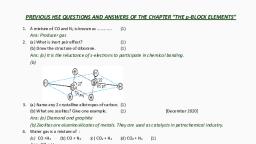
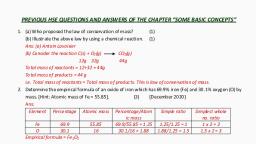
Page 3 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , CHAPTER 1, SOME BASIC CONCEPTS OF CHEMISTRY, PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL : CHEM DSM, =======================================================, 1. What is Chemistry? Which are different branches of chemistry?, Chemistry is the branch of science that deals with the composition, structure and properties of matter., , Branches of chemistry :, Organic chemistry, Inorganic chemistry, Physical chemistry, Analytical chemistry, Biochemistry, Polymer chemistry, Industrial chemistry etc., 2. What is Matter? How will you classify it?, Matter is anything that has mass and occupies space., Chemically matter is classified as two. (i) Pure substances and (ii) Mixtures., 3. What are Pure substances? How will you classify it? Explain each ., Pure substances contain only one substance. It cannot be physically separated., Pure substances are classified as (i) elements and (ii) Compounds., Elements contain only one type of atoms. eg. Hydrogen , Oxygen , Carbon , Iron , Gold etc., Compounds contain different types of atoms. Examples: marble (CaCO3), ice (H2O) etc., 4. What are Mixtures? How will you classify it? Differentiate them., Mixtures contain more type of substances. It can be physically separated., These are classified as (i) homogeneous mixture and (ii) heterogeneous mixture., , Homogeneous mixture, , Heterogeneous mixture, , Mixtures having uniform composition through out, The components of the mixture cannot be seen by, microscope, Eg:-air, sugar solution, kerosene, petrol, alloys, (Brass),916 gold, , Mixtures having different composition in different parts, The components of the mixture can be seen with naked, eye., Iron and sulphur, muddy water, sand, smoke, gun, powder, soil, , 5. Chemical classification of matter, Matter, Pure substances, Elements, , compounds, , mixtures, homogeneous, , heterogeneous
Page 4 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 6. Define precision and accuracy. Give example., Precision is the closeness of various measurements for the same quantity., Accuracy is the agreement of a particular value to the true value of the result., Example:- If true value is 2 g, Student A → 1.95, 1.93 precise , not accurate., Student B → 1.94, 2.05, Neither precise nor accurate., Student C → 1.99, 2.01 Both precise and accurate., 7. What are Significant figures?, Significant figures are the number of digits in a measurement about which we are certain plus one, additional digit which is uncertain., 8. Which are the rules for determining the number of significant figures?, (I), All non-zero digits are significant., (II), Trapped zeros are significant., (III), Leading zeros (Before zeros) are never significant., (IV), Trailing zeros (End zeros) are significant if it is after the decimal point., (V), Trailing zeros (End zeros) are non significant if there is no decimal point., (VI), In scientific notation, powers of ten are non- significant., (VII), Exact numbers have infinite number of significant figures., 9. Which are Laws of chemical combination? Explain each with example., , (I), , Law of conservation of mass :Matter can neither be created nor destroyed. OR, In a chemical reaction, total mass of reactants is equal to total mass of products., Lavoisier proposed this law., Eg., C + O2 → CO2, 12g, 32 g, 44 g, Total mass of reactants = 12+32=44 g, Total mass of products =44 g, , (II), , Law of definite proportion:A chemical compound always contains the same elements combined together in the same, proportion by mass. Proposed by Joseph proust., Example:- Carbon dioxide can be obtained by many methods., Its formula → CO2, Mass ratio→ 12:32, Simple ratio →3:8, , (III), , Law of multiple proportion:When two elements combine to form two or more compounds, the different masses of one, element that combine with a fixed mass of the other element, are in the small whole number ratio., Proposed by John Dalton., Example:- Hydrogen and oxygen combine to form two compounds, water and hydrogen peroxide., H2 + 1/2 O2 → H2O, ,, H2 + O2 → H2O2, 2g, 16 g, 18 g, 2g, 32 g, 34 g, Here hydrogen has fixed mass ( 2 g) . Oxygen has different masses ( 16 and 32), Its ratio → 16:32 = 1:2, It is simple whole number ratio, More examples:- (i) CO , CO2 (ii) NO , NO2
Page 5 :
