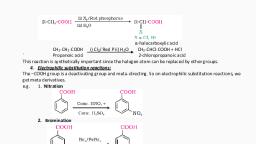
Page 1 :
[Hsslive.in], +, , c) All alkali metals have oxidation number of 1 and all alkaline earth metals have an oxidation number of +2., Aluminium shows an oxidation number of +3 in all of its compounds., d) The common oxidation number of oxygen is –2. But in peroxides (e.g., H2O2, Na2O2), oxidation number of, oxygen is –1 and in superoxides (e.g., KO2, RbO2), it is –½. In oxygen difluoride (OF2) and dioxygen difluoride, (O2F2), the oxygen is assigned an oxidation number of +2 and +1 respectively., e) The common oxidation number of hydrogen is +1. But in hydrides, H shows an oxidation number of -1., f) The common oxidation number of halogens is -1. Fluorine shows only -1 oxidation number in all of its, compounds. But other halogens show positive oxidation numbers also in their oxides and oxoacids., g) The algebraic sum of the oxidation number of all the atoms in a compound is zero., h) In polyatomic ion, the sum of the oxidation numbers all the atoms is equal to the charge on the ion., , 9. HYDROGEN, 1. Justify the position of hydrogen in the periodic table., Hydrogen shows resemblance with both Alkali metals of the first group and halogens of the 17th group. Like, alkali metals it has one electron in the outer most shell and forms unipositive ions. Like halogens, it requires only, one electron to complete the valence shell configuration. So it gains one electron to form uninegative ion. At the, same time it shows some differences from alkali metals and halogens. So it is placed separately in the periodic, table., 2. What is water gas or syn gas?, A mixture of CO (carbon monoxide) and H2., 3. What is ‘coal gasification’?, The process of producing 'syngas' from coal is called 'coal gasification'., C(s) + H2O(g) 1270K CO(g) + H2(g), 4. How is dihydrogen produced by ‘water gas shift reaction’?, The production of dihydrogen can be increased by reacting carbon monoxide of syngas mixtures with steam, in the presence of iron chromate as catalyst. This is called water-gas shift reaction., CO(g) + H2O(g) 673K, catalyst CO2(g) + H2(g), 5. Write any two uses of dihydrogen., a) It is used as a rocket fuel in space research., b) It is used in fuel cells for generating electrical, c) It is used in the manufacture of vanaspati fat by the hydrogenation of vegetable oils., 6. Water is an amphoteric substance. Justify., Water can act both as acid and base. So it is an amphoteric substance., e.g.: H2O(l) + NH3(aq), OH-(aq) + NH4+(aq), H2O(l)+H2S(aq), �H3O+(aq) + HS– (aq), In the first e.g. water acts as an acid and in the second it acts as a base., 7. Which are the different types of hydrides? Give one example for each., There are three types of hydrides – ionic hydride (e.g. NaH), covalent hydride (e.g. H2O) and metallic hydride, (e.g. VH0.56), 8. What is mean by hardness of water? What is the reason for hardness of water?, Water which does not easily form lather with soap is called hard water. It is due to the presence of calcium, and magnesium salts in the form chlorides, sulphates and bicarbonates., 9. Hard water is harmful for boilers. Why?, , Prepared by Anil Kumar K.L, Govt HSS, Ashtamudi, Kollam [Hsslive.in], , Page 18
Page 2 :
[Hsslive.in], On boiling hard water, the soluble Mg(HCO3)2 is converted into insoluble Mg(OH)2 and Ca(HCO3)2 is changed, to insoluble CaCO3. These salts are deposited in the boilers in the form of scales. This reduces the efficiency of, boilers., 10. What is mean by temporary hardness? Explain the different methods used for its removal?, Hardness which can be removed by simple boiling is called temporary hardness. It is due to the presence of, bicarbonate of calcium and magnesium. The following methods are used to remove temporary hardness., 1. Boiling: During boiling, the soluble Mg(HCO3)2 is converted into insoluble Mg(OH)2 and Ca(HCO3)2 is changed to, insoluble CaCO3, which can be removed by filtration., Mg(HCO3)2 ⎯⎯⎯⎯→Mg(OH)2 ↓ + 2CO2 ↑, Ca(HCO3)2 ⎯⎯⎯⎯→CaCO3 ↓ + H2O + CO2 ↑, 2. Clark’s method: In this method calculated amount of lime is added to hard water. It precipitates out calcium, carbonate and magnesium hydroxide which can be filtered off., Ca(HCO3)2 + Ca(OH)2 → 2CaCO3 ↓ + 2H2O, 11. What is mean by permanent hardness? Explain the different methods used for its removal?, Hardness which cannot be removed by boiling is called Permanent hardness. It is due to the presence of, soluble chlorides and sulphates of calcium and magnesium in water. It can be removed