Page 1 :
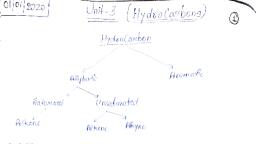
Hydrocarbons, , Classification of Hydrocarbons :, Hydrocarbons, Acyclic or open chain, or aliphatic, , Cyclic or closed chain, Alicyclic, , Saturated, , Aromatic, , Unsaturated, , Alkanes Alkenes Alkynes, , Unsaturated, , Saturated, Cycloalkanes, , Benzenoid Non-Benzenoid, , Cycloalkenes, , Cycloalkynes, , Alkanes, Saturated means, that each carbon, is bonded to, four other atoms, through single, covalent bonds., Hydrogen atoms, usually occupy, all available, bonding positions, after the carbons, have bonded to, each other., , Alkynes, Unsaturated hydrocarbons, contain either double or, triple bonds. Since the, compound is unsaturated, with respect to hydrogen, atoms, the extra electrons, are shared between two, carbon atoms forming, double or triple bonds., , Paraffins which, is derived from, a Latin word,, meaning "little, affinity", and, means that the, compounds are, very unreactive., , Alkenes are, also called, , Alkanes :Alkanes are saturated hydrocarbons, with general formula CnH2n+2, where, n is, equal to 1, 2, 3…. e.g ; CH4(methane), C2H6, (ethane), C3H8 (propane), etc., X Structure : Each carbon atom of alkanes, is in sp3 state of hybridization with its, four bonding orbitals directed towards, the four corners of a regular tetrahedron., All the C — C and C — H bonds are strong, sigma (s) bonds. Every C — C and C — H, bond has a length of 1.54 Å and 1.09 Å, respectively. All bond angles are of 109°28'., , Alkenes, , Alkynes, are also, Olefins, generally, because they known as, form oily, Acetylenes, product (i.e., from, ethylene, the first, chloride) on compound in, reaction with the series., chlorine gas., , Bond length and, bond angle in, methane, , Overlap of four sp3 orbitals, of carbon with the 1s orbitals, of four hydrogen atoms, , X, , Nomenclature : Use the following procedure, step-by-step to write the IUPAC names from, the structural formulae. Consider the following, structural formula :, CH3 CH3 CH2 CH3, CH3—CH—CH—CH—CH2—CH2—CH3, , Step 1. Identify the longest chain :, In the given example, longest chain has seven, carbons. The seven carbon chain is named as, heptane., CH3 CH3 CH2 CH3, , 2, 3-dimethyl, 4-ethyl, heptane, , CH3—CH—CH—CH—CH2—CH2—CH3, 1, , 2, , 3, , 4, , 5, , 6, , 7
Page 2 :
Step 2. Number the chain : The chain, is numbered from left to right. This gives, lowest numbers to the attached alkyl, group., , – Decarboxylation :, RCOONa + NaOH, , 2RCOONa + 2H2O, , –, , 2R—C—O, Acetate ion, , , CH2, CH + 2H2, , CH, , CH2 + H2, Pt/Pd/Ni, 523-573 K, , Pt/Pd/Ni, 523-573 K, , CH3, , CH3, , – Reduction of alkyl halides :, X, , – Wurtz reaction :, dry, , RX + 2Na + RX ether, , CH3, , X, R – R + 2NaX, , CH3, , O, , –2e, , –, , 2R—C—O, Acetate free radical, , 2R + 2CO2↑, , R+R, R —R ↑, At cathode :, –, H 2O + e –, OH + H, , X, , –, , –, , –, , Preparation :, , R–R, , Alkyl free, radical, , –, , X, , Electrolysis, , + 2CO2 + 2NaOH + H 2, , At anode :, O, , –, , – By hydrogenation of unsaturated, hydrocarbons (Sabatier–Senderens, reaction or reduction) :, , R– H + Na2CO3, , – Kolbe's electrolysis :, , Step 3. Identify the alkyl group :, There are two methyl groups at C-2 and, C-3, there is one ethyl group of C-4., Step 4. Write the IUPAC name : In, this case, the IUPAC name is 4-ethyl-2,, 3-dimethyl heptane. Always keep in mind, – Numbers are separated from each other, by commas., – Numbers are separated from names by, hyphens., – Prefixes di, tri are not taken into account, in alphabetising substituent names., X Isomerism : Alkanes exhibit chain, isomerism. Although the first three, members (methane, ethane and propane), do not exhibit isomerism, the number of, isomers in other alkanes increases with the, increase in the number of carbon atoms., Thus, butane has two isomers, pentane, has three, hexane has five, heptane has, nine, octane has eighteen, and decane as, many as seventy five isomers., , CaO, 630 K, , 2H, H2↑↑, Methane cannot be prepared by this, method., Physical properties :, Due to presence of weak van der Waals’, forces, the first four members, C1 to C4 are, gases, C5 to C17 are liquids and C18 and, above are solids at 298 K., All alkanes are colourless, odourless and, insoluble in water but dissolve in nonpolar solvents., The boiling point of alkanes increases with, increase in molecular mass and for the, same alkane the boiling point decreases, with branching., Alkanes with even number of carbon, atoms have higher melting points than, those with odd number of carbon atoms, due to symmetry., Incomplete combustion :, , – Catalytic oxidation :
Page 3 :
