principle,, concept of orbital, quantum numbers, shapes of s,p and d orbitals, rules for filling, electrons in orbitals- Aufbau principle, Pauli exclusion principles and Hund’s, rule, electronic configuration of atoms, stability of half filled and completely, filled orbitals., UNIT III: Classification of Elements and Periodicity in Properties, Details:• Modern periodic law and long form of periodic table, periodic trends in properties, of elements- atomic radii, ionic radii, ionization enthalpy, electron gain enthalpy,, electronegativity, valence., , UNIT IV: Chemical Bonding and Molecular Structure, Details:• Valence electrons, ionic bond, covalent bond, bond parameters, Lewis structure,, polar character of covalent bond, valence bond theory, resonance, geometry of, molecules, VSEPR theory, concept of hybridization involving s, p and d orbitals, and shapes of some simple molecules, molecular orbital theory of homonuclear, diatomic molecules (qualitative idea only). Hydrogen bond., , 13
Page 14 :
Final Syllabus for NEET-UG, UNITV: States of Matter: Gases and Liquids, Details:• Three states of matter, intermolecular interactions, types of bonding, melting and, boiling points, role of gas laws of elucidating the concept of the molecule,, Boyle’s law, Charle’s law, Gay Lussac’s law, Avogadro’s law, ideal behaviour of, gases, empirical derivation of gas equation. Avogadro number, ideal gas equation., Kinetic energy and molecular speeds (elementary idea), deviation from ideal, behaviour, liquefaction of gases, critical temperature., •, , Liquid State- Vapour pressure, viscosity and surface tension (qualitative idea, only, no mathematical derivations)., , UNITVI : Thermodynamics, Details:• First law of thermodynamics-internal energy and enthalpy, heat capacity and, specific heat, measurement of U and H, Hess’s law of constant heat, summation, enthalpy of : bond dissociation, combustion, formation, atomization,, sublimation, phase transition, ionization, solution and dilution., •, , Introduction of entropy as state function, Second law of thermodynamics, Gibbs, energy change for spontaneous and non-spontaneous process, criteria for, equilibrium and spontaneity., , •, , Third law of thermodynamics- Brief introduction., , UNIT VII: Equilibrium, Details:• Equilibrium in physical and chemical processes, dynamic nature of equilibrium,, law of chemical equilibrium, equilibrium constant, factors affecting equilibriumLe Chatelier’s principle; ionic equilibrium- ionization of acids and bases, strong, and weak electrolytes, degree of ionization, ionization of polybasic acids, acid, strength, concept of pH., Hydrolysis of salts (elementary idea)., buffer solutions,, Henderson equation, solubility product, common ion effect (with illustrative, examples)., UNIT VIII: Redox Reactions, Details:• Concept of oxidation and oxidation and reduction, redox reactions oxidation, number, balancing redox reactions in terms of loss and gain of electron and, change in oxidation numbers., , 14
Page 15 :
Final Syllabus for NEET-UG, , UNIT IX: Hydrogen, Details:• Occurrence, isotopes, preparation, properties and uses of hydrogen; hydridesionic, covalent and interstitial; physical and chemical properties of water, heavy, water; hydrogen peroxide-preparation, reactions, uses and structure;, UNIT X: s-Block Elements (Alkali and Alkaline earth metals), Details:• Group I and group 2 elements:, •, , General introduction, electronic configuration, occurrence, anomalous properties, of the first element of each group, diagonal relationship, trends in the variation of, properties (such as ionization enthalpy, atomic and ionic radii), trends in chemical, reactivity with oxygen, water, hydrogen and halogens; uses., , •, , Preparation and Properties of Some important Compounds:, , •, , Sodium carbonate, sodium chloride, sodium hydroxide and sodium, hydrogencarbonate, biological importance of sodium and potassium., , •, , Industrial use of lime and limestone, biological importance of Mg and Ca., , UNIT XI: Some p-Block Elements, Details:• General Introduction to p-Block Elements., •, , Group 13 elements: General introduction, electronic configuration, occurrence,, variation of properties, oxidation states, trends in chemical reactivity, anomalous, properties of first element of the group; Boron, some important compounds:, borax, boric acids, boron hydrides. Aluminium: uses, reactions with acids and, alkalies., , •, , General 14 elements: General introduction, electronic configuration, occurrence,, variation of properties, oxidation states, trends in chemical reactivity, anomalous, behaviour of first element. Carbon, allotropic forms, physical and chemical, properties: uses of some important compounds: oxides., , •, , Important compounds of silicon and a few uses: silicon tetrachloride, silicones,, silicates and zeolites, their uses., , 15
