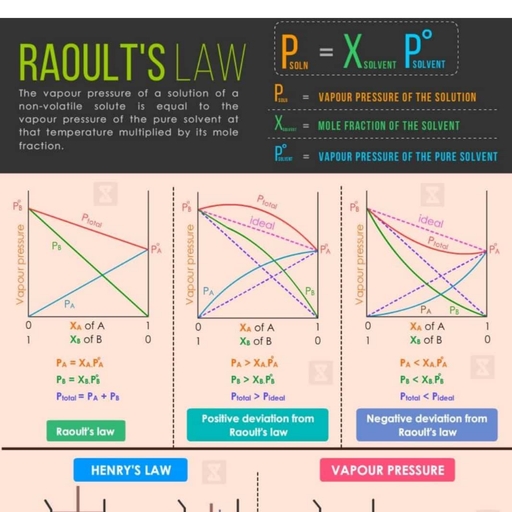
Page 1 :
p-BLOCK ELEMENTS, , p-BLOCK ELEMENTS, Introduction :, Group 13 to 18 of the periodic table of elements constitute the p–block., The p–block contains, metals, metalloids as well as non–metals., The p–block elements have general valence shell electronic configuration ns2 np1–6., The first member of each group from 13–17 of the p–block elements differ in many respects from the other, members of their respective groups because of small size, high electronegativity and absence of d–orbitals., The first member of a gorup also has greater ability to form pS–pS multiple bonds to itself (e.g. C = C, C {, C, N { N) and to element of second row (e.g C = O, C = N, C { N, N = O) compared to the other members of, the same group., The highest oxidation of p–block element is equal to the group number minus 10. Moving down the group,, the oxidation state two less than the highest group oxidation state becomes more stable in groups 13 to 16 due to, inert pair effect (reluctance of s-subshell electrons to participate in chemical bonding), TRENDS IN PROPERTIES OF p-BLOCK ELEMENTS., Electronegativity,, ionization enthalpy,, oxidizing power., , Covalent radius,, van der Waals' radius,, metallic character, , B, , C, , N, , O, , F, , Ne, , Al, , Si, , P, , S, , Cl, , Ar, , Ga, , Ge, , As, , Se, , Br, , Kr, , In, , Sn, , Sb, , Te, , I, , Xe, , Tl, , Pb, , Bi, , Po, , At, , Rn, , Electronegativity,, enthalpy of atomization, (except for N2, O2, F2),, ionization enthalpy,, oxidizing power., , Covalent radius,, van der Waals' radius,, enthalpy of atomization, (upto group 14),, metallic character, , (A) GROUP 13 ELEMENTS : THE BORON FAMILY, Boron is a typical non-metal, aluminium is a metal but shows many chemical similarities to boron , and gallium,, indium and thallium are almost exclusively metallic in character,, Electronic Configuration:, The outer electronic configuration of these elements is ns2 np1., Atomic Radii :, On moving down the group, for each successive member one extra shell of electrons is added and therefore, atomic, radius is expected to increases. Atomic radius of Ga is less than of Al. The presence of additional 10 d-electrons, offer only poor screening effect for the outer electrons from the increased nuclear charge in gallium. Consequentlym,, the atomic redius of gallium (135 pm) is less than that of aluminium (143 pm)., Ionization Enthalpy:, The ionisation enthalpy values as expected from the general trends do not decrease smoothly down the group . The, decreases from B to Al is associated with increases in size. The observed discontinuity in the ionisation enthalpy, values between Al and Ga, and between In and TI are due to inability of d- and f electrons, which have low screening, effect, to compensate the increase in nuclear charge. The sum of the first three ionisation enthalpies for each of the, elements is very high .
Page 2 :
p-BLOCK ELEMENTS, , Electronegativity:, Down the group, electronegativity first decreases from B to Al and then increases marginally. This is because of the, discrepancies in atomic size of the elements., Physical Properties, Boron is non-metallic in nature . It is extremely hard and black coloured solid. It exists in many allotropic forms., Due to very strong crystalline lattice, boron has unusually high melting point. Rest of the member are soft metals, with low melting point and high electrical conductivity. Gallium with low melting point (303 K), could exist in liquid, state during summer. Its high boiling point (2676 K) makes it a useful material for measuring high temperatures., Density of the elements increases down the group from boron to thallium., , Atomic & physical properties :, , Element, Atomic Number, , B, , Al, , Ga, , In, , Tl, , 5, , 13, , 31, , 49, , 81, , 10.81, , Atomic Mass, , 2, , Electronic configuration, , [He] 2s 2p, , Ionic Radius M, , Electronegativity, –3, , Density/[g cm, , (293 K)], , [Ne] 3s 3p, , 69.72, 1, , [Ar] 3d, , 10, , 114.82, 2, , 4s 4p, , 1, , [Kr] 4d, , 10, , 2, , 5s 5p, , 204.38, 1, , [Xe] 4f, , 14, , 5d10 6s26p1, , 143, , 135, , 167, , 170, , –, , 53.5, , 62, , 80, , 88.5, , ,, , 800, , 577, , 578, , 558, , 590, , ,,, , 2427, , 1816, , 1979, , 1820, , 1971, , ,,,, , 3659, , 2744, , 2962, , 2704, , 2877, , 2.0, , 1.5, , 1.6, , 1.7, , 1.8, , 2.35, , 2.70, , 5.90, , 7.31, , 11.85, , / pm, , Ionization enthalpy, / (kJ mol–1), , 2, , 85, , Atomic Radius / pm, 3+, , 26.98, 1, , Melting point / K, , 2453, , 933, , 303, , 430, , 576, , Boiling point / K, , 3923, , 2740, , 2676, , 2353, , 1730, , Chemical Properties :, Oxidation state and trends in chemical reactivity :, Due to small size of boron, the sum of its first three ionization enthalpies is very high. This prevents it to form + 3, ions and force it to form only covalent compounds. But as we move from B to Al, the sum of the first three ionisation, enthalpies of Al considerably decreases, and is therefore able to form Al+3 ions. However, down the group, due to, poor shielding, effective nuclear charge holds ns electrons tightly (responsible for inert pair effect) and thereby,, restricting their participation in bonding. As a result of this only p-orbital electron may be involved in bonding. In fact, in Ga, In and Tl, both + 1 and + 3 oxidations states are observed. The relative stability of + 1 oxidations state, progressively increases for heavier