– Isomerisation :, AlCl3 + HCl (conc.), CH3(CH2)2CH3, 573 K, 35 atm, n-Butane, , CH3, , 180°, , CH3—CH—CH3, , �, , iso-Butane, , – Aromatisation :, C6H14, , n-Hexane, , Cr2O3/Al2O3, 773 K/10-20 atm, , + 4H2, Benzene, , – Reaction with steam :, CH4 + H2O, X, , –, , –, , –, , –, , X, , CO + 3H2, , Conformational isomerism : The, different arrangements of atoms in space, that results from the free rotation of, groups about C — C bond axis are called, conformational isomers or rotational, isomers and the phenomenon is called, conformational or rotational isomerism., Torsional strain is a weak repulsive, interaction between the adjacent bonds, due to which rotation around a C—C, single bond is not free completely., Fully eclipsed conformation : In this, form, the bigger atoms are nearest to each, other. These conformers have maximum, energy and minimum stability., Staggered conformation : In this form,, the bigger atoms are farthest from each, other. These conformers have minimum, energy and maximum stability., Gauche or skew conformation :, Rotation between 0° to 60° generates one, of the many arrangements in between, staggered and eclipsed forms. These, arrangements are called Gauche or skew, conformation., , Sawhorse projection : It is a view of, molecule down a particular C — C bond, and groups connected to both the front, and back carbons are drawn using sticks, at 120° angle. The left-hand bottom end of, this locates atoms nearer to the observer, and right-hand top end locates atom, further away., , X, , Newman projection : In Newman, projection, the two carbon atoms forming, the s-bond are represented by two circles,, one behind the other, so that only front, carbon is seen. The atoms attached to the, front carbon are shown by the bonds from, the centre of the circle while the atoms, attached to the back carbon are shown by, the bonds from the circumference of the, circle., , – The order of stability of different, conformations of ethane is, Staggered > skew or Gauche > Eclipsed, X The potential energy difference among, these conformations of ethane is about, 3 kcal/mol (or 12.5 kJ mol–1), called, torsional strain (or energy barrier). Due, to small difference in energy the two, conformations are readily interconvertible, and that is why not possible to separate, the two conformations of ethane., Alkenes : Those unsaturated hydrocarbons, which have general formula ‘CnH2n’ are called, C), ‘alkenes’. They contain double bond (C, which is considered as a functional group., e.g., C2H4 (ethene or ethylene), C3H6 (propene, or propylene)., X Structure : In ethylene molecule, one of, the sp2-hybridised orbitals of one carbon, atom overlaps axially with one of the, sp2-orbitals of another carbon atom. Two of, the sp2-hybridised orbitals of each carbon, atom overlap separately and along the axes, with the 1s orbitals of the hydrogen atoms., The pure p-orbital on each of the two, carbon atoms overlap each other laterally, (sideways) and thus, a new type of bond (p), is formed between the two carbon atoms.
Page 4 :
compound and the other is closed chain., e.g.,, , Formation of ethylene molecule, , Nomenclature : The IUPAC names, of simple alkenes are derived from the, IUPAC names of the corresponding, alkanes by replacing the ending –ane by, –ene; while the names of higher alkenes, are obtained according to some rules., Alkene, Common, IUPAC, , name, name, CH2 CH2, Ethylene, Ethene, CH3, X, , Isobutylene 2-MethylCH3C CH2, , propene, CH2 CHCH2CH3 a-Butylene 1-Butene, CH3CH, CHCH3 b-Butylene 2-Butene, – In a case where the olefin contains two, or more double bonds, the ending ane of, alkanes is replaced by adiene or atriene,, etc., to get the name of the olefin., – Positions of double bonds are indicated by, the number of carbon atoms carrying the, double bond, e.g.,, , 6, , 5, , 3, , 4, , 2, , 1, , CH3 CH CH CH 2 CH CH 2, 1,4-Hexadiene, , Isomerism : Alkenes show following types, of isomerism :, – Chain isomerism : This type of isomerism, is shown by all alkenes having four or more, carbon atoms, e.g., pentene has following, two chain isomers., X, , – Position isomerism : The two isomers, differ in the position of double bond,, known as position isomers. e.g.,, CH2 CH CH2 CH3 ;, 1-Butene, , CH3, , CH, , CH, , 2-Butene, , CH3, , – Ring chain isomerism : In this type, of isomerism, one isomer is open-chain, , – Geometrical isomerism : A carboncarbon double bond is made up of one, s-bond and one p-bond. The presence of, p-bond prevents free rotation about the, double bond with the result of the type, Cab can exist in two different, abC, geometrical isomeric forms, i.e., cis-form, and trans-form., X Preparation :, – Partial hydrogenation of alkynes :, , – Birch reduction :, , – By dehydrohalogenation, halides :, , of, , alkyl, , + HX, , – From vicinal dihalides :, CH2 CH2, CH2Br—CH2Br + Zn, �, + ZnBr2, CH3CHBr—CH2Br + Zn, , �CH3CH CH2 + ZnBr2, – By dehydration of alcohols :, , Saytzeff rule : If a single starting compound can yield, two or more isomers then more substituted alkene is, formed in greater amount., X Physical properties :, – The first three members are gases, the, next fourteen are liquids and the higher, ones are solids., – All alkenes are colourless and odourless, except ethene which has faint sweet smell., – All alkenes are insoluble in water but, fairly soluble in non polar solvents., – Boiling points of alkenes increase regularly, with increase in size and straight chain, alkenes have higher boiling points than, isomeric branched chain compounds.