Page 16 :
Final Syllabus for NEET-UG, UNIT XII: Organic Chemistry- Some Basic Principles and Techniques, Details:• General introduction, methods of purification qualitative and quantitative, analysis, classification and IUPAC nomenclature of organic compounds., •, , Electronic displacements in a covalent bond: inductive effect, electromeric effect,, resonance and hyper conjugation., , •, , Homolytic and heterolytic fission of a covalent bond: free radials, carbocations,, carbanions; electrophiles and nucleophiles, types of organic reactions., , UNIT XIII: Hydrocarbons, Details:• Alkanes- Nomenclature, isomerism, conformations (ethane only), physical, properties, chemical reactions including free radical mechanism of halogenation,, combustion and pyrolysis., •, , Alkanes-Nomenclature, structure of double bond (ethene), geometrical isomerism,, physical properties, methods of preparation: chemical reactions: addition of, hydrogen, halogen, water, hydrogen halides (Markovnikov’s addition and, peroxide effect), ozonolysis, oxidation, mechanism of electrophilic addition., , •, , Alkynes-Nomenclature, structure of triple bond (ethyne), physical properties,, methods of preparation, chemical reactions: acidic character of alkynes, addition, reaction of- hydrogen, halogens, hydrogen halides and water., , •, , Aromatic hydrocarbons- Introduction, IUPAC nomenclature; Benzene; resonance,, aromaticity; chemical properties: mechanism of electrophilic substitutionNitration sulphonation, halogenation, Friedel Craft’s alkylation and acylation;, directive influence of functional group in mono-substituted benzene;, carcinogenicity and toxicity., , UNIT XIV: Environmental Chemistry, Details:• Environmental pollution: Air, water and soil pollution, chemical reactions in, atmosphere, smogs, major atmospheric pollutants; acid rain ozone and its, reactions, effects of depletion of ozone layer, greenhouse effect and global, warming-pollution due to industrial wastes; green chemistry as an alternative tool, for reducing pollution, strategy for control of environmental pollution., , 16
Page 17 :
Final Syllabus for NEET-UG, , CONTENTS OF CLASS XII SYLLABUS, UNIT I: Solid State, Details:• Classification of solids based on different binding forces; molecular, ionic, covalent and metallic solids, amorphous and crystalline solids (elementary idea),, unit cell in two dimensional and three dimensional lattices, calculation of density, of unit cell, packing in solids, packing efficiency, voids, number of atoms per unit, cell in a cubic unit cell, point defects, electrical and magnetic properties, Band, theory of metals, conductors, semiconductors and insulators., UNIT II: Solutions, Details:• Types of solutions, expression of concentration of solutions of solids in liquids,, solubility of gases in liquids, solid solutions, colligative properties- relative, lowering of vapour pressure, Raoult’s law, elevation of boiling point, depression, of freezing point, osmotic pressure, determination of molecular masses using, colligative properties abnormal molecular mass. Van Hoff factor., UNIT III: Electrochemistry, Details:• Redox reactions, conductance in electrolytic solutions, specific and molar, conductivity variation of conductivity with concentration, kohlrausch’s Law,, electrolysis and Laws of electrolysis (elementary idea), dry cell- electrolytic cells, and Galvanic cells; lead accumulator, EMF of a cell, standard electrode potential,, Relation between Gibbs energy change and EMF of a cell, fuel cells; corrosion., UNIT IV: Chemical Kinetics, Details:• Rate of a reaction (average and instantaneous), factors affecting rates of reaction;, concentration, temperature, catalyst; order and molecularity of a reaction; rate law, and specific rate constant, integrated rate equations and half life (only for zero and, first order reactions); concept of collision theory ( elementary idea, no, mathematical treatment). Activation energy, Arrhenious equation., , 17
Page 18 :
