Page 1 :
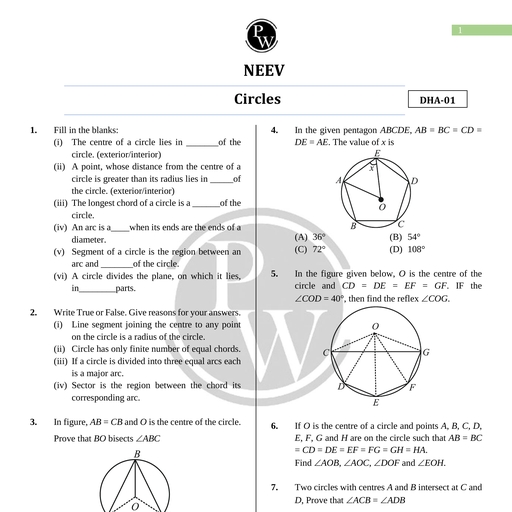
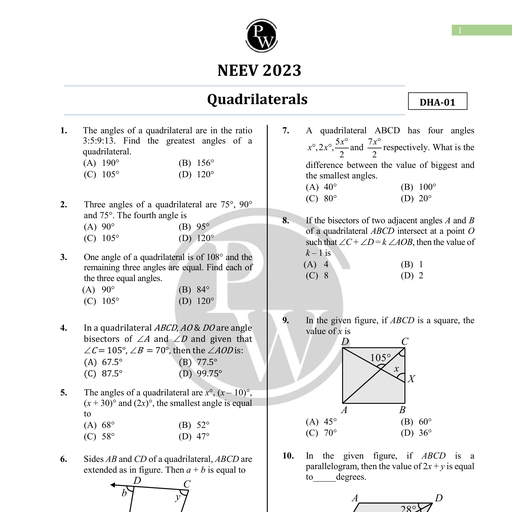
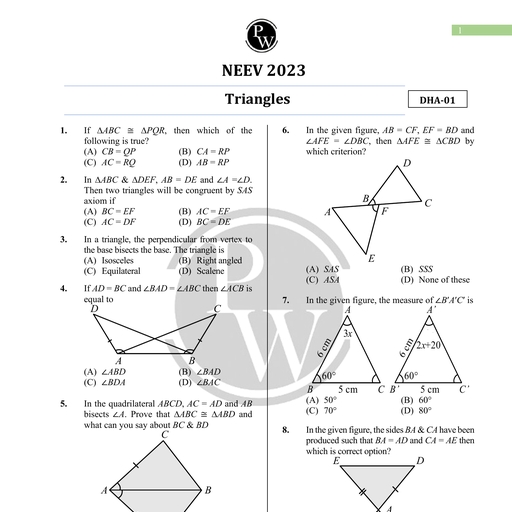
Chapter 11, , Dual Nature of Radiation, and Matter, , Chapter Contents, , e Introduction Introduction, , e Electron Emission Various phenomena like interference, diffraction and polarisation of, , light were explained by the wave nature of light. Wave nature of light, ° Pho Effect is further supported by Maxwell's equations of electromagnetism and, , e Experimental Study of production and detection of electromagnetic waves in 1986 by Hertz., Photoelectric Effect Many historic discoveries took place during the study of discharge, through gases at low pressure. The discovery of cathode ray by, , © Photoelectric Effect and William Crookes in 1879, X-rays by Roentgen in 1895 and electrons, Wave Theory of Light by J.J. Thomson in 1897 helped to understand the atomic structure., , ¢ Einstein's Photoelectric J.J. Thomson, using the mass spectrograph, experimentally, Equation : Energy Quantum determined the velocity and specific charge (charge/mass) of cathode, , of Radiation rays and predicted that the cathode rays are nothing but the stream, , Bi . of fast-moving electrons. He concluded that speed of cathode ray, © Particle Nature of Light: The oes from 0.1 to 0.2 times the speed of light and specific charge, , Enoway of cathode ray is 1.76 x 10!! C/kg, which is independent of, e Photocell (i) Nature of gas in the discharge tube (ii) Nature of material used, , as cathode means cathode ray particles are universally alike., © Wave Nature of Matter, It was found that the electrons with small velocities were emitted if, , © Davisson and Germer certain metals were irradiated by ultraviolet light or heated to high, Experiment for Wave Nature temperature. This observation suggested that electrons are, of Electron fundamental particles and are universal constituent of matter., , Millikan later on measured charge on the droplets and predicted, , Some Important Definitic, ° ani Definitions: are: on a diuplet in integral, mullipie af elementary chat of, , e Formulae Chart electron (~1.602 x 10-!® C) and concluded that the charge on a body, is quantised. From specific charge (e/m) and the value of charge (e)., © Quick Recap mass of the electron was determined (~ 9.11 x 10-3! kg)., , Photoelectric effect by Hertz, Compton effect by Compton, Stark effect, by Stark were discovered in 20 century and were explained by, quantum theory of light. According to which, the light consists of the, packets of energy. Each packet of energy is called photon, , or quantum of light (- ny =) where h is Planck's constant,, a, , Aakash Educational Services Pvt. Ltd. - Regd. Office : Aakash Tower, 8, Pusa Road, New Dethi-110005 Ph. 011-47623456
Page 2 :
182 Dual Nature of Radiation and Matter Board & Competitive Exams., , , , v is the frequency of light. c is the velocity of light and 4 is the wavelength of light and these packets of, energy travel in straight line with the speed of light. Thus, the particle nature of light was established., , Hence, it was concluded that light is of dual nature as some phenomena were explained by wave theory of, light and some by particle nature of light, In this unit, we shall study dual nature of radiation and matter., , ELECTRON EMISSION, The phenomenon of emission of electrons from the metal surface is called electron emission., , In metal, electrons are quite free to move easily within the metal. These electrons are responsible for the, conductivity of metals. These electrons in the outer shell (called valence electrons) of the atoms are loosely, bound. These loosely bound electrons are called free electrons., , However, the free electrons cannot normally leave the surface. When an electron try to come out of the metal,, the metal surface acquires a positive charge and attract the electron. The free electron is thus held inside, the metal. If it has got sufficient energy to overcome the attractive pull then only the electron can come out, of the metal surface., , Work Function : To pull out electron from the surface of the metal, a certain minimum amount of energy