Page 6 :
In case of alkynes the derived system, of nomenclature is already adopted in, practice according to which alkynes are, regarded as the derivatives of the first, member, i.e., acetylene., Common name, IUPAC name, Acetylene, Ethyne, Methyl acetylene, Propyne, Ethyl acetylene, 1-Butyne, Dimethyl acetylene 2-Butyne, X Isomerism :, – Ethyne does not show any type of isomerism., – Alkynes form chain, position, functional, and metamers., , X, , A and B are position isomers; A and C, are chain isomers; A and D are functional, isomers., CH3—CH2—C C—CH2—CH3 and, CH 3 — CH 2 — CH 2 — C C — CH 3 are also, said to be metamers., Structure : In acetylene molecule,, one of the sp-hybridised orbitals of one, carbon atom overlaps axially with one, of the sp-hybridised orbitals of the other, carbon atom; thus a C — C s-bond is, formed. Second sp hybridised orbitals, of each carbon atom overlaps axially, and separately with the 1s orbitals of, the two hydrogen atoms and thus two, C — H s-bonds are formed. The remaining, two pure p-orbitals of one carbon atom, overlaps sideways with the corresponding, p-orbitals of the other carbon atom and, thus, two C — C p-bonds are formed., , X Preparation :, – From calcium carbide :, CaCO3 D CaO + CO2, CaO + 3C, CaC2 + CO, Calcium carbide, , CaC2 + 2H2O, Ca(OH)2 + C2H2, – From vicinal dihalides :, H, H, H2C, Br, , C, Br, , alcohol, , H + KOH –KBr/–H O C, 2, , H, , NaNH2, –NaBr/–NH3, , H, C, Br, , CH, , CH, , X Physical properties :, – First three members are gases, the next, eight are liquids and the higher ones are, solids., – All alkynes are colourless and odourless, except ethyne which has characteristic, odour., – All alkynes are insoluble in water but, soluble in non polar solvents., – Melting points, boiling points and density, increase with increase in molar mass., X Chemical properties :
Page 7 :
Aromatic, hydrocarbons, :, Those, hydrocarbons which contain benzene ring, are called aromatic hydrocarbons or arenes., X Nomenclature :, – Aromatic compounds contain at least one, benzenoid ring. The simplest aromatic, hydrocarbon is benzene which has a, planar, hexagonal ring with alternate, double bonds., , – Hydrocarbons with more than one, ring :, , Exceptions to the above rule, e.g.,, , – Side chain substituted compounds :, When side chain attached to benzene, nucleus is substituted, compound is, named as phenyl derivative of aliphatic, compounds e.g.,, , – Disubstituted compounds :, • If the substituents are same then the, positions are given and prefix ‘di’ is added, before the name of the substituent, e.g.,, , – Alkyl benzenes : Common names are, given in brackets, • If the two substituents are different then, they are named successively in alphabetical, order, e.g.,, , • If one of the substituents gives a special, name to the compound then the molecule, is named as a derivative of that compound, with position 1 given to that functional, group, e.g.,, , – Aryl groups :, , CH, Benzal, (divalent), , C—, Benzo, (trivalent), , • There are many aromatic compounds, which are generally still having special, names. These names will be used during, the course of study, e.g.,, , – Mono-substituted derivatives are named, by prefixing the name of the substituent, before the name of hydrocarbon, e.g.,, , COOH, Phthalic, acid, , X, , COOH, , COOH, NH2, , Anthranilic, acid, , COCH3, , Acetophenone, , Isomerism : All six hydrogen atoms in, benzene are equivalent hence, only one, mono-substituted derivative is possible.
Page 8 :
– Position isomerism : When two, substituents are present (either same, or different), three position isomers are, possible., , Molecular formula = C7H8O, In the two compounds the properties of, the –OH group are different., – Tautomerism : Keto-enol isomerism is also, found to exist in acetophenone due to the, presence of mobile a-hydrogen atoms. e.g.,, , X Structure :, – In benzene, each carbon is sp2 hybridised., Two of the hybrid orbitals on each carbon, are used for the formation of s-bonds with, two adjacent carbon atoms whereas the, third sp2 hybrid orbital overlaps with the, s-orbital of H-atom to form a s-bond. All, these lie in the same plane. The unhybridised, orbital of each C-atom overlaps equally, with unhybridized p-orbital of both, the adjacent C-atoms to which it is bonded., – It has high degree of unsaturation and has, unusual stability due to resonance., , • When three substituents present are, identical, only three position isomers are, possible., X, X, X, X, X, X, , X, , X, , or, , X, , X, , • When two substituents are same and one, is different, six isomers are possible and if, all three groups are different, ten isomers, are possible., – Functional isomerism : This can be, seen in phenyl alcohols and phenols, e.g.,, , –, –, –, –, , •, •, •, •, , Aromatic, Cyclic, planar molecule, Complete delocalisation of, p-electrons, Follow Huckel’s rule (4n + 2) p, electrons, e.g., cycloheptatrienyl cation, (tropylium ion), cyclopropenyl, cation, benzene, etc., , Cyclopropenyl, cation (Aromatic), , •, •, •, •, , Aromaticity or Hückel rule : A, compound is said to be aromatic, if it, meets all of the following criteria :, Aromatic compounds contain one or more, rings that have a cyclic arrangement of, p-orbitals., Aromatic rings are planar., Aromatic systems are conjugated cyclic, systems., Aromatic systems must contain (4n + 2)p, electrons used in delocalisation, where, n = integer (0, 1, 2, ...)., , Anti-aromatic, Cyclic, planar molecule, Complete delocalisation of, p-electrons, Follow 4n p electrons, e.g., cyclopropenyl anion,, cyclopentadienyl cation, etc., , •, •, •, •, , +, Cyclopentadienyl cation, (Anti-aromatic), , Non-aromatic, Either non-cyclic,, non‑planar, No delocalisation of, p-electrons., May or may not follow, Huckel’s rule., e.g., cyclooctatetraene, (tub‑shaped), etc., , (Non-aromatic)
Page 9 :
Preparation :, – Cyclic polymerisation of ethyne :, , – Decarboxylation of aromatic acids :, , – Reduction of phenol :, , X, , X Physical properties :, – These are colourless liquids or solids with, characteristic aroma. These are immiscible, with water but miscible in organic solvents, and burn with sooty flame., – Boiling point of arenes increases with, increase in the molecular size due to, increase in van der Waals’ forces of, attraction. Melting point depends on, molecular size and symmetry. Among o-,, m- and p-xylenes, p-isomer has highest, melting point., , Chemical properties :, , X Directive influence :, – Groups with positive mesomeric effect, (+M) increases electron density at, o- and p-positions due to delocalisation., +, , +, , +, , positions because these are electron rich, positions., – Groups with negative mesomeric effect, (–M) decreases electron density on o- and, p-positions, so electrophile will attack on, m-position., +, +, , (Here, X may be —CH3,—C2H5, —OCH3,, —NH2, —NHR, NHCOCH3, —OH, —F,, —Cl, —Br, —I.), Thus, electrophile attacks on o- and p-, , +, , (Here, X may be —NO2, —CHO, —COR,, —COOH, —COOR,—SO3H, —CN.)
