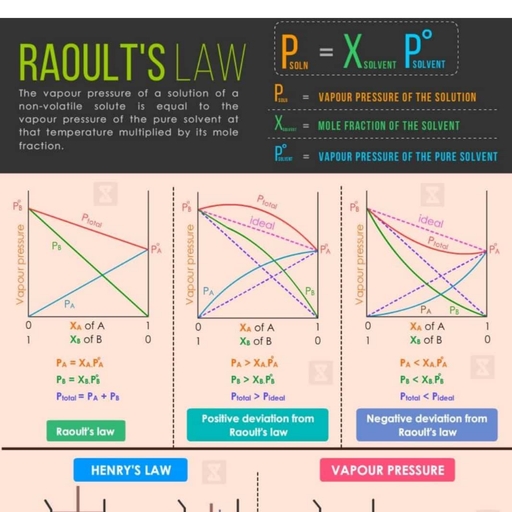
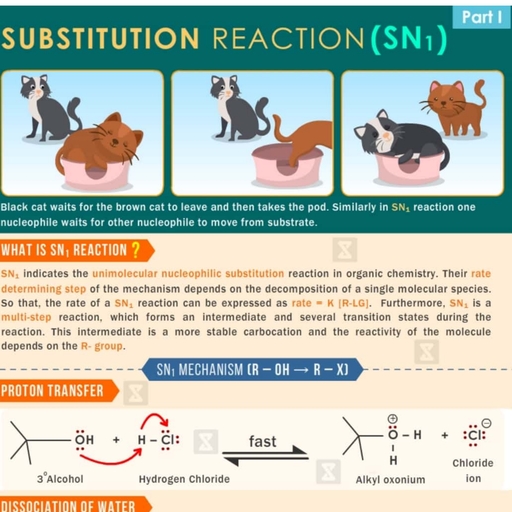
Page 2 :
Hydrocarbons, , 5., , By decarboxylation of carboxylic acid: The sodium salt of carboxylic acid is strongly heated with soda lime to, give alkane by elimination of CO2 as carbonate., Heat, R – COONa + NaOH →, RH + Na2CO3, CaO, , 6., , Kolbe’s electrolysis: Sodium or potassium salts of fatty acids are electrolyzed to give higher alkanes at node., 2CH3COONa + 2H2O → CH3 – CH3 + 2CO2 + 2NaOH + H2, Methane can’t be prepared by this method., , 7., , By action of water on aluminium carbide or beryllium carbide:, Al4C3 + 12H2O 4Al(OH)3 + 3CH4↑, Aluminium carbide, Be2C + 4H2O → 2Be(OH)2 + CH4↑, Beryllium carbide, , Physical Properties, 1. State: Due to weak forces, the alkanes upto four carbon atoms are colourless, odourless gases, the next, thirteen members are colourless, odourless liquids. Alkanes from C18 onwards are colourless and odourless, solids., In alkenes, except ethene, all are odourless and follow some trend as alkanes. Ethene has a pleasant odour. All, are colourless. Alkynes also follow the same trend as alkanes., 2. Density: The density of alkanes increases very slowly with the rise of molecular mass until it becomes constant, at 0.8., 3., , Solubility: They are generally insoluble in polar solvents such as water but insoluble in non-polar solvents like, ether, CCl4, benzene etc., , 4., , Boiling and melting points: The boiling point of straight chain alkanes increase regularly with increasing, number of carbon atoms. The melting points of alkanes do not follow a very smooth gradation with the increase, of molecular size. Alkenes and alkynes also show a gradual increase in boiling and melting points with the, increase of molecular mass in homologous series. They are less volatile than alkanes, i.e., their boiling point, and melting point are higher than corresponding alkanes., , Chemical Properties, Alkanes are extremely stable and inert substance due to presence of non-polar C – C and C – H bonds. Alkanes are, saturated compounds with strong sigma bonds which doesn’t break under ordinary conditions. Alkanes react at high, temperature by free radical mechanism., 1., , Halogenation (free radical substitution):, Alkanes react with halogens (Cl2, Br2) in presence of light or in dark at high temperature to form corresponding, substituted products., Cl, , Cl, , Cl, , Cl, , 2 →, 2 →, 2 → CHCl , 2 → CCl, CH4 , CH3Cl , CH2Cl 2, , 3, 4, ( − HCl ), ( − HCl ), ( − HCl ), ( − HCl ) methyl chloride, methene, methylene chloride, carbon, chloroform, , tetrachloride, , The relative reactivity of halogens and alkanes follows this order,, F2 > Cl2 > Br2 > I2 and 3° > 2° > 1° > CH3, 2., , Nitration: Nitration is possible for alkanes having three or more carbon atoms. Nitration of propane yields, mixture of nitro products.