Page 4 :
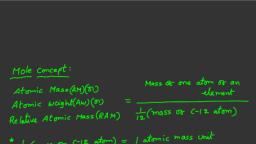
One mole Ie an amount of substance containing Avogadro's number of particles, Avogad, equa’ to 602,218 189,000,000,000,000,000 of more simply, 6.02214198 « 10” 5, A mole bol mol) is defined as the amount of substance that contains ag, srateagpen, 1080, electrons of any other elementary entities as there are carbon ‘tore n't, om of “C, Tho number of atoms in 12 gm of "C is callad Avogadro's number (M4)., , N 22 = 1, From mass spectrometer wn found that there are 6.022 x 10°" atoms present i 12 gm of C=?, Sy bgy, , The number of entities in 1 mol is so impartant that it is given 8 separate name and, Avogadre constant denoted by Na. Le, on the whole we can say that 1 mole Is The collection of ¢, entibes. Here entities may represent atoms, lons, molecules or even pens, Chairs , paper ete aig, thes but as this number (Na) is very large therefore its used only for very small things,, , HOW BIG IS A MOLE?, One mole of marbles would cover the entire Earth (oceans included) for 8 depth of thas, mole of $109 bills stacked one on top of another would reach from the Sun to Pluto g, mition times. It would take light 9500 years to Iravel from the bottom to the top of a sta, , of $1 bills, ', , + The alornic mass of an element expressed in grams is called gram atomic mass of that ele, * Itis also defined as mass of 6.022 x 10” atoms., , * [tis also defined as the mass of one mole atoms., * itis also cefined as the mass of 1 grarn atom of the element., , For example for oxygen atom:, Atomic mass of ‘O" atom = mass of one ‘O' atom = 16 amu, , Gram stomic mass = mass of 6.022 « 10” ‘0! atoms, = 16 amu x 6.022 x 10”, = 16x 1.66 « 10™ «6,022 x 10" = 16 gmimole, , 91.66% 107 6.022107 1, , Ex. 4: How many atoms of oxygen are there in 16 g oxygen., x 1, , Sol, xx 1.66%10™ x16 =16; ¥=————, , : e 16610 =, , , , GRAM MOLECULAR MASS :, + The molecular mass of a substance expressed in gram is called the gram-molecu, substance., + itis also defined as mass of 6.022 x 10” molecules., + Itis also defined as the mass of 1 mole molecules., + [tis also defined as the mass of 1 gram molecule,, , For examples for ‘O,’ molecules :, , Molecular mass of ‘Oy' molecule = mass of one ‘0,’ molecule, = 2 x mass of one ‘O' atom, =2 x 16 amu, = 32 amu, , Gram molecular mass = mass of 6,022 x 10**'O,' molecules, = 32 amu x 6,022 « 10”, = 32 x 1.66 x 10% gm x 6.022% 10%, = 32 gm/mole, , CENTERS: MUMBAWDELHI/AKOLA ‘LUCKNOW /N