Page 10 :
Practice Time, , 1. In the following structures, which two forms, are staggered conformation of ethane?, H, H, H, H, H, H, H, H, H, H, H, H, H, H, H H, H, H, H, H, H, H, H, H, (1), , (2), , (a) 1 and 4, (c) 1 and 2, 2., the, left, (a), (b), (c), (d), 3., , (a), (b), (c), (d), , (4), , (3), , (b) 2 and 3, (d) 1 and 3, , Which of the following molecules represents, order of hybridisation sp2 , sp2 , sp, sp from, to right atoms?, HC ≡ C — C ≡ CH, CH2 == CH — C ≡ CH, CH2 == CH — CH == CH2, CH3 — CH == CH — CH3, The IUPAC name of the given compound is, , 4. Arrange the following in decreasing order of, their boiling points., (I) n-Butane, (II) 2-Methylbutane, (III) n-Pentane, (IV) 2,2-Dimethylpropane, (a) I > II > III > IV, (b) II > III > IV > I, (c) IV > III > II > I, (d) III > II > IV > I, 5., , IUPAC name of, O 2N, , 1-chloro-2-methyl-4-nitrobenzene, 2-chloro-1-methyl-5-nitrobenzene, 1-nitro-1-methyl-4-nitrobenzene, 2-methyl-1-chloro-4-nitrobenzene., , 6. The alkene that exhibits geometrical, isomerism is, (a) propene, (b) 2-methylpropene, (c) 2-butene, (d) 2-methyl-2-butene., 7. During ozonolysis of CH2 CH2 if hydrolysis, is made in absence of Zn dust the products, formed are, (a) HCHO, (b) HCOOH, (d) CH3OH, (c) CH2OHCH2OH, 8., the, (a), (b), (c), (d), , Among the three conformations of ethane,, order of stability follows the sequence :, eclipsed > gauche > staggered, eclipsed > staggered > gauche, staggered > gauche > eclipsed, gauche > staggered > eclipsed, , 9. In the following sequence of reactions, the, compound B is, , 5-formylhex-2-en-3-one, 5-methyl-4-oxohex-2-en-5-al, 3-keto-2-methylhex-5-enal, 3-keto-2-methylhex-4-enal, , CH3, , (a), (b), (c), (d), , Cl, , is, , (a), (c), 10., (a), (c), , CH3CHO, (b) CH3CH2CHO, (d) CH3CH2COCH3, CH3COCH3, Which of the following is Baeyer’s reagent?, (b) acidic K2Cr2O7, alkaline KMnO4, alkaline Na2Cr2O7 (d) MnO2, , 11. The function of anhydrous AlCl3 in Friedel–, Crafts reaction is, (a) to absorb water, (b) to absorb HCl, (c) to produce attacking electrophile, (d) to produce nucleophile., 12. Meta-directing and deactivating group in, aromatic electrophilic substitution is, (b) – OH, (a) – CH3, (d) – Cl, (c) – NO2
Page 12 :
(a), (b), (c), (d), , (c) CH3, , 5-(2′, 2′-Dimethylpropyl)decane, 4-Butyl-2,2-dimethylnonane, 2,2-Dimethyl- 4-pentyloctane, 2,2-Dimethyl- 4-butylnonane, , CH3, , 31. An alkene on ozonolysis gave 2-pentanone, and acetaldehyde. The alkene was, (a) CH3—CH2—CH2—C CH—CH3, CH3, , (b) CH3—CH2—CH, , C—CH2—CH3, , (d) CH3, , (I) CH3, , CH2, CH2, , CH 3, , 32. Which of the following compounds will not, undergo Friedal-Crafts reaction easily?, (a) Nitrobenzene, (b) Toluene, (c) Cumene, (d) Xylene, 33. The reaction of HBr with CH3C(CH3), , in the presence of peroxide will give, CBr, , CH2, , CH3, , CH3, (b) CH3CH2CH2CH2, , Br, , CH, an 3 C, OC, hy, d., Al l, Cl, 3, , Case I : Read the following and answer the, questions from 36 to 40 given below., Compound (A) is an important industrial feed, stocks, but it’s largest use as the fuel for the, oxyacetylene torch. It is a colourless, foul smelling, gas that burns in air with a yellow, sooty flame., Red hot Fe, Cl 2, CaC2 + H2O A tube, B anhy. AlCl, C, 3, , Conc. H2SO4, �, , D, E, 36. Identify the product A., (a) ethane, (b) ethyne, (c) ethene, (d) methane, , 37. The compound (B) formed is, (a) cyclohexane, (b) benzene, (c) hexane, (d) cyclopentane., Cl 2, 38. The reaction B , → C follows, Anhy.AlCl, 3, , H, C, , CH3, , H, , (c) CH3—CH2—CH2—C—CH2—CH3, , (a) CH3, , CH2, , CH2Br, , Br, 34. Arrange the following alkyl halides in, decreasing order of the rate of b-elimination, reaction with alcoholic KOH., , CH3, , (d) CH3—CH2—CH2—CH—CH, , CH, , (a) nucleophilic substitution mechanism, , (II) CH3, , (III) CH3, , C, CH3, , CH2, , CH2, , (a) I > II > III, (c) II > III > I, , CH2Br, Br, CH2, , Br, (b) III > II > I, (d) I > III > II, , Benzene easily shows, ring fission reactions since it is unstable, addition reactions since it is unsaturated, electrophilic substitution reactions due to, stable ring and high p electron density, (d) nucleophilic substitution reactions due to, stable ring and minimum electron density., 35., (a), (b), (c), , (b) electrophilic addition mechanism, (c) electrophilic subsituition mechanism, (d) elimination reaction mechanism., 39., (a), (b), (c), , The product E is, nitrobenzene, benzene sulphonic acid, both (a) and b, (d) none of these., , 40. Identify the product ‘D’., (a) Chlorobenzene, (b) Bromobenzene, (c) Toluene, (d) Acetophenonone, Case II : Read the following and answer the, questions from 41 to 45 given below., Compound ‘A’ is the simplest and ideal aromatic, compound. It is also one of the most basic, petrochemicals which is mainly used to prepare, a number of important chemicals such as, toluene, phenol, aniline, biphenyl etc, which are, used in manufacture of dyes, detergents, drugs,, explosives, pesticides etc. But it is carcinogenic.