elements: Al < Ga < In < Tl. In thallium +1 oxidation state is predominant and, + 3 oxidation state highly oxidising in character. The compound in +1 oxidation state , as expected from energy, considerations, are more ionic than those in + 3 oxidations state., In trivalent state, the number of electrons around the central atom in a molecule of the compounds of these, elements (e.g., boron in BF3) will be only six., Such electron deficient molecules have tendency to accept a pair of electrons to achieve stable electronic, configuration and thus, behave as Lewis acids. The tendency to behave as Lewis acid decreases with the increases, in the size down the group. BCl3 easily accepts a lone pair of electrons from ammonia to form BCl3. NH3. In trivalent, state most of the compounds being covalent are hydrolysed in water. The trichloride on hydrolysis in water form, tetrahedral [ M (OH)4]– species; Aluminium chloride in acidified aqueous solution form octahedral [ Al(H2O)6]3+ ion., (i), , Reactivity towards air, Boron is unreactive in crystalline form. Aluminium forms a very thin oxide layer on the surface which protects the, metal from further attack. Amorphous boron and aluminium metal on heating in air form B2O3 and Al2O3 respectively., With dinitrogen at high temperature they form nitrides., ', ', 2E(s) + 3 O2 (g) o 2 E2O3(s) ; 2E(s) + N2(g) o 2 EN (s).
Page 3 :
p-BLOCK ELEMENTS, The nature of these oxides varies down the group. Boron trioxide is acidic and reacts with basic (metallic) oxides, forming metal borates. Aluminium and gallium oxides are amphoteric and those of indium and thallium are basic in, their properties., (ii), , Reactivity towards acids and alkalies, Boron does not react with acids and alkalies even at moderate temperature; but aluminium dissolves in mineral, acids and aqueous alkalies and thus shows amphoteric character., Aluminimum dissolved in dilute HCl and liberates dihydrogen. However, concentrated nitric acid renders aluminium, passive by forming protective oxide layer on the surface . Aluminium also reacts with aqueous alkali and liberates, dihydrogen ., 2 Al(s) + 6 HCl(aq) , o 2 Al3+ (aq) + 6 Cl–(aq) + 3 H2(g), 2Al(s) + 2NaOH (aq) + 6H2O (1) o 2Na+ [Al(OH)4]– (aq) + 3H2(g), Sodium tetrahydroxoaluminate (III), (iii), Reactivity towards halogens, These elements react with halogen to form trihalides (except Tl I3 ).., 2E(s) + 3X2 (g) o 2EX3 (s), (X = F, Cl Br, I), IMPORTANT TRENDS AND ANOMALOUS PROPERTIES OF BORON, The tri-chlordes, bromides and iodies of all these elements being covalent in nature are hydrolysed in water., Species like tetrahedral [M(OH)4]– and octahedral [M(H2O)6]3+ ,except in boron, exist in aqueous medium., It is due to the absence of d orbitals that the maximum covalence of boron is 4. Since the d-orbitals are available, with Al and other elements, the maximum covalence can be expected beyond 4., , �z, , BORON (B):, , , OCCURRENCE :, , (i), (iii), , Boron occurs in nature in the form of the following minerals:, Borax (Na+)2B4O72- .10H2O.(Boron is part of an anionic complex), (ii) Boric acid H3BO3,, Kernite Na2B4O7 . 4H2O & (iv) Colemanite Ca2 B6O11. 5H2O, , , , EXTRACTION OF BORON :, , (i), , By the reduction of B2O3 with magnesium, sodium or potassium in the absence of air :, Na2B4O7 + 2HCl + 5H2O o 4H3BO3 + 2NaCl, ', B2O3 + 3Mg o 2B + 3MgO, 2H3BO3 o, B2O3 + 3H2O ;, The product thus obtained is boiled with HCl and filtered when K2O or MgO dissolves leaving, behind elemental boron. It is thoroughly washed to remove HCl and then dried finally., Brown amorphous powder of B is obtained in this way., , (ii), , From potassium fluoroborate (KBF4) by heating it with potassium metal., ', KBF4 + 3K o, 4KF + B., It is then treated with dilute HCl to remove KF and B is then washed and dried., , (iii), , In small quantities in pure form (crystalline boron) by the, (i) Reduction of BBr3 with H2 on a heated titanium metal filament at 1275-1475 K, The vapours of Br2 are absorbed in Cu and the residual vapours of boron are condensed., (ii) Decomposition of BI3 vapours by means of high tension arc (80 kV) through tungsten, electrodes., 2BI3 o 2Bn + 3 I2n, , (Van Arkel mehod).
Page 5 :
p-BLOCK ELEMENTS, , , , PREPARATION :, , (i), , It is precipitated by treating a concentrated solution of borax with sulphuric acid., Na2B4O7 + H2SO4 + 5H2O o Na2SO4 + 4H3BO3 p, , (ii), , From Colemanite: Powdered colemanite is suspended in water and excess SO2 is passed through it., On filtering and cooling the filtrate, white crystals of H3BO3 are obtained., Ca2B6O11 + 2SO2 + 11H2O o 2Ca(HSO3)2 + 6H3 BO3, , , , PROPER TIES:, , (i), , It is a weak monobasic acid and in aqueous solution the boron atom completes its octet by, removing OH- from water molecules:, B(OH)3(aq) + 2H2O(A) o B(OH)4– (aq) + H3O+(aq)., It, therefore, functions as a Lewis acid and not as a proton donor., It behaves as strong acid when a polyhydroxy compound such as glycol or glycerol is added to its, aqueous solution. The acidity is due to the high stability of the conjugate bone chelate complex., , Ethanol does not form similar complex but catechol, salicylic acids form similar complexes., , When heated it first forms metaboric acid (HBO2) and then boron trioxide., , Orthoboric acid is greasy to touch less soluble in cold water but more soluble in hot water. It has a layered, structure in which planar BO3 units are joined by hydrogen bonds.