Final Syllabus for NEET-UG, , UNIT V: Surface Chemistry, Details:• Adsorption-physisorption and chemisorption; factors affecting adsorption of gases, on solids, catalysis homogeneous and heterogeneous, activity and selectivity:, enzyme catalysis; colloidal state: distinction between true solutions, colloids and, suspensions; lyophillic, lyophobic multimolecular and macromolecular colloids;, properties of colloids; Tyndall effect, Brownian movement, electrophoresis,, coagulation; emulsions- types of emulsions., UNIT VI: General Principles and Processes of Isolation of Elements, Details:• Principles and methods of extraction- concentration, oxidation, reduction, electrolytic method and refining; occurrence and principles of extraction of, aluminium, copper, zinc and iron., UNIT VII: p- Block Elements, Details:• Group 15 elements: General introduction, electronic configuration, occurrence,, oxidation states, trends in physical and chemical properties; preparation and, properties of ammonia and nitric acid, oxides of nitrogen (structure only);, Phosphorous- allotropic forms; compounds of phosphorous: preparation and, properties of phosphine, halides (PCI3, PCI5) and oxoacids (elementary idea, only)., •, , Group 16 elements: General introduction, electronic configuration, oxidation, states, occurrence, trends in physical and chemical properties; dioxygen:, preparation, properties and uses; classification of oxides; ozone. Sulphur –, allotropic forms; compounds of sulphur: preparation, preparation, properties and, uses of sulphur dioxide; sulphuric acid: industrial process of manufacture,, properties and uses, oxoacids of sulphur (structures only)., , •, , Group 17 elements: General introduction, electronic configuration, oxidation, states, occurrence, trends in physical and chemical properties; compounds of, halogens: preparation, properties and uses of chlorine and hydrochloric acid,, interhalogen compounds oxoacids of halogens (structures only)., , •, , Group 18 elements: General introduction, electronic configuration, occurrence,, trends in physical and chemical properties, uses., , 18
Page 19 :
Final Syllabus for NEET-UG, , UNIT VIII: d and f Block Elements, Details:• General introduction, electronic configuration, characteristics of transition metals,, general trends in properties of the first row transition metals- metallic character,, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property,, magnetic properties, interstitial compounds, alloy formation. Preparation and, properties of K2Cr2O7 and KMnO4., •, , Lanthanoids- electronic configuration, oxidation states, chemical reactivity, and, lanthanoid contraction and its consequences., , •, , Actinoids: Electronic configuration, oxidation states and comparison with, lanthanoids., , UNIT IX: Coordination Compounds, Details:• Coordination compounds: Introduction, ligands, coordination number, colour,, magnetic properties and shapes, IUPAC nomenclature of mononuclear, coordination compounds, isomerism (structural and stereo) bonding, Werner’s, theory VBT,CFT; importance of coordination compounds (in qualitative analysis,, biological systems)., UNIT X: Haloalkanes and Haloarenes, Details:• Haloalkanes: Nomenclature, nature of C –X bond, physical and chemical, properties, mechanism of substitution reactions. Optical rotation., •, , Haloarenes: Nature of C-X bond, substitution reactions (directive influence of, halogen for monosubstituted compounds only)., , •, , Uses and environment effects of – dichloromethane, trichloromethane,, tetrachloromethane, iodoform, freons, DDT., , UNIT XI: Alcohols, Phenols and Ethers, Details:• Alcohols: Nomenclature, methods of preparation, physical and chemical, properties (of primary alcohols only); identification of primary, secondary and, tertiary alcohols; mechanism of dehydration, uses with special reference to, methanol and ethanol., , 19
Page 20 :
Final Syllabus for NEET-UG, •, , Phenols: Nomenclature, methods of preparation, physical and chemical, properties, acidic nature of phenol, electrophillic substitution reactions, uses of, phenols., , •, , Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses., , UNIT XII: Aldehydes, Ketones and Carboxylic Acids, Details:• Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of, preparation, physical and chemical properties; and mechanism of nucleophilic, addition, reactivity of alpha hydrogen in aldehydes; uses., •, , Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical, and chemical properties; uses., , UNIT XIII: Organic Compounds Containing Nitrogen, Details:• Amines: Nomenclature, classification, structure, methods of preparation, physical, and