Page 13 :
In the given sequence of reaction, compound A, undergoes a number of changes, C6H5OH, F, , Zn, , A, , CH3CH2CH2Cl, AlCl3, , 3H i. D, 3N dark, 2, , 6 Cl2, cold Anhy.AlCl3, , B, Conc.HNO3, + conc.H2SO4, , 43. The name of product F is, (a) benzene, , (b) cyclohexene, , (c) cyclohexane, , (d) cyclohex-1 4-diene, , 44. The major product when ‘A’ reacts with, propyl chloride in the presence of AlCl3 is, , (a) propyl benzene, , 41., (a), (b), (b), (d), , D, E, The name of compound A is, cyclohexane, benzene, cyclohexene, none of these., , 42., (a), (b), (c), (d), , 2, 2, B is an example of, The reaction A 3 AlCl, 3, elimination reaction, addition reaction, electrophilic substitution reaction, nucleophilic substitutional reaction., , (b) isopropyl benzene, (c) chloro benzene, (d) ethyl benzene., , CH CH CH Cl, , 45. The major product ‘D’ in the given series of, reaction is, (a) 4-nitro isopropyl benzene, (b) 2-nitro isopropyl benzene, (c) 3-nitro propyl benzene, (d) none of these., , Assertion & Reasoning Based MCQs, For question numbers 46-55, a statement of assertion followed by a statement of reason is given. Choose, the correct answer out of the following choices., (a) Assertion and reason both are correct statements and reason is correct explanation for assertion., (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion., (c) Assertion is correct statement but reason is wrong statement., (d) Assertion is wrong statement but reason is correct statement., 46. Assertion : Boiling point of alkanes, increases with increase in molecular weight., Reason : van der Waal’s forces increase with, increase in molecular weight., 47. Assertion : Acetylene is acidic in nature., Reason : Acetylene is sp hybridised., 48. Assertion : Trans-pent-2-ene is polar but, trans-but-2-ene is non-polar., Reason : The polarity of cis-isomer is more than, trans which are either non-polar or less polar., 49. Assertion : Benzene on heating with conc., H2SO4 gives benzenesulphonic acid which when, heated with superheated steam under pressure, gives benzene., Reason : Sulphonation is a reversible process., 50. Assertion : Sodium acetate on Kolbe’s, electrolysis gives methane., Reason : Methyl free radical is formed at anode., 51. Assertion : HC, CH–., H 2C, , C – is more stable, , than, , Reason : HC, H2C CH–., , C– has more s-character than, , 52. Assertion : All the hydrogen atoms in, CH2, C, CH2 are attached to sp2 hybridised, carbon atom., Reason : All the carbon atoms in its are, sp2 hybridized., 53. Assertion : Methane cannot be obtained by, Wurtz reaction., Reason : Wurtz reaction leads to the formation, of symmetrical alkane having an even number of, carbon atoms., 54. Assertion : Saturated hydrocarbons are, chemically less reactive., �Reason : All isomeric paraffins have same, parent name., 55. Assertion : Nitrobenzene does not undergo, Friedel Crafts reaction., Reason : Nitrobenzene is a m-director.
Page 14 :
SUBJECTIVE TYPE QUESTIONS, , Very Short Answer Type Questions (VSA), 1. When alkyne is treated with bromine water, then what will be the colour of product?, 2. What product would you get from acid catalysed, hydration of 1-methylcyclohexene? Explain., , 5. Acetylene is acidic but it does not react with, NaOH or KOH. Give reason., 6., , Complete the following reaction :, , CH, , H3 C, , C, , CH2 + HBr, , Organic peroxide, , 7. Write structures of A and B in the following, reaction :, H, , 3. Explain why the branching of an alkane, chain lowers its boiling point., 4. Give a brief account for the following, statement :, CH4 cannot be synthesized by Wurtz reaction., , CH3, , CH3, , Alc., KOH, , A, , HBr, Benzoyl peroxide, , B, , Br, 8. Name the chain isomer of C5H12 which has, a tertiary hydrogen atom., 9. Explain why dry ether is used as a solvent, in Wurtz reaction., 10. Draw the Newmann projection formula for, staggered and eclipsed conformation of ethane., , Short Answer Type Questions (SA-I), 11., , OH, H2SO4, , (A), , Major, , Identify A, 12. How will you convert methyl bromide to, ethane?, 13. Why is Wurtz reaction not preferred for the, preparation of alkanes containing odd number, of carbon atoms?, 14. Ethyne reacts with dil. H2SO4 in presence, of mercury salt to give acetaldehyde but with, dil. HCl under similar conditions, it gives vinyl, chloride. Explain why., 15. Which alkyne would you start with and, what reagents would you use to prepare :, (i) cis-but-2-ene, (ii) trans-pent-2-ene, , 16. What do the following compounds produce, when passed through Cr 2 O 3 supported over, aluminia at 600°C?, (i) n-Hexane, (ii) n-Heptane, 17. What will, , CH2 produce on ozonolysis?, , 18., Identify X and Y ., 19. Arrange benzene, n-hexane and ethyne in, decreasing order of acidic behaviour. Also, give, reason for this behaviour., 20. Explain Friedel–Crafts alkylation reaction, with chemical equation., , Short Answer Type Questions (SA-II), 21. An alkyne (X) has molecular formula, , 2O,HgSO 4 /H 2SO 4 → B, (ii) HC ≡ CH H, , C5H8. It reacts neither with sodamide nor with, , (iii) CH3C, , 22. Complete the following reactions :, , 23. An alkene ‘A’ contains three C — C, eight, C — H s bonds and one C — C p bond. ‘A’ on, ozonolysis gives two moles of an aldehyde of, molar mass 44 u. Write IUPAC name of ‘A’., , ammoniacal cuprous chloride. Identify X., (i), , NaNH ,CH Br, , 2, 3 →A, HC ≡ CH , , CH + H2, , Pt/ Pd/Ni, , C, , H2, , D
Page 15 :
24. (a) Explain the order of stability of, carbocations giving reason., (b) Addition of HBr to propene in the presence of, benzoyl peroxide yields 1-bromopropane. Explain, with suitable mechanism., 25. (a) Write chemical reactions to illustrate, the Kolbe’s reaction, (b) Name the compound that will be required to, obtain butane using Kolbe’s electrolysis process., 26. Why cis-but-2-ene has higher boiling point, than trans-but-2-ene?, 27. Despite their –I effect, halogens are o- and, p-directing in haloarenes. Explain., 28. An organic compound A with molecular, formula C 3 H 8 O reacts with conc. H 2 SO 4 to, give B, which on reaction with HCl gives C., Compound C reacts with metallic sodium to give, D. Identify compounds A, B, C and D., , 29. Explain ortho- and para-directing influence, of monosubstituted benzene giving suitable, example., 30. Draw the Newman projections of the eclipsed, and staggered conformers of ethane. Which of, the two is stable and why?, 31. Explain anti-Markovnikov addition or, peroxide effect or Kharash effect with example., 32. What does ozonolysis of benzene yield?, 33. Give two reactions to show