Page 9 :
p-BLOCK ELEMENTS, , (ix), , Reduction of oxides of metals: When oxides of less reactive metal than aluminium is heated with, aluminium, the other metal is liberated., ', 2Al2O3 + 3Mn;, 3MnO2 + 4Al + o, , , , ', Cr2O3 + 2Al + o, Al2O3 + 2Cr, , , , USES :, , (i), (ii), (iii), (iv), , It is extensively used, for manufacture of cooking and household utencils., as aluminium plating for tanks, pipes, iron bars and other steel objects to prevent corrosion., for manufacture of aluminium cables., for making precision instruments, surgical apparatus, aircraft bodies, rail coaches, motorboats, car, , COMPOUNDS OF ALUMINIUM :, , �� ALUMINIUM OXIDE (Al 2 O 3 ) :, , It is also called alumina. It occurs in nature in the form of bauxite and corundum. It is also found in the form, of gems. Some important aluminium oxide gems are:, (A) Topaz-yellow, (B) Sapphire-blue, (C) Ruby-red, (D) Amethyst-violet, (E) Emerald-green, , , PREPARATION:, , Pure Al2O3 is obtained by igniting Al2(SO4)3, Al(OH)3 or ammonium alum., ', Al2O3 + 3SO3n ;, Al2(SO4)3 + o, , ', Al2O3 + 3H2On, 2Al(OH)3 + o, , ', (NH4)2SO4.Al2(SO4)3.24H2O o, 2NH3n + Al2O3 + 4SO2 n + 25H2On, , , , PROPERTIES :, It is a white amorphous powder insoluble in water but soluble in acids (forming eg., AlCl3) as well as, alkalies (forming NaAlO2) , Thus amphoteric in nature. It is a polar covalent compound., , , , USES :, , (i), (ii), (iii), (iv), (v), , It is used, for the extraction of aluminium., for making artificial gems., for the preparation of compounds of aluminium., in making furnace linings. It is a refractory material., as a catalyst in organic reactions., , , , ALUMINIUM CHLORIDE (AlCl 3 .6H 2 O) :, , It is a colourless crystalline solid, soluble in water. It is covalent. Anhydrous AlCl3 is a deliquescent white, solid., , , PREPARATION :, , (i), , By dissolving aluminium, Al2O3, or Al(OH)3 in dilute HCl :, , 2Al + 6HCl o 2AlCl3 + 3H2n�, , Al2O3 + 6HCl o 2AlCl3 + 3H2O; Al(OH)3 +, 3HCl o AlCl3 + 3H2O, The solution obtained is filtered and crystallized when the crystals of AlCl3.6H2O are obtained., , (ii), , Anhydrous AlCl3 is obtained by the action of Cl2 on heated aluminium., , (iii), , By heating a mixture of Al2O3 and coke and passing chlorine over it., Al2O3 + 3C + 3Cl2 o 2AlCl3 (anhydrous) + 3COn
Page 10 :
p-BLOCK ELEMENTS, , , , PROPERTIES :, , (i), , Action of heat:, Hydrated salt when heated strongly is converted to Al2O3., , (ii), , (iii), , (iv), , (v), , (vi), , ', Al2O3 + 6HCln + 9H2O, 2AlCl3.6H2O o, Action of moisture on anhydrous AlCl3:, When exposed to air, anhydrous AlCl3 produces white fumes of HCl, AlCl3 + 3H2O Al(OH)3 + 3HCln, , Action of NH3:, Anhydrous AlCl3 absorbs NH3 since the latter is a Lewis acid., AlCl3 + 6NH3 o AlCl3.6NH3 (white solid), Action of NaOH solution:, When NaOH solution is added dropwise to an aqueous AlCl3 solution, a gelatinous precipitate of, Al(OH)3 is first formed which dissolves in excess of NaOH solution to give a colourless solution of, sodium aluminate., AlCl3 + 3NaOH o Al(OH)3p + 3NaCl ;, Al(OH)3 + NaOH o NaAlO2 + 2H2O, This reaction is important as a test to distinguish between an aluminium salt from salts of Mg, Ca, Sr,, and Ba. (When NaOH solution is added to their salt solutions, a white precipitate of hydroxide, forms which does not dissolve in excess of NaOH)., Action of NH4OH solution:, When NH4OH solution is added to a solution of AlCl3, a white precipitate of Al(OH)3 is formed, which does not dissolve in excess of NH4OH., AlCl3 + 3NH4OH o Al(OH)3 p�(white gelatinous) + 3NH4Cl, This reaction is important as a test to distinguish an Al salt from a Zn salt. (With a Zn salt a white, precipitate of Zn(OH)2 is formed which dissolves in excess of NH4OH solution)., Hydrolysis with water:, When AlCl3 is dissolved in water, it undergoes hydrolysis rapidly to produce Al(OH)3 which is a, weak base and HCl which is a strong acid. Hence the solution is acidic to litmus., [Al(H2O)5OH]+2 + H+, [Al(H2O)6]3+, The complex cation has a high tendency to get dimerised., 2[Al(H2O)5OH]2+ o [(H2O)4, , (vii), , 4LiH + AlCl3 o LiAlH4 + 3LiCl, , , , USES :, , (H2O)4 ]+4 + 2H2O, , It is used :, (i) as catalyst for cracking of petroleum., (ii) as catalyst in Friedel-Crafts reactions., (iii) for preparing aluminium compounds., , �, , ALUMS ; M 2 SO 4 . M, Mcc 2 (SO 4 ) 3 . 24H 2 O OR MMc, MM c (SO 4 ) 2 . 12H 2 O, , Alums are transparent crystalline solids having the above general formula where M is a univalent metal or, positive radical and Mc is a trivalent metal. Some important alums are:, (i) Potash alum K2SO4 . Al2(SO4)3 . 24H2O (ii) Chrome alum K2SO4 . Cr2(SO4)3 . 24H2O, (iii) Ferric alum K2SO4 . Fe2(SO4)3. 24H2O (iv) Ammonium alum (NH4)2SO4 . Al2(SO4)3 . 24H2O, Alums are double salts which when dissolved in water produce metal ions(or ammonium ions) and the, sulphate ions.