chemical properties, uses, identification of primary secondary and tertiary, amines., •, , Cyanides and Isocyanides- will be mentioned at relevant places., , •, , Diazonium salts: Preparation, chemical reactions and importance in synthetic, organic chemistry., , UNIT XIV: Biomolecules, Details:• Carbohydrates- Classification (aldoses and ketoses), monosaccharide (glucose, and fructose), D.L. configuration, oligosaccharides (sucrose, lactose, maltose),, polysaccharides (starch, cellulose, glycogen): importance., •, , Proteins- Elementary idea of – amino acids, peptide bond, polypeptides, proteins,, primary structure, secondary structure, tertiary structure and quaternary structure, (qualitative idea only), denaturation of proteins; enzymes., , •, , Hormones- Elementary idea (excluding structure)., , •, , Vitamins- Classification and function., , •, , Nucleic Acids: DNA and RNA, , 20
Page 21 :
Final Syllabus for NEET-UG, , UNIT XV: Polymers, Details:• Classification- Natural and synthetic, methods of polymerization (addition and, condensation), copolymerization. Some important polymers: natural and, synthetic like polyesters, bakelite; rubber, Biodegradable and non-biodegradable, polymers., UNIT XVI: Chemistry in Everyday Life, Details:• Chemicals in medicines- analgesics, tranquilizers, antiseptics, disinfectants,, antimicrobials, antifertility drugs, antibiotics, antacids, antihistamines., • Chemicals in food- preservatives, artificial sweetening agents, elementary idea, of antioxidants., • Cleansing agents- soaps and detergents, cleansing action., , 21
Page 23 :
Final Syllabus for NEET-UG, , CONTENTS OF CLASS XI SYLLABUS, UNIT I: Diversity in Living World, Details:, •, , What is living? ; Biodiversity; Need for classification; Three domains of life;, Taxonomy & Systematics; Concept of species and taxonomical hierarchy;, Binomial nomenclature; Tools for study of Taxonomy – Museums, Zoos,, Herbaria, Botanical gardens., , •, , Five kingdom classification; salient features and classification of Monera; Protista, and Fungi into major groups; Lichens; Viruses and Viroids., , •, , Salient features and classification of plants into major groups-Algae, Bryophytes,, Pteridophytes, Gymnosperms and Angiosperms (three to five salient and, distinguishing features and at least two examples of each category); Angiospermsclassification up to class, characteristic features and examples)., , •, , Salient features and classification of animals-nonchordate up to phyla level and, chordate up to classes level (three to five salient features and at least two, examples)., , UNIT II: Structural Organisation in Animals and Plants, Details:, • Morphology and modifications; Tissues; Anatomy and functions of different parts, of flowering plants: Root, stem, leaf, inflorescence- cymose and recemose,, flower, fruit and seed (To be dealt along with the relevant practical of the, Practical Syllabus)., •, , Animal tissues; Morphology, anatomy and functions of different systems, (digestive, circulatory, respiratory, nervous and reproductive) of an insect, (cockroach). (Brief account only), , UNIT III: Cell Structure and Function, Details:, •, , Cell theory and cell as the basic unit of life; Structure of prokaryotic and, eukaryotic cell; Plant cell and animal cell; Cell envelope, cell membrane, cell, wall; Cell organelles-structure and function; Endomembrane system-endoplasmic, reticulum, Golgi bodies, lysosomes, vacuoles; mitochondria, ribosomes, plastids,, , 23
Page 24 :
Final Syllabus for NEET-UG, micro bodies; Cytoskeleton, cilia, flagella, centrioles (ultra structure and, function); Nucleus-nuclear membrane, chromatin, nucleolus., •, , •, , Chemical constituents of living cells: Biomolecules-structure and function of, proteins, carbodydrates, lipids, nucleic acids; Enzymes-types, properties, enzyme, action., B Cell division: Cell cycle, mitosis, meiosis and their significance., , UNIT IV: Plant Physiology, Details:• Transport in plants: Movement of water, gases and nutrients; Cell to cell, transport-Diffusion, facilitated diffusion, active transport; Plant – water relations, – Imbibition, water potential, osmosis, plasmolysis; Long distance transport of, water – Absorption, apoplast, symplast, transpiration pull, root pressure and, guttation; Transpiration-Opening and closing of stomata; Uptake and, translocation of mineral nutrients-Transport of food, phloem transport, Mass flow, hypothesis; Diffusion of gases (brief mention)., •, , Mineral nutrition: Essential