acidic character, of alkynes., 34. Identify a reagent which can easily distinguish, between 1-butyne and 2-butyne., 35. A hydrocarbon (Z) has molecular formula, C8H10. It does not decolourise bromine water, and is oxidised to benzoic acid on heating with, K2Cr2O7. It can also have three other isomers A,, B and C. Write the structures of Z, A, B and C., , Long Answer Type Questions (LA), 36. An alkyl halide C 5 H 11 Br (A) reacts with, ethanolic KOH to give an alkene ‘B’, which, reacts with Br2 to give a compound ‘C’, which, on dehydrobromination gives an alkyne ‘D’. On, treatment with sodium metal in liquid ammonia, one mole of ‘D’ gives one mole of the sodium, salt of ‘D’ and half a mole of hydrogen gas., Complete hydrogenation of ‘D’ yields a straight, chain alkane. Identify A,B,C and D. Give the, reactions involved., 37. A hydrocarbon ‘Y’ decolourises bromine, water. On ozonolysis it gives 3-methylbutanal and, , formaldehyde. Give the name of the compound., Identify Y., 38. Explain why the following systems are not, aromatic., (a), , CH2, , (b), , (c), 39. Draw the resonating structure of C6H5OH, (phenol) and C6H5CHO (benzaldehyde)., , 40. �Give mechanism for the following reaction., + Cl2, , Anhy. AlCl3, , OBJECTIVE TYPE QUESTIONS, 1. (c) : In staggered conformation, the rotation, about the C – C bond is such that the hydrogen atoms, attached tetrahedrally to the two carbon atoms are, completely staggered i.e. they are at maximum distance, apart in space., 2., , (b) :, , 3., , (d) :, , 4. (d) : Boiling point increases with increase in molecular, mass. Straight chain hydrocarbons have higher boiling, points than branched chain hydrocarbons of comparable, molecular mass.
Page 16 :
5., , (a), , 6. (c) : When two groups attached to a double bonded, carbon atom are same, the compound does not exhibit, geometrical isomerism., Compounds in which the two groups attached to a double, bonded carbon are different, exhibit geometrical isomerism,, thus, only 2-butene exhibits cis-trans isomerism., , 7., , (b) :, , . Electron donating group, , stabilizes the cation., 16. (c) : The decreasing order of reactivity of halo acids with, propene is HI > HBr > HCl. As the size of halogen increases,, the strength of H X bond decreases and hence, reactivity, increases., 17. (c) :, , HCOOH + HCOOH, Reductive hydrolysis of ozonide with Zn dust gives carbonyl, compound whereas oxidative hydrolysis yields carboxylic acid., 8. (c) : The eclipsed conformation is least stable because, the hydrogens and bonding pairs of electrons on adjacent, carbon atoms are as close to one another as possible. This, causes maximum repulsion and least stability. Staggered, conformation is most stable because of minimum repulsion., Gauche conformation lie between these two in stability. Thus,, order of stability is : staggered > gauche > eclipsed., 9., , 15. (d) :, , (a) : The complete reaction sequence is as follows:, , 18. (c) : CH4 + O2 → C + 2H2O, Complete combustion will give CO2 and H2O., 19. (c), 20. (b) :, trans-pent-2-ene is unsymmetrical, therefore, show net dipole, moment., While 2,2-dimethylpropane, trans-but-2-ene and 2,2,3,3tetramethylbutane are symmetrical, therefore do not show net, dipole moment., 1°, , 1°, , 21. (d) : CH3 CH3, 22. (b) :, , 23. (a) :, 10. (a) : Baeyer’s reagent is alkaline solution of cold, potassium permanganate (KMnO4)., 11. (c) : AlCl3 produces attacking electrophile., –, , , 12. (c) : – CH3, – OH, – NO2, – Cl, 13. (a) : H, , �, , H, , �, , 14. (d) :, , :, :, :, :, , Activating and o, p-directing, Activating and o, p-directing, Deactivating and m-directing, Deactivating and o, p-directing, , H H, �, , �, , �, , �, , C�C�C�C, , H, �, �, , H, , 24. (c) : Anti-Markownikoff addition of HBr is observed, only with unsymmetrical alkenes i.e., propene, 1-butene,, 2-pentene. As, 2-butene is symmetrical so in its case antiMarkownikoff addition will not be observed., 25. (a) : Aromatic are most stable followed by non-aromatic, and anti-aromatics are least stable., 26. (d) : The directive influence order is, O– > NR2 > NHR > NH2 > OH > OCH3 ≈ NHCOCH3 > CH3 > X, Electrolysis, , →, 27. (b) : 2CH3COONa + 2H2O (Kolbe's, method), , Sodium ethanoate, , CH3 CH3 + 2CO2 + 2NaOH + H2, Ethane, , 28. (c) : Only acetylene has acidic hydrogens and hence, reacts with Na to evolve H2 gas., –, – +, CH + 2Na, 2Na+ C, CNa + H2, 2HC
Page 17 :
29. (a) : In general electron-releasing groups activate, and electron withdrawing groups deactivate the benzene, ring towards electrophilic substitutions. Thus order of, reactivity is:, , 36. (b) :, , 30. (a) :, 37. (c) , 39. (b) , 41. (b) , , 38. (c), 40. (d), 42. (c), Ni,∆, , + 3H2 →, , 43. (c) :, , CH3, 31. (a), 32. (a) : Nitrobenzene is strongly deactivated, hence will, not undergo Friedel-Crafts reaction., 33. (c) :, , 45. (a) :, , C, , AlCl, , 3, →, + CH3CH2CH2Cl , , 44. (b) :, CH3, , CH3, , CH—CH3, , CH—CH3, , H, CH3, , Conc.HNO +, , 3, Conc, , →, .H SO, 2, , 4, , NO2, , 34. (d) :, , > CH3CH2CH2Br > CH3CH2Br, , is the order of rate of b-elimination with alcoholic KOH., b, , CH3, , a, , CH2, , Br, , (I) , (II), (has 2 b-substituents) (has no b-substituent), b, , a, , Br, CH3 CH2 CH2, (III), (has 1 b-substituent), More the number of b-substituents (alkyl groups), more, stable alkene it will form on b-elimination and more, will be the reactivity. Thus, the decreasing order of, the rate of b-elimination reaction with alcoholic KOH, is : I > III > II., 35. (c) : The most common reactions shown by benzene, are electrophilic substitution reactions., , 46. (a) : Greater is the molecular mass, greater is the, magnitude of van der Waal’s forces of attraction and hence, higher the boiling point., 47. (b) : The acidic nature in acetylene is described on the, basis of higher electronegativity of sp hybridized carbon atom, which pulls the C – H bond pair more effectively to lose H+., 48. (b) : The vector sum of all polar bonds in trans-pent-2ene is not zero but the vector sum is zero in trans-but-2-ene., CH3 – C – H, CH3 CH2 – C – H, H – C – CH3, , trans-2-pentene, (µ ≠ 0), , H – C – CH3, , trans-2-butene, (µ = 0), , 49. (a) : Sulphonation of benzene is an electrophilic, substitution reaction in which SO3 acts as the electrophile., SO3H, 80°C, + HOSO3 H, , + H2 O, , 50. (d) : Sodium acetate on Kolbe’s electrolysis gives ethane., It is formed at anode., 51. (a) : HC C– has 50% s-character and H2C CH– has, 33% s-character. Stability of carbanions increases with an, increase in the s-character at the carbanion. So, HC C– is, more stable than H2C CH–.
