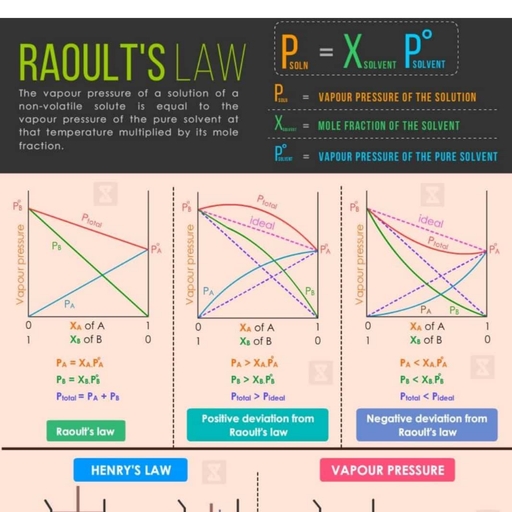
Page 1 :
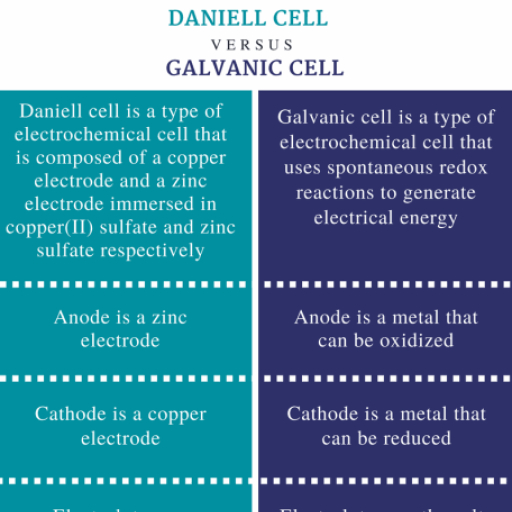
s-BIOCK ELEMENTS, s-BLOCK ELEMENTS, 1.1.5 Ionization Energy, 1. ALKALI METALS (GROUP 1), The group 1 elements have ns' electronic configuration, The first ionisation energies for the atoms in this group, and are highly reactive metals, are lower than those for any other group in the periodic, table. The atoms are very large so the outer electron are, Elements, Atomic, Electronic, held weakly by the nucleus hence the ionisation energy is, not large. Ionization energy decreases on moving down, the group., Number, Configuration, Lithium (Li), 3, [He] 2s', Sodium (Na), [Ne] 3s', 1.1.6 Flame Test, 11, Potassium (K), 19, [Ar] 4s', Alkali metals have large size, when heated on the flame, the electrons present in the valence shell move from lower, Rubidium (Rb), 37, [Kr] 5s', energy level to higher energy level by absorption of heat, from the flame. When they come back to the ground state,, Cesium (Cs), 55, [Xe] 6s', they emit the extra energy in the form of visible light to, provide colour to the flame, Francium (Fr), 87, [Rn] 7s', Element, Colour, 1.1 Physical Properties, Li, Red, 1.1.1 Atomic Size, Na, Golden yellow, The atoms are largest in their corresponding periods., Atomic size increases in going down the group., K, Violet, Rb, Red Violet, 1.1.2 Oxidation State, Cs, Blue, The group 1 elements exhibit +1 oxidation state, 1.1.7 Standard Oxidation Potential, 1.1.3 Density, The measure of the tendency of donating electrons of a, metal in water is called its electrode potential. If the, Alkali metals have large size which accounts for their low, density, concentration of metal ions is unity, then it is called as, standard electrode potential. Lithium has the highest, ionization potential but has an highest electrode potential, due to highest hydration energy., Atomic mass, Density =, Atomic volume, 1.1.8 Hydration of Ions, Atomic weight increases from Li to Cs in the group and, volume also increases but increase in atomic weight is, more than increase in volume. Therefore density increases, The ions are heavily hydrated. The smaller the size of the, ion, the greater is the degree of hydration. Thus the degree, of hydration decreases down the group from Li* to Cs*., Thus with the increase in hydration electrical conductivity, from Li to Cs., Exception : Density of Na is more than that of K, order : Li <K <Na <Rb <Cs, decreases., 1.1.4 Nature of Bonds, 1.1.9 Lattice Energy, Salts of alkali metals are ionic solids. Lattice energy of, salts of alkali metals having common anion decreases on, descending down the group., The electronegativity values being low, they combine with, other elements to form Ionic bond.