Page 11 :
p-BLOCK ELEMENTS, , , , PREPARATION :, , Alums can be prepared by fusing M2SO4 & M’2(SO4)3 in 1 : 1 molar ratio & the resulting mass is, dissolved into water. From the solution thus obtained, alums are crystallised., , , USES :, It is used, , (i), (ii), , as a mordant in dye industry, as a germicide for water purification, , (iii), , as a coagulating agent for precipitating colloidal impurities from water., , (B) GROUP 14 ELEMENTS : THE CARBON FAMILY, Carbon (C), silicon (Si), germanium (Ge), tin (Sn) and lead (Pb) are the members of group 14. Naturally occurring, carbon contains two stable isotopes:12C and 13C. In addition to these third isotopes, 14C is also presents , it is a, radioactive isotope with half-life 5770 years and used for radiocarbon dating. Silicon is a very important component, of ceramices, glass and cement. Germanium exists only in traces. Tin occurs mainly as cassiterite, SnO2 and, lead as galena, PbS. Ultrapure form of germanium and silicon are used to make transistors and semiconductor, devices., Electronic Configuration :, The valence shell electronic configuration of these elements is ns2 np2., Covalent Radius :, There is a considerable increase in covalent radius from C to Si, thereafter from Si to Pb a small increase in radius, is observed. This is due to the presence of completey filled d and f orbitals in heavier members., Ionization Enthalpy :, The first ionization enthalpy of group 14 members is higher than the corresponding members of group 13. The, influence of inner core electron is visible here also. In general the ionisation enthalpy decreases down the group.Small, decreases in 'iH from Si to Ge to Sn and slight increase in 'iH from Sn to Pb is the consequence of poor shielding, effects of intervening d and f–orbitals and increases in size of the atom., Electronegativity :, Due to small size, the elements of this group are slightly more electronegative than group 13 elements. The, electronegativity value for elements from Si to Pb are almost the same., Physical Properties :, All group 14 members are solids. Carbon and silicon are non-metals, germanium is metalloid whereas tin and lead, are soft metals with low melting points. Melting points and boiling points of group 14 elements are much higher than, those of corresponding elements of group 13.
Page 12 :
p-BLOCK ELEMENTS, , ATOMIC & PHYSICAL PROPERTIES, Element, , C, , Si, , Ge, , Sn, , Pb, , Atomic Number, , 6, , 14, , 32, , 50, , 82, , Atomic Mass, , 12.01, , 28.09, , 72.60, , 118.71, , 207.2, , Electronic configuration, , [He] 2s 2p, , Atomic Radius / pm, , 77, , 118, , 122, , 140, , 146, , –, , 40, , 53, , 69, , 78, , ,, , 1086, , 786, , 761, , 708, , 715, , ,,, , 2352, , 1577, , 1537, , 11411, , 1450, , ,,,, , 4620, , 3228, , 3300, , 2942, , 3081, , Electronegativity, , 2.5, , 1.8, , 1.8, , 1.8, , 1.9, , Melting point / K, , 4373, , 1693, , 1218, , 505, , 600, , Boiling point / K, , –, , 3550, , 3123, , 2896, , 2024, , Ionic Radius M, , +4, , 2, , / pm, , Ionization enthalpy, / (kJ mol–1), , 2, , 2, , [Ne] 3s 3p, , 2, , 10, , 2, , [Ar] 3d 4s 4p, , 2, , [Kr] 4d, , 10, , 2, , 5s 5p, , 2, , [Xe] 4f, , 14, , 5d, , 10, , 2, , 6s 6p, , 2, , Chemical Properties :, Oxidation states and trends in chemical reactivity :, The group 14 elements have four electrons in outermost shell. The common oxidation states exhibited by these, elements are + 4 and + 2. Carbon also exhibits negative oxidation states.Since the sum of the first four ionization, enthalpies is very high, compound in +4 oxidation state are generally covalent in nature. In heavier members the, tendency to show +2 oxidation state increases in the sequence Ge < Sn < Pb. It is due to the inability of ns2, electrons of valence shell to participate in bonding. The relative stabilities of these two oxidation states vary down, the group. Carbon cannot exceed its covalence more than 4. Other elements of the group can do so. It is because, of the presence of d orbital in them. Due to this, their halides undergo hydrolysis and have tendency to form, complexes by accepting electron pairs from donor species. For example, the species like ,SiF62– . [GeCl6]2–, ,[Sn(OH)6]2– exist ., (i), , Reactivity towards oxygen :, All members when heated in oxygen form oxides. There are mainly two types of oxides, i.e. monoxide and dioxide, of formula MO and MO2 respectively. SiO only exists at high temperature. Oxides in higher oxidation states of, elements are generally more acidic than those in lower oxidation state. The dioxides – CO2, SiO2 and GeO2 are, acidic, whereas SnO2 and PbO2 are amphoteric in nature. Among monoxides, CO is neutral, GeO is distinctly, acidic whereas SnO and PbO are amphoteric ., , (ii), , Reactivity towards water :, Carbon , silicon and germanium are not affected by water . Tin decomposes steam to form dioxide and dihydrogen, gas. Lead is unaffected by water, probably becauses of a protective oxide film formation., , (iii), , Reactivity towards halogen :, These elements can form halides of formula MX2 and MX4 (where X = F, Cl Br, I). Except carbon all other members, react directly with halogen under suitable condition to make halides. Most of the MX4 are covalent in nature., Exceptions are SnF4 and PbF4, which are ionic in nature . PbI4 does not exist because Pb–I bond initially formed, during the reaction does not release enough energy to unpair 6s2 electrons and excite one of them to higher orbital, to have four unpaired electrons around lead atom. Heavier members Ge to Pb are able to make halides of formula, MX2 . Stability of dihalides increases down the group. Except CCl4 other tetrachlorides are easily hydrolysed by, water because the central atom can accommodate the lone pair of electrons from oxygen atom of water molecules, in d orbital.