Page 18 :
52. (c) : The two H-atom on first carbon and the two, H-atoms on the third carbon atom are attached to, sp2 hybridised carbon atoms. The central carbon atom is, sp-hybridized., 53. (a) : Wurtz reaction involves the formation of alkanes, by heating alkyl halide with sodium in ether., In this reaction two alkyl radicals join together to form an, alkane. The net result in this reaction is the formation of, symmetrical alkane (R–R) having an even number of carbon, atoms., 54. (c) : Less reactivity of saturated hydrocarbons is due to, the presence of single bonds between carbon atoms. Paraffins, (alkanes) may have straight chain or branched chain isomers, which have different parent names., 55. (b) : The Friedel Crafts reaction does not take place, with nitrobenzene because the ring has been too greatly, deactivated. Moreover, any coordination of AlCl 3 with, unshared electrons of oxygen of NO2 group would further, deactivate the ring making –NO2 electrophilic., , SUBJECTIVE TYPE QUESTIONS, 1. The product will be colourless., 2. 1-Methylcyclohexanol will be formed because a 3°, carbocation will be formed as an intermediate., , 8. 2-Methylbutane, (CH3)2CH — CH2 — CH3., 9. In Wurtz reaction, pure sodium is used which is highly, violent towards water therefore, dry ether is used., 10., , 11., , OH, , H2SO4, –OH, , 2, , 1, , 1,2 methyl shift, , –H, , +, , 12. Two moles of methyl bromide react with sodium metal in, presence of dry ether as solvent to give ethane. This reaction, is known as Wurtz reaction., CH3 Br + 2Na + Br, Methyl bromide, , H3, , ether, , H3, , H3 + 2NaBr, , Ethane, , 13. It is because mixture of alkanes will be formed e.g.,, Dry, , → CH3—CH3, 3CH3Cl + 6Na + 3C2H5Cl ether, , Chloromethane Chloroethane , , �, , , Ethane, , + CH3CH2CH2CH3 + CH3—CH2—CH3 + 6NaCl, Butane Propane, , 14. Mercuric ion forms a complex (I) with acetylene. Since,, H2O is more nucleophilic than SO2−, 4 ion, it attacks the complex, (I) to form first vinyl alcohol which further tautomerises to, give acetaldehyde., 3. Boiling point decreases with increase in branching due, to decrease in surface area of the molecule., 4. �, Wurtz reaction occurs between two alkyl halides to yield, alkane. Methane has only one carbon atom, hence cannot, be prepared by using Wurtz reaction., 5. Due to sp-hybridisation of C-atom in acetylene, proton is, strongly attracted by nucleus and cannot be abstracted easily, therefore, it does not react with NaOH or KOH., 6., H, 7., , H3C, , C, Br, , CH3, , Alc.KOH, , In case of dil. HCl, Cl– ion is more nucleophilic than H2O, it, reacts with complex (I) to form vinyl chloride., , H, H2C = C – CH3, (A), , Benzoyl peroxide, , HBr, , H2C – CH2 – CH3, Br, (B), , 15. (i)
Page 19 :
(ii), , 16. �, (i), , Na/ liq. NH3, C2H5OH, , + 4H2, , 21. �Alkyne X is C5H8. Since it does not react with sodamide, or ammoniacal cuprous chloride, the triple bond cannot be, terminal., \ X is CH3CH2C ≡ CCH3 (Pent-2-yne), 22. (i), , (ii) CH ≡≡ CH, (ii), , dil. H 2SO4, , HgSO4, , Tautomerisation, , �, 17., , 18. �, On heating with alc. KOH in inert solvent, the triple, bond of 1-alkyne is shifted towards the centre to form an, isomeric 2-alkyne. On heating with sodamide (NaNH2 in liq., NH3) the triple bond shifts towards end., 19., , Since s-orbitals are closer to the nucleus, hence due to more, s-character in ethyne (sp hybridised), the hybridised orbital, is nearest to this carbon atom in comparison to sp3 or sp2, hybridised carbon. This leads to the movement of C — H, bond pair more towards sp hybridised carbon, leading to, the development of partial positive charge on the hydrogen, attached to sp hybridised carbon. Thus, such a hydrogen, behaves as acidic hydrogen. Hence, order of acidic nature is,, ethyne > benzene > n-hexane., 20. Friedel–Crafts alkylation is a Lewis acid-catalyzed, electrophilic aromatic substitution reaction that allows the, synthesis of alkylated products via the reaction of arenes, with alkyl halides. With anhydrous aluminium chloride as, a catalyst, the stable alkyl carbocation is generated which, attacks the benzene ring. An example of this type of reaction, is shown below :, , (B), , (iii), , 23. Alkene A contains 3C — C, 8C — H and one C C, bonds. An aldehyde containing one —CHO group and having, molar mass of 44 amu has to be CH 3CHO and since two, moles of CH3CHO are obtained by ozonolysis of alkene A,, the alkene has to be joined by two CH3CH— groups by a, double bond. It has to be CH3 — CH CH — CH3, i.e.,, but-2-ene. But-2-ene contains 3C—C s bonds, 8C — H s, bonds and one C C bond., , 24. (a) Stability of carbocations decreases as 3° > 2° > 1°., Alkyl groups have +I effect. when an alkyl group is attached, to positively charged carbon atom of a carbocation, it tends to, release electrons towards that carbon and reduces the positive, charge on the carbon. Thus, positive charge gets dispersed. This, dispersal of the positive charge stabilises the carbocation., �(b) Mechanism : Peroxide effect proceeds via free radical, mechanism as given below :, O, , (i), , O, , C6H5 C O O C C6H5, O, 2C6H5 C O, , (ii), , Homolysis, , 2C6H5 + 2CO2