is, required. This minimum energy required by the electron is called the work function of the metal., , Work function is generally denoted by 0, and it depends on the properties of the metal and nature of its, surface. It is very sensitive to surface impurities., , Work function is measured in eV (electron volt). One electron volt is the energy acquired by an electron, when, it has been accelerated by 1 volt potential difference. (1 eV = 1.602 = 10-19 J), , Work function for caesium (¢, = 2.14 eV) is the lowest and highest (0, = 5.65 eV) for platinum. Work function, for some metal is given in the following table., , , , , , Cs 2.14 Al 4.28, K 2.30 Hg 4.49, Na 2.75 Cu 4.65, Ca 3.20 Ag 4.70, Mo 417 Ni 5.15, Pb 4.25 Pt 5.65, , , , Table : Work functions of some metals, , The required minimum energy for the electron emission from the metal surface can be supplied to the free, electrons by the following physical process :, , (i) Thermionic emission : The process of emission of electrons when a metal is heated is known as, thermionic emission. Sufficient thermal energy can be given to the free electrons of metal to enable them, to come out of the metal by suitably heating the metal. The emitted electrons are called thermions., Emitted number of thermions depends on the temperature of the metal surface., , (il) Field emission : The process of emission of free electrons when a strong electric field (= 108 V/m) is, applied across the metal surface is known as field emission. Field emission is also known as cold, emission or cold cathode emission. One of the examples of cold emission is spark plug., , (ili) Photoelectric emission : The process of emission of electrons when light of suitable frequency is incident, on a metal surface is known as photoelectric emission. These photo (light) - generated electrons are called, photo electrons. The number of photo electrons emitted depends on the intensity of the incident light., , (iv) Secondary emission : The process of emission of free electrons when highly energetic electron beam, is incident on a metal surface is called secondary emission. The electrons so emitted are called, secondary electrons., , Aakash Educational Services Pvt. Ltd. - Regd. Office : Aakash Tower, 8, Pusa Road, New Delhi-110005 Ph. 011-47623456
Page 3 :
Board & Competitive Exams. Dual Nature of Radiation and Matter 183, , , , PHOTOELECTRIC EFFECT, , The phenomenon of emission of electrons from (preferably) metal surface exposed to light energy of suitable, frequency is known as photoelectric effect., , The emitted electrons are called photo electrons and the current so produced is called photoelectric current., , Alkali metals (lithium, sodium, potassium, caesium etc.) show photoelectric effect with visible light, whereas, the metals like zinc, cadmium, magnesium etc. are sensitive only to ultraviolet light., , , , Hertz’s Observations, The phenomenon of photoelectric emission was discovered in 1887 by Heinrich Hertz (1857-1894) while, studying experimentally the production of electromagnetic waves by means of spark discharge. He found that, when the emitter plate was illuminated by ultraviolet light, high-voltage sparks across the detector loop were, enhanced. This observation led him to conclude that light facilitated the emission of some electrons., , From this it was concluded that when suitable radiation falls on a metal surface, some electrons near the, surface absorb enough energy from the incident radiation to overcome the attraction of the positive ions in, the material of the surface., , Hallwachs’ and Lenard’s Observations, , Wilhelm Hallwachs and Philipp Lenard studied in detail the phenomenon of photoelectric effect during, 1886-1902., , Lenard (1862—1947) observed flow of current when ultraviolet radiations is exposed on the emitter plate of an, evacuated glass tube enclosing two electrodes (metal plate). The current flow stops as soon as the ultraviolet, radiations were stopped. These observation indicate that electrons are ejected from emitter plate C, when, ultraviolet radiations fall on it which are attracted towards the positive, collector plate A by the electric field., , Thus current