Page 13 :
p-BLOCK ELEMENTS, IMPORTANT TRENDS AND ANOMALOUS BEHAVIOUR OF CARBON, Like first member of other groups, carbon also differs from rest of the members of its group. It is due to its smaller, size, higher electronegativity, higher ionisation enthalpy and unavailability of d orbitals. Accommodate only four, pairs of electrons around it. This would limit the maximum covalence to four whereas other members can expand, their covalence due to the presence of d orbitals, Carbon also has unique ability to form pS-pS multiple bonds with, itself and with other atoms of small size and high electronegativity. Few example of multiple bonding are C = C, C, {�C, C = O C = S and C {�N. Heavier elements do not form pS-pS bonds because their atomic orbital are too large, and diffuse to have effective overlapping ., Carbon atoms have the tendency to link with one another through covalent bonds to form chains and rings. This, property is called catenation. This is becauses C–C bonds are very strong. Down the group the size increases, tendency to show catenation decreases. This can be clearly seen from bond enthalpies values. The order of, catenation is C > > Si > Ge | Sn. Lead does not show catenation. Due to the property of catenation and pS-pS bonds, formation, carbon is able to show allotropic forms., , ALLOTROPES OF CARBON, Carbon exhibits many allotropic forms; both crystallic as well as amorphous. Diamond and graphite are two wellknown crystalline forms of carbon. In 1985 third form of carbon known as fullerenes was discovered by H.W. Kroto,, E Smalley and R.F.Curl., Diamond :, It has a crystalline lattice. In diamond each carbon atom undergoes sp3 hybridisation and linked to four other carbon, atoms by using hybridised orbitals in tetrahedral fashion. The C–C bond length is 154 pm. The structure extends in, space and produces a rigid three dimensional network of carbon atoms .In this structure directional covalent bonds, are presents throughout the lattice., It is very difficult to break extended covalent bonding and therefore, diamond is a hardest substance on the earth. It, is used as an abrasive for sharpening hard tools in making dyes and in the manufacture of tungsten filament for, electric light bulbs., Graphite :, Graphite has layered structure. Layers are held by van der Waal’s forces and distance between two layers is 340, pm. Each layer is composed of planar hexagonal rings of carbon atoms. C – C bond length within the layer is 141.5, pm Each carbon atom in hexagonal ring undergoes sp2 hybridisation and make three sigma bonds with three, neighbouring carbon atoms. Fourth electron forms a S bond. The electrons are delocalised over the whole sheet., Electrons are mobile and , therefore graphite conducts electricity along the sheet. Graphite cleaves easily between, the layers and therefore, it is very soft and slippery. For this reason graphite is used as a dry lubricant in machines, running at high temperature, where oil cannot be used as a lubricant., Fullerenes :, Fullerenes are made by the heating of graphite in an electrical arc in the presence of inert gases such as helium or, argon. Fullerences are the only pure form of carbon becauses they have smooth structure without having ‘dangling’, bonds. Fulleren are cage like molecules. C60 molecule has a shape like soccer ball and called Buckminsterfullerene., It contains twenty six -membered rings and twelve five membered rings. A six membered ring is fused with six or five, membered rings but a five membered ring can only fuse with six membered rings. All the carbon atoms are equal, and they undergo sp2 hybridisation. Each carbon atom forms three sigma bonds with other three carbon atoms. The, remaining electron at each carbon atom is delocalised in molecular orbitals, which in turn give aromatic character, to molecule. This ball shaped molecule has 60 vertices and each one is occupied by one carbon atom and it also, contains both single and double bonds with C – C distance of 143.5 pm and 138.3 pm respectively. Spherical, fullerenes are also called bucky balls in short. It is very important to know that graphite is thermodynamically most, stable allotrope of carbon and, therefore, 'fH(–) values of diamond and fullerene, C60 are 1.90 and 38.1 kJ mol–1,, respectively. Carbon black is obtained by burning hydrocarbons in a limited supply of air.