Page 20 :
(iii) CH3, , CH CH2 + Br, , Homolysis, , CH3, , CH CH2, (more stable free radical), , Br, , (iv), , 29. —OH group attached to benzene ring release electrons, and activate the benzene ring, direct the incoming groups to, ortho and para positions., OH, OH, OH, , 25. �, �(a) Kolbe’s reaction : In this reaction, an aqueous, solution of sodium or potassium salt of carboxylic acid on, electrolysis gives alkane having even number of carbon atoms, at anode., 2CH3COO–Na+ + 2H2O, , electrolysis, , CH3, , OH, , OH, , CH3 +, , Ethane, , 2CO2 + H2 + 2NaOH, , (b) Sodium propanoate,, 2CH3CH2COONa + 2H2O, �CH3CH2, , 30., , Newman projections, , CH2CH3 + 2CO2 + H2 + 2NaOH, , 26. Due to higher dipole moment, the boiling point of cisisomer is higher than the corresponding trans-isomer., 27. In case of aryl halides, halogens are little deactivating, because of their strong –I-effect. Therefore, overall electron, density on the benzene ring decreases. In other words,, halogens are deactivating due to –I-effect. However, because, of the +R-effect, i.e., participation of lone pairs of electrons, on the halogen atom with the p-electrons of the benzene, ring, the electron density increases more at o- and p-positions, than at m-positions., As a result, halogens are o, p-directing. The combined result, of +R-effect and –I-effect of halogens is that, halogens are, deactivating but o, p-directing., , In staggered form of ethane, the electron clouds of carbonhydrogen bonds are as far apart as possible. Thus, there are, minimum repulsive forces, minimum energy and maximum, stability of the molecule. On the other hand, when the staggered, form changes into the eclipsed form, the electron clouds of the, carbon-hydrogen bonds come closer to each other resulting in, increase in electron cloud repulsions. Thus, the molecule has, more energy and therefore, has lesser stability., 31. �, Peroxide effect : Addition of HBr in presence of peroxide, gives products opposite to Markovnikov rule., Markovnikov, rule, , 32., , 33. CH CH + Na, 28. Compound A is an alcohol which on reaction with H2SO4, gives alkene B., CH3CH2CH2OH, (A), , Conc., H2SO4, , Propanol, , CH3—CH—CH—CH3, |, |, CH3 CH3, (D), 2, 3-Dimethylbutane, , CH3CH == CH2, (B), , Propene, Markovnikov, HCl, addition, , Na, , CH3—CH—CH3, |, Cl, , CH, , CH + NaNH2, , 1, H2, 2, –, +, C Na + NH3, , C–Na+ +, CH, , 34. �, There will be no reaction between 2-butyne and Cu2Cl2, because it has no acidic hydrogen. In 1-butyne the terminal, hydrogen is acidic (CH3CH2 – C CH) so it will give a red, ppt. with ammoniacal Cu2Cl2., 35. �, Since, it does not decolourise bromine water, it is arene., Thus,, CH2CH3, COOH, [O], K2Cr2O7, H+, , (C), 2-Chloropropane, , HC, , (Z), , Benzoic acid
Page 21 :
infact it is non-aromatic compound., , The other three isomers are :, , (A), , (B), , 39. Resonating structures of phenol :, —OH group attached to benzene ring release electrons and, activate the benzene ring, direct the incoming groups to, ortho- and para-positions., OH, OH, OH, , (C), , 36., ., , OH, Since, (D) an alkyne reacts with sodium in liq. NH3, to give, alkylide, it shows that (D) is a terminal alkyne. It yields straight, chain alkane on hydrogenation it shows it is a straight chain, terminal alkyne. Hence,, (A) CH3CH2CH2CH2CH2Br, (B) CH3CH2CH2CH CH2, , OH, , Resonating structures of benzaldehyde :, H—C==O, , H—C—O, , H—C—O, , (C), H—C—O, (D) CH3CH2CH2C, , H—C—O, , H—C== O, , CH, , n-Pentyne, , 37. �, H ydrocarbon ‘Y ’ is alkene because it decolourises, bromine water. From the products of ozonolysis, the structure, of alkene can be predicted., H, , CH3CHCH2C, CH3, , 3-Methylbutanal, , H, , O +O, , C, , 40. The halogenation of benzene proceeds by the following, mechanism :, Step 1. Generation of an electrophile., , H, , Formaldehyde, , CH3CHCH2CH, , Step 2. Formation of s-complex or carbocation intermediate., , CH2, , CH3, , 4-Methylpent-1-ene, , 38. �, (a), , CH2 it has 6p-electrons but not in the ring, , and one carbon atom has sp3-hybridisation, hence it is nonaromatic., 1 2, (b) In, 3, due to the presence of sp3-hybridised carbon, 5 4, (carbon 3) and only four p electrons, it does not contain planar, delocalised cloud of (4n + 2)p electrons. Hence, it is nonaromatic compound., (c) Cyclooctatetraene (COT) is not aromatic because of, its non-planar tub-shaped structure. Athough according to, electron-count it seems to be an anti-aromatic compound but,, , This step is slow and hence is the rate-determining step of, the reaction., Step 3. Loss of a proton from the carbocation intermediate., , This step is fast and hence does not affect the rate of the, reaction., ,