in the external circuit is due to light falling on the surface of the emitter., , Hallwachs and Lenard studied variation of photocurrent with collector plate potential and with frequency and, intensity of incident light., , Hallwachs, in 1888 for further study, connected a negatively charged zinc plate to an electroscope. He found, that when zinc plate was illuminated by ultraviolet light it has lost its charge. When uncharged zinc plate was, illuminated by ultraviolet light, it became positively charged. Further when positively charged zinc plate was, illuminated by ultraviolet light it was found to be further enhanced. He concluded from these observations that, under the action of ultraviolet light negatively charged particles were emitted from the zinc plate., , It became evident after the discovery of the electron in 1897 that the incident light causes electrons to be, emitted from the emitter plate. The emitted electrons due to its negative charge are pushed towards the, collector plate by the electric field. Hallwachs and Lenard also observed that when the frequency of the incident, light was smaller than a certain minimum value, no electrons were emitted at all from the emitter plate. This, minimum frequency is called the threshold frequency and it depends on the nature of the material of the emitter, plate., , It was found that some alkali metals such as lithium, sodium, potassium, caesium and rubidium were sensitive, even to visible light whereas certain metals like zinc, cadmium, magnesium etc. responded only to ultraviolet, light, having short wavelength for electron emission from the surface. Electrons are emitted, when photosensitive, substances are illuminated by light. These electrons were termed as photoelectrons after the discovery of, electrons. This phenomenon is known as photoelectric effect. The electric current constituted by photo-electrons, is known as photoelectric current., , EXPERIMENTAL STUDY OF PHOTOELECTRIC EFFECT, , The experimental set-up to study photoelectric effect is shown in figure. It consists of an evacuated glass or, quartz tube having two electrodes. The electrode ‘C’ is a photosensitive plate, which emits photoelectrons when, exposed to ultraviolet radiation. The electrode ‘A’ is a charge-collecting plate. The tube has a side window, which, will allow the light of a particular wavelength to pass through it and falls on the photosensitive plate ‘C’., , , , Aakash Educational Services Pvt. Ltd. - Regd. Office : Aakash Tower, 8, Pusa Road, New Delhi-110005 Ph. 011-47623456
Page 4 :
184 Dual Nature of Radiation and Matter Board & Competitive Exams., , , , , , , , , , Fig. : Experimental arrangement for study of photoelectric effect., , The window is made of quartz covered with a filter. The electrons collected by the plate A (collector), are emitted, by the plate C. Battery creates the electrical field between collector and emitter. The potential difference, between the plates C and A is maintained by the battery, which can be varied. From a commutator the polarity, of the plates C and A can be reversed. Thus with respect to emitter C, the plate A can be maintained at a, desired positive or negative potential. The electrons are attracted, when the collector plate A is positive with, respect to the emitter plate C. Electron emission causes flow of electric current in the circuit. Voltameter (V), measures the potential difference between the emitter and collector plates. Microammeter (A) measures the, resulting photocurrent flowing in the circuit. The current flowing in the circuit can be increased or decreased, by varying the potential between collector plate A and emitter plate C. We can also vary the intensity and, frequency of the incident light., , To study the variation of photocurrent with (a) intensity of radiation (b) frequency of incident radiation (c) the, potential difference between the plates A and C, and (d) the nature of the material of plate C, the experimental, arrangement of above figure is used., , To get different frequency of light falling on the emitter C, suitable-coloured filter or coloured glass is used., The change in distance of light source