Page 14 :
p-BLOCK ELEMENTS, , Uses of carbon :, Graphite fibres embedded in plastic material form high strength, lightweight composites. The composites are used, in products such as tennis rackets, fishing rods, aircrafts and canoes. Being good conductor, graphite is used for, electrodes in batteries and industrial electrolysis. Crucibles made from graphite are inert to dilute acids and alkalies. Being highly porous, activated charcoal is used in adsorbing poisonous gases; also used in water filters to, remove organic contaminators and in air conditioning system to control odour. Carbon black is used as black, pigment in black ink and as filler in automobile tyres. Coke is used as a fuel and largely as a reducing agent in, metallurgy. Diamond is a precious stone and used in jewellery. It is measured in carats (1 carat = 200 mg.)., , , , PROPERTIES OF CARBON :, , (i), , Carbon in any form will react with oxygen at a sufficiently high temperature to give carbon dioxide;, in a deficiency of oxygen, carbon monoxide is formed as well., , (ii), , C(s) + 2S(s) o CS2(l), , (iii), (iv), , C(s) + 2F2(g) o CF4(g), It will reduce steam, forming water gas, and many oxides of metals; these reductions are of industrial, importance., ', C + H2O(g) o, CO + H2 ; Fe2O3 + 3C o 2Fe + 3CO, It is not attacked by dilute acids, but concentrated nitric acid and sulphuric acid are reduced if, warmed with carbon according to the equations:, , (v), , (iii) Ca(s) + 2C(s) o CaC2(s), , C(s) + 4HNO3(aq) o 2H2O(l) + 4NO2(g) + CO2(g) ;, + CO2(g), z, , OXIDES OF CARBON :, , �, , CARBON DIOXIDE (CO 2 ) :, , C(s) + 2H2SO4(l) o 2H2O(l) + 2SO2(g), , , , PREPARATION :, , (i), , In the laboratory it can be conveniently made by the action of dilute hydrochloric acid on marble, chips:, CO32-(aq) + 2H+(aq) o CO2(g) + H2O(l), Industrially it is produced as a by-product during the manufacture of quicklime and in fermentation, processes:, CaCO3(s) o CaO(s) + CO2(g) ; C6H12O6(aq){glucose}o 2C2H5OH(aq) + 2CO2(g), , (ii), , , , PROPER TIES:, , (i), , It is a colourless, odourless and heavy gas which dissolves in its own volume of water at ordinary, temperature and pressure. Like all gases, it dissolves much more readily in water when the pressure, is increased and this principle is used in the manufacture of soda water and fizzy drinks., , (ii), , CO2 is easily liquefied (critical temperature = 31.1oC) and a cylinder of the gas under pressure is a, convenient fire extinguisher. When the highly compressed gas is allowed to expand rapidly solid, carbon dioxide (‘dry ice’) is formed. Solid carbon dioxide sublimes at –78oC and, since no massy, liquid is produced, it is a convenient means of producing low temperatures.
Page 15 :
p-BLOCK ELEMENTS, , (iii), , Carbon dioxide is the acid anhydride of carbonic acid, which is a weak dibasic acid and ionises in to, steps as follows :, H2CO3(aq) + H2O (l) (reversible) HCO3– (aq) + H3O+ (aq), HCO3– (aq) + H2O (l) (reversible) CO32– (aq) + H3O+ (aq), H2CO3 / HCO3– buffer system helps to maintain pH of blood between 7.26 to 7.42., A solution of carbonic acid in water will slowly turn blue litmus red and when the solution is boiled,, all the CO2 is evolved., , (iv), , Carbon dioxide readily reacts with alkalies forming the carbonate and, if CO2 is in excess, the, hydrogen carbonate. This is the basis of the lime-water test for CO2 gas., Ca(OH)2(aq) + CO2(g) o CaCO3(s) + H2O(liq) ;, CaCO3(s) + H2O(liq) + CO2(g) o Ca(HCO3)2(aq), The above reaction accounts for the formation of temporarily hard water., , (v), , Carbon dioxide, which is normally present to the extent of ~ 0.03% by volume in the atmosphere, is, removed from it by the process known as photosynthesis. It is the process by which green plants, convert atmospheric CO2 into carbohydrates such as glucose. The overall chemical change can be, expressed as :, hv, 6 CO2 + 12 H2O o, C6H12O6 + 6 O2 + 6 H2O, Chlorphyll, , (vi), �, , By this process plants make food for themselves as well as for animals and human beings.But the, increase in combustion of fossil fuels and decomposition of limestone for cement manufacture in, recent years seem to increase the CO2 content of the atmosphere. This may lead to increase in, green house effect and thus, raise the temperature of the atmosphere which might have serious, consequences., Gaseous CO2 is extensively used to carbonate soft drinks. Being heavy and non–supporter of, combustion it is used as fire extinguisher. A substantial amount of CO2 is used to manufacture urea., , CARBON MONOXIDE (CO) :, , PREPARATION:, , (i), , It forms together with CO2, when carbon or carbonaceous matter is oxidized by air or oxygen. It is, also produced when CO2 is reduced by red- hot carbon; this reaction is of importance in metal, extractions., C(s) + CO2(g) o 2CO(g), , (ii), , In the laboratory it can be prepared by dehydrating methanoic acid with concentrated sulphuric acid:, o CO(g) + H2O, HCOOH (liq) , conc .H SO, 373 K, 2, , (iii), , 4, , If oxalic acid is dehydrated in the same way, CO2 is formed as well., conc . H2SO 4 , ', o CO + CO, H2C2O4 –H, 2, O, 2, , (iv), , On commercial scale it is prepared by the passage of steam over hot coke. The mixture of CO and, H2 thus produced is known as water gas or synthesis gas.sss
Page 16 :