minerals, macro and micronutrients and their role;, Deficiency symptoms; Mineral toxicity; Elementary idea of Hydroponics as a, method to study mineral nutrition; Nitrogen metabolism-Nitrogen cycle,, biological nitrogen fixation., , •, , Photosynthesis: Photosynthesis as a means of Autotrophic nutrition; Site of, photosynthesis take place; pigments involved in Photosynthesis (Elementary, idea); Photochemical and biosynthetic phases of photosynthesis; Cyclic and non, cyclic and photophosphorylation; Chemiosmotic hypothesis; Photorespiration C3, and C4 pathways; Factors affecting photosynthesis., , •, , Respiration: Exchange gases; Cellular respiration-glycolysis, fermentation, (anaerobic), TCA cycle and electron transport system (aerobic); Energy relationsNumber of ATP molecules generated; Amphibolic pathways; Respiratory, quotient., , •, , Plant growth and development: Seed germination; Phases of Plant growth and, plant growth rate; Conditions of growth; Differentiation, dedifferentiation and, redifferentiation; Sequence of developmental process in a plant cell; Growth, regulators-auxin,gibberellin, cytokinin, ethylene, ABA; Seed dormancy;, Vernalisation; Photoperiodism., , 24
Page 25 :
Final Syllabus for NEET-UG, UNIT IV: Human Physiology, Details:• Digestion and absorption; Alimentary canal and digestive glands; Role of digestive, enzymes and gastrointestinal hormones; Peristalsis, digestion, absorption and, assimilation of proteins, carbohydrates and fats; Caloric value of proteins,, carbohydrates and fats; Egestion; Nutritional and digestive disorders – PEM,, indigestion, constipation, vomiting, jaundice, diarrhea., • Breathing and Respiration: Respiratory organs in animals (recall only); Respiratory, system in humans; Mechanism of breathing and its regulation in humans-Exchange of, gases, transport of gases and regulation of respiration Respiratory volumes; Disorders, related to respiration-Asthma, Emphysema, Occupational respiratory disorders., • Body fluids and circulation: Composition of blood, blood groups, coagulation of, blood; Composition of lymph and its function; Human circulatory system-Structure of, human heart and blood vessels; Cardiac cycle, cardiac output, ECG, Double, circulation; Regulation of cardiac activity; Disorders of circulatory systemHypertension, Coronary artery disease, Angina pectoris, Heart failure., • Excretory products and their elimination: Modes of excretion- Ammonotelism,, ureotelism, uricotelism; Human excretory system-structure and fuction; Urine, formation, Osmoregulation; Regulation of kidney function-Renin-angiotensin, Atrial, Natriuretic Factor, ADH and Diabetes insipidus; Role of other organs in excretion;, Disorders; Uraemia, Renal failure, Renal calculi, Nephritis; Dialysis and artificial, kidney., • Locomotion and Movement: Types of movement- ciliary, fiagellar, muscular;, Skeletal muscle- contractile proteins and muscle contraction; Skeletal system and its, functions (To be dealt with the relevant practical of Practical syllabus); Joints;, Disorders of muscular and skeletal system-Myasthenia gravis, Tetany, Muscular, dystrophy, Arthritis, Osteoporosis, Gout., • Neural control and coordination: Neuron and nerves; Nervous system in humanscentral nervous system, peripheral nervous system and visceral nervous system;, Generation and conduction of nerve impulse; Reflex action; Sense organs; Elementary, structure and function of eye and ear., • Chemical coordination and regulation: Endocrine glands and hormones; Human, endocrine system-Hypothalamus, Pituitary, Pineal, Thyroid, Parathyroid, Adrenal,, Pancreas, Gonads; Mechanism of hormone action (Elementary Idea); Role of hormones, as messengers and regulators, Hypo-and hyperactivity and related disorders (Common, disorders e.g. Dwarfism, Acromegaly, Cretinism, goiter, exopthalmic goiter, diabetes,, Addison’s disease)., (Imp: Diseases and disorders mentioned above to be dealt in brief.), , 25
Page 26 :