Join Telegram Channel: https://t.me/hsslive, , (IV), , (V), , Downloaded from www.Hsslive.in ®, , Gay Lussac’s law of gaseous volume:-, , When gaseous reactants combine to form gaseous products, there exist a simple whole number, ratio between their volumes., Example :H2 + Cl2 → 2 HCl, Volume ratio of reactants and products → 1: 1: 2, , Avogadro law :-, , Equal volumes of all gases under similar conditions of temperature and pressure contain equal, number of molecules. This law is proposed by Avogadro., For example, if we take hydrogen, nitrogen and oxygen in different flasks of same capacity, we will, find that all flasks contain the same number of molecules., 10. What is atom?, An atom is the smallest particle of an element which may or may not have free existence., Atoms of Helium and Neon can exist freely. Atoms of hydrogen, oxygen, nitrogen etc. cannot exist freely, 11. What are the postulates of Dalton’s atomic theory?, (I), Matter is made up of extremely small, indivisible particles called atoms., (II), Atoms of the same element are identical. i.e size, shape and mass., (III), Atoms of different elements are different., (IV), Atoms of different elements may combine with each other in a simple whole number ratio to form, compounds., (V), Atoms can neither be created nor destroyed., 12. Define atomic mass ., The atomic mass of an element means how many times an atom of an element is heavier than one-twelfth, of a carbon-12 atom., , Element, , Relative atomic mass, , Element, , Relative atomic mass, , Hydrogen , H, Carbon , C, Nitrogen , N, Oxygen , O, Sodium , Na, Phosphorus , P, , 1, 12, 14, 16, 23, 31, , Sulphur , S, Chlorine , Cl, Potassium , K, Calcium , Ca, Iron , Fe, Bromine , Br, Silver , Ag, , 32, 35.5, 39, 40, 56, 80, 108, , 13. Define molecule, Molecule is the simplest particle of an element or a compound which has free existence., 14. Define molecular mass, The molecular mass of a substance( element or compound) means how many times the mass of a molecule, is heavier than one-twelfth ( 1/12) of a carbon-12 atom., It is obtained by multiplying the atomic mass of each element by the number of its atoms and adding them, together., , Molecular mass of glucose ( C6H12O6) = (12 X6) + (1 X 12 ) + (16 X 6) = 72 + 12 + 96 = 180, Molecular mass of sulphuric acid ( H2SO4) = (1 X2) + (32 X 1 ) + (16 X 4) = 2 + 32 + 64 = 98, Molecular mass of Calcium carbonate ( CaCO3) = (40 X1) + (12 X 1 ) + (16 X 3) = 40 + 12 + 48 = 100, Molecular mass of Ethanol( C2H5OH) = (12 X2) + (1 X 6 ) + (16 X 1) = 24 + 6 + 16 = 46, Molecular mass of ammonium sulphate (NH4)2SO4 = (14 X2) + (1 X 8 ) + (32 X 1) + (16 X 4) = 132, 15. Define Atomic mass unit (amu) or unified mass (u). What is its value ?, One atomic mass unit is equal to one-twelfth( 1/12 ) the mass of an atom of carbon-12 ., 𝟏 𝒂𝒎𝒖 =, , 𝟏, 𝟏𝟐, , 𝑿, , 𝟏𝟐, =, 𝟔.𝟎𝟐𝟐 𝑿 𝟏𝟎𝟐𝟑, -24, , 1 amu (u) = 1.66056 x 10, , g, , 𝟏. 𝟔𝟔𝟎𝟓𝟔 𝐱 𝟏𝟎−𝟐𝟒 𝒈
Page 8 :
Join Telegram Channel: https://t.me/hsslive, , CHAPTER, , Downloaded from www.Hsslive.in ®, , 2, , STRUCTURE, , OF ATOM, , PREPARED BY: YOOSAFALI T K, GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL : CHEM DSM, =======================================================, 1. Electron was discovered by J J Thomson by cathode ray discharge tube experiment., 2. Compare the properties of cathode rays and anode rays., Cathode rays, Anode rays ( Canal rays), They start from cathode, more rays are produced, They are produced from the space between cathode and, from the space between cathode and anode and, anode and move towards cathode, move towards anode, They are material particles, They are material particles, They travel in straight lines., They travel in straight lines., They are deflected by both electric and magnetic, They are deflected by both electric and magnetic field., field. Deflection in the electric field is towards, Deflection in the electric field is towards negative plate, positive plate shows that they are negatively, shows that they are positively charged particles, charged particles, They does not depend on the nature of the gas, They depend on the nature of the gas inside discharge, inside discharge tube, tube, The charge to mass ratio (e/m) is same for all gases The charge to mass ratio is different for different gases, 3. Calculate the mass of electron., The charge to mass ratio of electron is determined by JJ Thomson by using cathode ray discharge tube., e/m =1.758 x 1011 Ckg-1, The charge of the electron is determined by Millikan’s oil drop experiment . e = -1.6022 x 10 -19 C, ∴, , 𝑴𝒂𝒔𝒔 𝒐𝒇 𝒆𝒍𝒆𝒄𝒕𝒓𝒐𝒏 =, , 𝒆, 𝒆⁄, 𝒎, , =, , 𝟏.