p-BLOCK ELEMENTS, 473 �1273 K, C (s) + H2O (g) , o CO (g) + H2(g) (water gas)., When air is used instead of steam, a mixture of CO and N2 is produced, which is called producer, gas., 1273 K, o 2 CO (g) + 4 N2 (g) (Producer gas)., 2 C (s) + O2 (g) + 4 N2 (g) , Water gas and producer gas are very important industrial fuels. Carbon monoxide in water gas or, producer gas can undergo further combustion forming carbon dioxide with the liberation of heat., , (v), , Zn + CO2 o ZnO + CO, , (vi), (vii), , ', K4Fe(CN)6 + 6H2SO4 (conc.) + 6H2O o, 2K2SO4 + FeSO4 + 3(NH4)2SO4 + 6CO, HCN + 2H2O o HCOOH + 2NH3 (absorbed by H2SO4), ', HCOOH o, H2O + CO, , , , PROPERTIES :, , (i), , Carbon monoxide is a colourless, odourless gas which burns in air with a blue flame, forming CO2., It is exceedingly poisonous, combining with the haemoglobin in the blood more readily than oxygen,, so that normal respiration is impeded very quickly. Ordinary gas masks are no protection against the, gas, since it is not readily adsorbed on active charcoal. In the presence of air, a mixture of, manganese (IV) oxide and copper(II) oxide catalytically oxidizes it to CO2, and this mixed catalyst, is used in the breathing apparatus worn by rescue teams in mine disasters., , (ii), , Carbon monoxide is a powerful reducing agent, being employed industrially in the extraction of iron, and nickel:, Fe2O3(s) + 3CO(g) o 2Fe(s) + 2CO2(g) ; NiO(s) + CO(g) o Ni(s) + CO2(g), , (iii), , It reacts with many transition metals, forming volatile carbonyls; the formation of nickel carbonyl, followed by its decomposition is the basis of the Mond’s process for obtaining very pure nickel:, 90 º C, 180 º C, o Ni(CO)4(liq) , Ni(s) + 4CO(g) , , o Ni(s) + 4CO(g), , (iv), , In addition to reacting with oxygen, carbon monoxide combines with sulphur to give carbonyl, sulphide and with chlorine in the presence of light to give carbonyl chloride (phosgene), used in the, production of polyurethane foam plastics. Phosgene is an exceedingly poisonous gas., CO(g) + Cl2(g) o COCl2(g) (carbonyl, CO(g) + S(s) o COS(s) (carbonyl sulphide) ;, chloride), , (v), , Although carbon monoxide is not a true acid anhydride since it does not react with water to produce, an acid, it reacts under pressure with fused sodium hydroxide to give sodium methanoate:, dil. HCl, NaOH(liq) + CO(g) o HCOONa(s) , , o HCOOH(aq), , (vi), , With hydrogen under pressure and in the presence of zinc oxide or chromium (III) oxide catalyst it, reacts to give methanol; this reaction is of industrial importance., CO(g) + 2H2(g) o CH3OH(liq), , (vii), , CO is readily absorbed by an ammoniacal solution of copper (I) chloride to give CuCl.CO.2H2O. It, reduces an ammonical solution of silver nitrate to silver (black) and, in the absence of other gaseous, reducing agents, this serves as a test for the gas. It can be estimated by reaction with iodine, pentoxide, the iodine which is produced quantitatively being titrated with standard sodium, thiosulphate solution., 5CO(g) + ,2O5(s) o ,2(s) + 5CO2(g)
Page 18 :
p-BLOCK ELEMENTS, , (iii) Interstitial or metallic carbides, Such carbides are formed by transition metals in which carbon atoms occupy interstitials in the crystal, structure of metals., �, , CARBORUNDUM (SiC) :, , , , PREPARATION:, elect ., o SiC + 2CO, SiO2 + 3C furnace, 2000 º C, , �, , , , PROPERTIES :, , (i), , It is a very hard substance (Hardness = 9.5 Moh), , (ii), , On heating it does not melt rather decomposes into elements., , (iii), , Not attacked by acids. However, it gives the following two reactions at high temperature., ', ', Na2SiO3 + CO2 + H2O ; SiC + 4Cl2 o, SiCl4 + CCl4, SiC + 2NaOH + 2O2 o, , ), , It has a diamond like structure in which each atom is sp3 hybridized. Therefore each atom is tetrahedrally, surrounded by 4 atoms of other type., , SILICON :, , Silicon is the second most abundant element occurring in the earth’s crust (about 28 per cent by weight) as, the oxide, silica, in a variety of forms, e.g., sand, quartz and flint, and as silicates in rocks and clays., , , PREPARATION :, , (i), , (ii), , The element is obtained from silica by reduction with carbon in an electric furnace:, SiO2(s) + 2C(s) o Si(s) + 2CO(g), Extremely pure silicon is obtained from ‘chemically’ pure silicon by the method of zone refining., ', 2MgO + Si, SiO2 + 2Mg o, , , , PROPERTIES :, , Silicon is a very high melting-point solid with the same structure as diamond. The non-existence of an, allotrope with the graphite structure clearly shows the inability of silicon atoms to multiple bond with themselves. In, the massive form, silicon is chemically rather unreactive but powdered silicon is attacked by the halogens and, alkalies:, (i), Si(powdered) + 2Cl2(g) o SiCl4(liq), (ii), Si(powdered) + 2OH-(aq) + H2O(liq) o SiO32-(aq) + 2H2(g), (iii), It is not attacked by acids except hydrofluoric acid, with which it forms hexafluorosilicic acid:, Si(s) + 6HF(g) o H2SiF6(aq) + 2H2(g), , z, , (iv), , Si + 2KOH + H2O o K2SiO3 + 2H2, , (vi), , ', 2Mg + Si o, Mg2Si (Magnesium silicide), , (v), , ', Na2CO3 + Si o, Na2SiO3 + C, , COMPOUNDS OF SILICON:, Silicon Dioxide SiO2, Silicon dioxide, commonly known as silica, occurs in several crystallographic forms. Quartz, cristobalite and, tridymite are some of the crystalline forms of silica, and they are interconvertable at suitable temperature. Silicon, dioxide is a covalent, three-dimensional network solid in which each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms. Each oxygen atom in turn covalently bonded to another silicon atoms. Each, corner is shared with another tetrahedron. The entire crystal may be considered as giant molecule in which eight
Page 19 :