Page 2 :
s-BLOCK ELEMENTS, participates in bonding and of the large size and diffusing, 1.1.10 Solubility in Liquid Ammonia, nature of the outer bonding electron. The atoms become, larger on descending down the group, so the bonds are, weaker, the cohesive energy decreases and the softness, of metal increases. Hence the melting point decreases, down the group. Boiling point also decreases down the, M+n NH, →, [M (NH,),J* + e¯ (NH,),, (n = x+y), Dilute solutions of alkali metals in liquid ammonia are dark, group., blue in colour and the main species present are solvated, metal ions and solvated electrons. If the blue solution is, 1.2 Chemical Properties, allowed to stand, the colour fades until it dissappears, owing to the formation of metal amide. The solutions of, metal in liquid conduct electricity because of the presence, Some common reactions of Group 1 metals, of solvated electrons. The dilute solutions are, Reaction, Comment, paramagnetic because they contain free electrons., М+Н,О->МОН + Н,, Hydroxides are strongest, base known, 1.1.11 Electronegativity Values, Li+O,→Li,0, Monoxide formed by Li and, The electronegativity values are small which decrease from, to a small extent by Na, Li to Cs., Na + O,→ Na,0,, Peroxide formed by Na and, to a small extent by Li, 1.1.12 Reactivity, K+0, >ко,, Superoxide formed by K, Rb,, Cs., The reactivity of alkali metals goes on increasing in the, following order., M+ H, → MH, Ionic salt like hydrides, Li+N, →Li,N, Nitride formed only by Li, Li <Na <K< Rb<Cs, M +S→ M,S, All metals form sulphides, 1.1.13 Colourless and Diamagnetic ions, M+X, →MX, All the meatls form halide, M+ NH, -, MNH,, All the metals form amides., The property of an ion being colourless or coloured, depends on the number of unpaired electrons present in, the ion. If unpaired electrons are present in anion then, these electrons can be excited by energy from light and, show colour on coming back to the ground state. The ion, which have unpaired electrons show magnetic properties, 1.2.1 Reaction with Air, Group 1 elements are very reactive and tarnish rapidly in, air. Cs burns spontaneously in air., whereas the ions having paired electrons nullify the, magnetic fields of each other. Such ions are called, These metals form alkaline carbonates in moist air., diamagnetic ions., 2Na + 0, →2 Na,0, Super oxides are para magnetic and coloured due to the, Na,O+H,O (mositure) →2 NAOH, presence of unpaired electrons., 2NAOH + CO, → Na, CO,+ H,O, 1.1.14 Melting and Boiling Point, 1.2.2 Reaction with O,, The cohesive energy is the force holding the atoms or, ions together in the solid. The cohesive energy depends, on the number of electrons that can participate in bonding., Li forms Li,0, Na forms two type of oxide, (M,0, M, O,) and K, Rb, Cs forms superoxides (MO,)., The cohesive force decreases down the group in alkali, metals group as they have only one valence electron which
Page 3 :
s-BIOCK ELEMENTS, The bases liberate ammonia from ammonium salts, 1.2.2.1 Basic Nature, Ionic Nature of the Oxides, NaOH + NH,CI –→NH, + NaCl + H,0, Basic nature of oxides increases from Li to Cs due to, KOH + NH,CI → NH, + KCI + H,0, increase in the size of cation, KOH resembles NaOH in all its reaction. but as KOH is, • Size of cation increases from Li to Cs. According to, much more expensive it is seldom used. However KOH is, Fajan's Rule, ionic character of these oxides increases, more soluble in alcohol, thus producing C,H,O ions by, the equilibrium, from Li to Cs., Solubility in water increases from Li to Cs oxides, due, C,H,OH + OH=C,H̟O¯ +H,O, to increase in ionic character of these metal oxides., This accounts for the use of alcoholic KOH in organic, 1.2.3 Reaction with water, chemistry Group 1 hydroxides are thermally stable., 1.3.2 Oxides, Peroxides, Superoxides, Group 1 metals react with water liberating H, and forming, hydroxides., Normal oxides - monoxide : The monoxides are ionic. They, 2 Li+2H,0→2LİOH +H,, are strongly basic oxides and they react with water form, 2Na + 2 H,O→ 2NAOH + H,, strong bases., 2K +2 Н,О ->2 КОН + Н,, Na,O + H,О >NaОН, K,O+H,0→KOH, 1.2.4 Reaction with Hydrogen, Peroxides, Preparation:, Group 1 metals reacts with H, to form ionic hydrides., Thermal stability of LiH is high., 2Na + 0, (excess), 300°C, Na,O,, Stability of hydrides :, 2Na,0 7400°C, Na,O, + Na (vapour), LiH >NaH >ΚΗ> RbH > CsΗ, Properties, 1.2.5 Reaction with dilute acids, Na,0, + H,SO, (dil) →Na,SO, +H,O,, Due to alkaline nature, these metals react rapidly with, Na,O, + 2H,0→2N2OH + H,O,, dilute acids and the rate of reaction increases from Li to Cs, Na,0, is a powerful oxidant Because it reacts with CO, in, because of increase in basic character., the air it has been used to purify the air in submarines, 1.3 Compounds of Alkali Metal, Na,0, + CO→ Na CO,, Na,0, + 2CO, →Na,CO, +0,, 1.3.1 Нydroxides, Na,O, + Cr³* → Cro;, NaOH is often called as caustic soda. KOH is called caustic, Structure, potash because of their corrosive properties. The caustic, alkali are the strongest base in aqueous solution. The, solubility of hydroxides increases down the group., peroxide ion, The bases react with acids to form salt and H,O., КОН + НCI > КCІ+Н,О, O is sp' hydridised. The peroxide ion has 18 electrons, NaOH + HCI → NaCl+H,O, which occupy the molecular orbitals as shown., 2NAOH + CO, → Na,CO, + H,O, ols, o*ls, o2s', o*25°,02p; . n2p; = 12p;,a*2p; = n*2P;
Page 4 :
s-BLOCK ELEMENTS, Bond order is 1 and it is diamagnetic, (a) small size and strong polarisation of Li distorts the e, Superoxides (O7), cloud of the near by oxygen atom of the large CO-, to such an extent that the C-O bond gets weakened., Superoxides are ionic oxides M*O,, A, Li,CO,, Li,0+CO,, Preparation:, МM+0, (ехcess) мо,, (b) Replacement of the larger carbonate ion by a smaller, ion leads to increased lattice energy and thus favours, (M = K, Rb, Cs), the decomposition., Superoxides are stronger oxidizing agents than peroxides., The stability of these superoxides is in the order, A, M,CO,, M,0 + CO,t, KO, < RbO, < CsO,, Na,CO, is used as washing soda. NaHCO, is used as, baking soda. The crystal structure of NaHCO, and KHCO,, Reactions, ко, + Н,О > коН + Н,О, + 1/20,, both show hydrogen bonding. In NaHCO,, the HCO,, KO, is used in space capsules, submarines and breathing, masks because it produces O, and removes CO,, linked into an infinite chain whilst in KHCO, a dimeric, anion is formed., 4KO, + 2CO, →2 K,CO, +30,, 4KO, + 4CO, +2 H,O→4KHCO,+O,, Reactions, 2HNO, + K, CO, → 2KNO, +CO,+H,O, sodium superoxide cannot be prepared by burning metal, 2NaHCO,, Na,CO, + H,O+CO,, in oxygen but it can be prepared by reacting sodium, peroxide with O, at high temperature and pressure, M,CO, + H,0 = 2M* + HCO; +OH-, Na,0 +0,→ 2NAO,, They hydrolyze to give basic solution., Structure, :ö *** ö:, 1.3.4 Halides, All the metals in this group form halides of type MX., The presence of one unpaired electron in 3 electron bond, explains paramagnetic character. The superoxide has 17, electrons which give a bond order of 1.5 which occupy, Lit is the smallest ion in the group, it would be expected to, form hydrated salts more readily than other metals., the molecular orbitals as shown, Properties :, ols', o*1s', o2s', o*2s°,02p , 12p, = n2p;,7*2p, = n*2P;, As evident from their following properties, alkali metal, Stability of oxides : Normal oxide >peroxide> superoxide., halides are ideal ionic compounds., (i), All alkali halides except lithium fluoride are freely, 1.3.3 Carbonates and Bicarbonates, soluble in water (LiF is soluble in non-polar, solvents)., Group 1 metals form solid bicarbonates (MHCO,). All alkali, metals form carbonates of type M,CO,. Due to highly, (ii), They have high melting and boiling points., electro positive nature of the alkali metals their carbonates, (a) For the same alkali metal, the melting and boiling, and bicarbonates are highly stable to heat (Li,CO,, points decrease regularly in the order, decomposes easily by heat)., Fluoride > chloride > bromide > iodide, The exceptional behaviour of Li,CO, can be explained by
Page 5 :