Final Syllabus for NEET-UG, CONTENTS OF CLASS XII SYLLABUS, , UNIT I: Reproduction, Details:• Reproduction in organisms: Reproduction, a characteristic feature of all, organisms for continuation of species; Modes of reproduction – Asexual and, sexual; Asexual reproduction; Modes-Binary fission, sporulation, budding,, gemmule, fragmentation; vegetative propagation in plants., •, , Sexual reproduction in flowering plants: Flower structure; Development of male, and female gametophytes; Pollination-types, agencies and examples; Outbreeding, devices; Pollen-Pistil interaction; Double fertilization; Post fertilization eventsDevelopment of endosperm and embryo, Development of seed and formation of, fruit; Special modes-apomixis, parthenocarpy, polyembryony; Significance of, seed and fruit formation., , •, , Human Reproduction: Male and female reproductive systems; Microscopic, anatomy of testis and ovary; Gametogenesis-spermatogenesis & oogenesis;, Menstrual cycle; Fertilisation, embryo development upto blastocyst formation,, implantation; Pregnancy and placenta formation (Elementary idea); Parturition, (Elementary idea); Lactation (Elementary idea)., , •, , Reproductive health: Need for reproductive health and prevention of sexually, transmitted diseases (STD); Birth control-Need and Methods, Contraception and, Medical Termination of Pregnancy (MTP); Amniocentesis; Infertility and assisted, reproductive technologies – IVF, ZIFT, GIFT (Elementary idea for general, awareness)., , UNIT II: Genetics and Evolution, Details:• Heredity and variation: Mendelian Inheritance; Deviations from MendelismIncomplete dominance, Co-dominance, Multiple alleles and Inheritance of blood, groups, Pleiotropy; Elementary idea of polygenic inheritance; Chromosome, theory of inheritance; Chromosomes and genes; Sex determination-In humans,, birds, honey bee; Linkage and crossing over; Sex linked inheritance-Haemophilia,, Colour blindness; Mendelian disorders in humans-Thalassemia; Chromosomal, disorders in humans; Down’s syndrome, Turner’s and Klinefelter’s syndromes., , 26
Page 27 :
Final Syllabus for NEET-UG, , •, , Molecular basis of Inheritance: Search for genetic material and DNA as genetic, material; Structure of DNA and RNA; DNA packaging; DNA replication; Central, dogma; Transcription, genetic code, translation; Gene expression and regulationLac Operon; Genome and human genome project; DNA finger printing., , •, , Evolution: Origin of life; Biological evolution and evidences for biological, evolution from Paleontology, comparative anatomy, embryology and molecular, evidence); Darwin’s contribution, Modern Synthetic theory of Evolution;, Mechanism of evolution-Variation (Mutation and Recombination) and Natural, Selection with examples, types of natural selection; Gene flow and genetic drift;, Hardy-Weinberg’s principle; Adaptive Radiation; Human evolution., , UNIT III: Biology and Human Welfare, Details:• Health and Disease; Pathogens; parasites causing human diseases (Malaria,, Filariasis, Ascariasis. Typhoid, Pneumonia, common cold, amoebiasis, ring, worm); Basic concepts of immunology-vaccines; Cancer, HIV and AIDS;, Adolescence, drug and alcohol abuse., •, , Improvement in food production; Plant breeding, tissue culture, single cell, protein, Biofortification; Apiculture and Animal husbandry., , •, , Microbes in human welfare: In household food processing, industrial production,, sewage treatment, energy generation and as biocontrol agents and biofertilizers., , UNIT IV: Biotechnology and Its Applications, Details:• Principles and process of Biotechnology: Genetic engineering (Recombinant, DNA technology)., •, , Application of Biotechnology in health and agriculture: Human insulin and, vaccine production, gene therapy; Genetically modified organisms-Bt crops;, Transgenic Animals; Biosafety issues-Biopiracy and patents., , 27
Page 28 :
Final Syllabus for NEET-UG, UNIT V: Ecology and environment, Details:• Organisms and environment: Habitat and niche; Population and ecological, adaptations; Population interactions-mutualism, competition, predation,, parasitism; Population attributes-growth, birth rate and death rate, age, distribution., • Ecosystem: Patterns, components; productivity and decomposition; Energy flow;, Pyramids of number, biomass, energy; Nutrient cycling (carbon and, phosphorous); Ecological succession; Ecological Services-Carbon fixation,, pollination, oxygen release., •, , Biodiversity and its conservation: Concept of Biodiversity; Patterns of, Biodiversity; Importance of Biodiversity; Loss of Biodiversity; Biodiversity, conservation; Hotspots, endangered organisms, extinction, Red Data Book,, biosphere reserves, National parks and sanctuaries., , •, , Environmental issues: Air pollution and its control; Water pollution and its, control; Agrochemicals and their effects; Solid waste management; Radioactive, waste management; Greenhouse effect and global warning; Ozone depletion;, Deforestation; Any three case studies as success stories addressing environmental, issues., , 28