p-BLOCK ELEMENTS, membered rings are formed with alternates silicon and oxygen atoms. Silica in its normal form is almost nonreactive because of very high Si – O bond enthalpy. It resists the attack by halogens, dihydrogen and most of the, acids and metals even at elevated temperatures. Howevers it is attacked by HF and NaOH., SiO2 + 2 NaOH o Na2SiO3 + H2O, SiO2 + 4 HF o SiF4 + 2 H2O, Quartz is extensively used as a piezoelectric material ; it has made possible to develop extremely accurate clocks,, modern radio and television broadcasting and mobile radio communications. Silica gel used as a drying agent and, as a support for chromatographic materials and catalysts. Kieselghur, an amorphous form of silica is used in, filtration plants., , �, , SILICATES :, , Binary compouds of silicon with oxygen are called silicates but they contain other metals also in their structures., (i), , Since the negativity difference b/w O & Si is about 1.7, so Si–O bond can be considered 50% ionic &, 50% covalent., , (ii), , If we caluclate the radius ratio, , rSi � 4, , rO 2 �, , = 0.29, , It suggests that the co-ordination no. of silicon must be 4 and from VBT point of view we can say, that Si is sp3 hybridized. Therefore silicate structures must be based upon SiO4–4 tetrahedral units, (iii), , SiO4–4 tetrahedral units may exist as discrete units or may polymerise into larger units by sharing, corners., , z, , CLASSIFICATION OF SILICATES :, , (A), , Orthosilicates :, These contain discrete [SiO4]4– units i.e., there is no sharing of corners with one another as shown is figure., , e.g. Zircon (ZrSiO4), Forsterite of Olivine (Mg2SiO4), Willemite (Zn2SiO4), (B), , Pyrosilicate :, In these silicates two tetrahedral units are joined by sharing oxygen at one corner thereby giving [Si2O7]6– units., , ) (–) charge will be present on the oxygen atoms which is bonded with one Si atom., e.g. Thorteveitite (Sc2Si2O7), Hemimorphite (Zn3(Si2O7) Zn(OH)2H2O), (C), , Cyclic silicates :, If two oxygen atoms per tetrahedron are shared to form closed rings such that the structure with general, formula (SiO32–)n or (SiO3)n2n– is obtained, the silicates containing these anions are called cyclic silicates. Si3O96– and, Si6O1812– anions are the typical examples of cyclic silicates.
Page 20 :
p-BLOCK ELEMENTS, , (D), , Chain silicates :, Chain silicates may be further classified into simple chain & double chain compounds., In case of simple chains two corners of each tetrahedron are shared & they form a long chain of, tetrahedron. Their general formula is also same as the cyclic silicates i.e. (SiO3)n2n–, , Similarly, double chain silicates can be drawn in which two simple chains are joined together by shared, oxygen. Such compounds are also known as amphiboles. The asbestos mineral is a well known example of double, chain silicates. The anions of double chain silicates have general formula (Si4O11)n6n– ., , e.g., Synthetic silicates (Li2SiO3, Na2SiO3), Spondumene (LiAl(SiO3)2),, Enstatite (MgSiO3), Diopside (CaMg(SiO3)2 ), Tremolite (Ca2Mg5(Si4O11)2 (OH)2 ), etc., (E), , Two dimensional sheet silicates :, In such silicates, three oxygen atoms of each tetrahedral are shared with adjacent SiO44– tetrahedrals. Such, sharing forms two dimension sheet structure with general formula (Si2O5)n2n–, e.g. Talc (Mg(Si2O5)2 Mg(OH)2 , Kaolin Al2(OH)4 (Si2O5), (F), , Three dimenstional sheet silicates :, These silicates involve all four oxygen atom in sharing with adjacent SiO44– tetrahedral units., e.g. Quartz, Tridymite, Crystobalite, Feldspar, Zeolite and Ultramarines.
Page 22 :
p-BLOCK ELEMENTS, , The dichloro derivative will form a long chain polymer as usual. But the growth of this polymer can be, blocked at any stage by the hydrolysis product of mono-chloro derivative., , ), , Silicones from the hydrolysis of trichloro derivative, , When a compound like CH3SiCl3 undergoes hydrolysis, a complex cross-linked polymer is, obtained., , ), , The hydrocarbon layer along the silicon-oxygen chain makes silicones water-repellent., , Products having the physical properties of oils, rubbers, and resins can be produced using silicones. Silicone, fluids (say as hydraulic systems of planes) are thermally stable and their viscosity alters very little with temperature,, and silicone rubbers retain their elasticity at much lower temperatures than ordinary rubber. Silicone varnishes are, such excellent insulators and so heat-resistant that insulating wiring with them enabled motors to work over-loads, that would have set fire to the insulation formerly used. A whole new field of chemistry and technology, civilian as, well as military, has been opened up by the development of silicones., TIN AND LEAD :, �, ��, , COMPOUNDS OF TIN :, STANNOUS OXIDE (SnO) :, , , PREPARATION:, , (i), , (ii), , , By heating stannous hydroxide, Sn(OH)2, in absence of air., Sn(OH)2 o SnO + H2On, By heating stannous oxalate, SnC2O4 in absence of air., SnC2O4 o SnO + COn + CO2 n, , PROPER TIES:, , (i), SnO is an amphoteric dark grey or black solid oxide, insolulbe in water. It dissolves in, acids to form stannous salts., SnO + 2HCl o SnCl2 + H2O ; SnO + H2SO4 o SnSO4 + H2O, (ii), , SnO dissolves in hot NaOH solution to form (soluble) sodium stannite and water., SnO + 2NaOH o Na2SnO2 + H2O, , stannites are only known in aqueous solutions. Stannites absorb oxygen from air and are oxidised to, stannate which are stable in nature., 2 Na2SnO2 + O2 o 2 Na2SnO3, , , , USES :, , For the preparation of stannous chloride and stannous sulphate., , , STANNOUS CHLORIDE (SnCl 2 ∙2H 2 O) :, , It is a colourless solid soluble in water. Its solution becomes milky on standing due to its hydrolysis, to Sn(OH)2 and HCl. It aqueous solution is acidic to litmus. It is a strong reducing agent. It is soluble, in alcohol and ether also.