by the following methods:, 1. Treatment with washing soda (Sodium carbonate): Washing soda reacts with soluble calcium and magnesium, chlorides and sulphates in hard water to form insoluble carbonates., CaCl2 + 2Na2CO3 → CaCO3 ↓ + 2NaCl, MgCl2 + 2Na2CO3 → MgCO3 ↓ + 2NaCl, 2. Calgon’s method: Sodium hexametaphosphate (Na6P6O18) is commercially called ‘calgon’. When it is added to hard, water, the Ca and Mg ions in hard water are replaced by Na+ ions., 3. Ion-exchange method: This method is also called zeolite/permutit process. Zeolite /permutit is hydrated sodium, aluminium silicate which can be written as NaZ. When this is added to hard water, exchange reactions take place., 2NaZ + M2+ → MZ2 + 2Na+ (where M = Mg or Ca), 4. Synthetic resins method: Cation exchange resins contain large organic molecule with -SO3H group and are water, insoluble. Ion exchange resin (RSO3H) is changed to RNa by treating it with NaCl. The resin exchanges Na+ ions with Ca2+ and, Mg2+ ions present in hard water and make the water soft., 2RNa + M2+ → R2M + 2Na+, This method is more suitable to get pure demineralised water., 12. Give the industrial preparation of Hydrogen peroxide., Industrially it is prepared by the auto-oxidation of 2-alklylanthraquinols., 2 ethylanthraquinol, H2O2 + oxidised product, 13. Give the structure of Hydrogen Peroxide (H2O2)., Hydrogen peroxide has a non-planar structure (open book-like structure)., , 14. Explain why hydrogen peroxide is not stored in glass vessels., OR, Hydrogen peroxide is stored in plastic vessels in dark. Why?, Ans: H2O2 decomposes slowly on exposure to light., 2H2O2(l) → 2H2O(l) + O2(g), , Prepared by Anil Kumar K.L, Govt HSS, Ashtamudi, Kollam [Hsslive.in], , Page 19
Page 3 :
[Hsslive.in], In the presence of traces of alkali (present in glass containers), the above reaction is catalysed. So it is stored in, wax-lined glass or plastic vessels in dark., 15. What is heavy water? Give one of its uses., Deuterium oxide (D2O) is called heavy water. It is used as a moderator in nuclear power plant., , 10., , THE S-BLOCK ELEMENTS, , 1. Alkali metals and their salts give characteristic colour to non-luminous flame. Why?, This is because the heat from the flame excites the outer most orbital electron to a higher energy level., When this electron comes back to the ground level, they emit the radiation in the visible region., 2. How do alkali metals react with air?, Alkali metals react with air to form oxides, peroxides and super oxides. Li forms only monoxide, sodium, forms monoxide and peroxide and other alkali metals form monoxide, peroxide and super oxide., 3. Solutions of alkali metals in liquid ammonia are blue in colour. Why?, The alkali metals dissolve in liquid ammonia to give deep blue solutions. The blue colour of the solution is due to, the formation of ammoniated electron., 4. Give any two anomalous properties of Lithium., a) Li is the least reactive but the strongest reducing agent among all the alkali metals., b) It forms only monoxide with oxygen., 5. What is mean by diagonal relationship? Give any two similarities in properties shown by Lithium and Magnesium., The similarity in properties shown by diagonally placed elements of second and third periods in modern, periodic table is called diagonal relationship., Li shows the following similarities in properties with Mg of the second group., a) Both react slowly with water., b) They do not form superoxides., 6. Name the process used for the industrial preparation of sodium carbonate. Explain the process., Sodium carbonate is manufactured by Solvay process (Ammonia-Soda Process). In this process, CO2 is, passed through a concentrated solution of NaCl saturated with ammonia. Ammonium carbonate first formed then, converted to ammonium bicarbonate and finally reacts with NaCl to form NaHCO3., 2NH3 + H2O + CO2 → (NH4)2CO3, (NH4)2CO3 + H2O + CO2 → 2NH4HCO3, NH4HCO3 + NaCl → NH4Cl + NaHCO3, Sodium bicarbonate crystals are separated and heated to get sodium carbonate., 2NaHCO3 → Na2CO3 + CO2 + H2O, 7. Give any two uses of sodium carbonate., a) It is used in water softening, laundering and cleaning., b) It is used as a laboratory reagent., 8. What are the uses of sodium hydroxide (NaOH)?, a) in petroleum refining., b) in the purification of bauxite, c) as a laboratory reagent., 9. Give the structure of beryllium chloride (BeCl2)., It has a chain structure in the solid state., , Prepared by Anil Kumar K.L, Govt HSS, Ashtamudi, Kollam [Hsslive.in], , Page 20