s-BIOCK ELEMENTS, This is explained on the basis of lattice energy* of, these metal halides. For the same metal, lattice, (a) Lattice energy :- Lattice energy is the energy, released during the formation of a crystal lattice, energy decreases with the decrease in, electronegativity of the halogen. For example,, from the respective gaseous cations and anions;, or it is the energy required to separate one mole of, the solid ionic compound into its gaseous ions., Metal Halide :, NaF, NaCI, NaBr, Nal, Thus lattice energy (the force of attraction among, Lattice energy, the ions) is a direct measure of the stability of ionic, (kJ/mole) :, 910, 769, 732, 682, crystals; higher the lattice energy of a compound, lower will be its solubility in water., Melting point (K):, 1298, 1081, 1028, 934, When a crystal of an ionic compound comes in, (b) For the same halide ion, the melting point of lithium, contact with a polar solvent such as water, the, halides are lower than those of the sodium halides., hydrogen end (positive pole) of the water molecule, is attracted to a negative ion while the oxygen end, However, after sodium the melting points of halides, decrease as we move down the group from Na to, Cs. This abnormal behaviour shown by lithium, (negative pole) is attracted to a positive ion. This, halides is probably due to its covalent nature, attachment of polar solvent molecules to the ions, whereas sodium and other halides are ionic in, is known as solvation (or hydration, if the solvent, nature. Amongst ionic halides, melting point, is water) of the ions. With the stabilization of the, decreases as lattice energy decreases as we move, ions by solvation, a large amount of solvation, energy (or hydration energy) is released which if, exceeds the lattice energy of the crystal causes the, down the group i.e.,, Nacl > KCi > RbCl > CSCI, dissolution of the ionic compound in the solvent., On the other hand, if the solvation energy is not, 1081 K, 1045 K 990 K, 918 K, enough to counteract the lattice energy, the, (iii), Solubility of halides of alkali metals :- The solubilities, of alkali metal halides show a gradation. For example,, substance remains insoluble as in case of lithium, fluoride. The high lattice energy of lithium fluoride, solubility of alkali metal fluorides in water increases, is due to the combination of small lithium ion with, regularly from lithium to caesium., small fluoride ion. In general, for a given ion, the, Metal fluoride :, LiF NaF KF, RbF CsF, lattice energy increases as the size of the oppositely, charged ion decreases., Solubility in water at, 2-7, 42, 1020 | 1310 | 3700, (b) Polarising power and polarisability (Fajan's rule), 298 K (gm/litre) :, :- Although an ionic bond in a compound like M*X-, is considered to be 100% ionic, in some cases (e.g.,, In case of chlorides, LiCl has much higher solubility in, lithium halides) it is found to have significant, water than NaCl. This is due to small size of Lit ion and, covalent character. According to Fajan, when the, much higher hydration energy. However, from NaCl to, CSCI, solubility in water increases regularly due to, decrease in their lattice energy., two oppositely charged ions approach each other,, the nature of the bond between them depends upon, the effect of one ion on the other., (iv), They are good conductors of electricity in the fused, When two oppositely charged ions approach each, state., other, the positive ion attracts electrons present, (v), They have ionic crystal structure. However, lithium, on the outermost shell of the anion and repels its, halides have partly covalent character due, to, positively charged nucleus. This results in the, distortion, deformation or polarisation of the anion., polarising power of Li ions., The structure and stability (solubility) of alkali metal, halides are explained by the lattice energy, and, Thus the power of a cation to distort the anion is, known as its polarisation power and the tendency, polarising power., of the anion to get polarised by the cation is known, as its polarisability. If the polarisation is quite small,