from the emitter varies the intensity of light., , Effect of Intensity of Light on Photocurrent, To attract ejected electron from C towards collector A, the collector A is maintained at a positive potential, with respect to emitter C. The intensity of light is varied, keeping the frequency of the incident radiation and, , the accelerating potential fixed and the resulting photoelectric current is measured each time. It is observed, that the photocurrent increases linearly with intensity of incident light as shown in the figure., , !, j, L<_, , Fig.: Variation of Photoelectric current with intensity of light., , As we know the photocurrent is directly proportional to the number of photo electrons emitted per second, so, the number of photo electrons emitted per second is directly proportional to the intensity of the incident radiations., , Aakash Educational Services Pvt. Ltd. - Regd. Office : Aakash Tower, 8, Pusa Road, New Dethi-110005 Ph. 011-47623456
Page 5 :
Board & Competitive Exams. Dual Nature of Radiation and Matter 185, , , , Effect of Potential of Photoelectric Current, , Illuminate the plate C with radiation of fixed frequency (greater than threshold frequency) and fixed intensity,, keeping the plate A at some accelerating positive potential with respect to plate C. Now we gradually vary, the positive potential of plate A and measure the resulting photocurrent each time. For fixed frequency and, fixed intensity of incident light, this photoelectric current increases with the increase in applied positive potential, of plate ‘A’. The photoelectric current has the maximum value, when all the photoelectrons emitted by, electrode ‘C’ reach the plate ‘A’. This maximum current is known as saturation current. Further increase in, accelerating potential of plate A does not increase the current., , When the polarity is reversed (meaning applying a negative (retarding) potential to the plate A with respect, to the plate C) and increase retarding potential gradually, then electrons are repelled. The photocurrent is found, to decrease rapidly and at a certain, sharply defined critical value of the negative potential V, on the plate, A, it drops to zero. The minimum negative (retarding) potential V, given to the plate A, for a particular frequency, of incident radiation, for which the photocurrent stops or becomes zero is called the cut-off or stopping, potential. At this stage photo electrons of maximum kinetic energy (the fastest photoelectron) cannot reach, the plate A, therefore, , K =eV, (Knax iS Maximum kinetic energy of photoelectron), , When we repeat this experiment with different intensity /,, /, and /, (/, > /, > /,) of incident radiation and of, the same frequency, we observe that the saturation currents have reached to higher values which implies that, more electrons are emitted in a unit time, proportional to the intensity of incident radiation but there is no, change in stopping potential for a given frequency of the incident radiation, graphically shown in figure., , , , =v,, <+— Retarding potential Collector plate —>, Potential, , Fig.: Variation of photocurrent with collector plate potential for different intensity of incident radiation., , Thus stopping potential is independent of its intensity for a given frequency of the incident radiation or the, maximum kinetic energy of photo electrons is independent of intensity of incident radiation but depends on, frequency (color) of the light source and the emitter plate material., , Effect of Frequency of Incident Radiation on Stopping Potential, , We now study the relation between stopping potential V, and the frequency v of the incident radiation. The, resulting variation of photocurrent with collector plate potential for same intensity of light radiation at various, frequencies is shown in figure., , ‘Saturation current, , , , , , , , Ves —Ve2 —Vor Collector plate potential—>, , +— Retarding potential, Fig.: Variation of photoelectric current with collector plate potential for different frequencies of incident, radiation., , Aakash Educational Services Pvt. Ltd. - Regd. Office : Aakash Tower, 8, Pusa Road, New Delhi-110005 Ph. 011-47623456