𝟔𝟎𝟐𝟐 𝑿 𝟏𝟎 𝟏𝟗, 𝟏.𝟕𝟓𝟖 𝑿 𝟏𝟎𝟏𝟏, , = 𝟗. 𝟏 𝑿 𝟏𝟎, , 𝟑𝟏, , kg, , 4. What is the Mass of proton ?, -27, Mass of proton =1.672 x 10 kg . It is equal to the mass of hydrogen atom, 5. How neutron is discovered? What is its mass?, Neutron is discovered by James Chadwick by bombarding beryllium sheet by alpha (α) particles., They are neutral particles., -27, , Mass of neutron =1.674 x 10 kg ., Mass of neutron is slightly greater than proton., 6. Give the equation of Atomic number and Mass number, Atomic number = Number of protons = No. of electrons, Mass number = Number of protons + Number of neutrons = No. of nucleons, 7. How will you find number of neutrons, Number of neutrons= Mass number - Atomic number, 8. Define isotopes and isobars. Give examples for each., (I), Isotopes are atoms of same element having the same atomic number but different mass number., 12, 13, 14, They contain different number of neutrons. e.g.,, ,, 6C ,, 6C, 6C, (II), Isobars are atoms of different elements which have the same mass number. e.g., 146C , 147N
Page 9 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 9. Explain Rutherford’s alpha ray scattering experiment . Give its important observations and conclusions., Rutherford bombarded thin gold foil by alpha particles (positive charge) and the movements of rays are, detected by circular zinc sulphide screen., , 1, 2, 3, , Observations, , Conclusions, , Most of the alpha particles passed through the, gold foil without any deflections., A few alpha particles were deflected through, small angles ., Very few alpha particles are deflected back (1800)., , Most of the space in an atom is empty., A heavy positive centre is present at the centre, of the atom called nucleus., Nucleus is very small in size., , 10. What are the postulates of Rutherford atom model?, (I), Most of the mass and all positive charge is concentrated at the centre of the atom called nucleus., (II), Electrons are revolving around the nucleus with very high speeds., (III), Most of the space inside the atom is empty., (IV), Electrons and nucleus are held by electrostatic forces of attraction., 11. What are the draw backs (failure) of Rutherford atom model?, (I), Failed to explain the stability of the atom., (II), Failed to explain hydrogen spectrum., (III), It does not say anything about the electronic structure of atom., 12. Arrange electromagnetic radiations in order of the wavelength., Gamma-rays < X-rays <UV rays <visible rays < infra red rays < Micro waves < radio waves, , 13.The relation connecting frequency (γ) , speed of light (c), and wave length (λ) is c = γ λ, 14. Give the postulates of planks quantum theory of radiation., (i), Radiant energy is emitted or absorbed not continuously but discontinuously in the form of small, packets of energy called quanta. In the case of light, it is called photon., (ii), The energy of a quantum of radiation is proportional to its frequency., Eαν, OR, E=hν, h= plank’s constant =6.626 x 10-34 Js, 15. What is Photoelectric effect? What are its characteristics?, When light falls on certain metals, electrons are emitted is called photoelectric effect., e.g., Alkali metals (Potassium, Rubidium, Caesium ) show photoelectric effect., (I), For the ejection of electrons, the incident light must have a minimum frequency called threshold, frequency (ν0). Corresponding minimum energy is called work function, (II), The kinetic energy of the ejected electrons depends on the frequency of the incident radiation., (III), The number of electrons ejected is proportional to the intensity or brightness of light., (IV), The electrons are ejected from the metal surface as soon as the beam of light strikes the surface., , Equation : h ν = h ν0 + ½ mv2, h ν = energy of incident light ,, h ν0 =threshold energy (work function) ,, ½ mv2 = kinetic energy, , Kinetic energy , ½ mv2, , = h ν - h ν0 = h( ν - ν0 )
Page 10 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 16. Explain line spectrum of hydrogen., When an electric discharge is passed through, hydrogen gas at low pressure, ressure taken in discharge tube,, H2 molecules dissociate to form hydrogen atoms. The, excited hydrogen atoms emit electromagnetic, radiations of certain frequencies. When this radiation, is passed through spectroscope,, we can see many, lines. It is called hydrogen spectrum., Hydrogen, ydrogen spectrum consists of several series., Their wave number and wavelength is determined by, the Rydberg equation., 𝝑=, , 𝟏, 𝟏, 𝟏, = 𝟏𝟎𝟗𝟔𝟕𝟕 𝟐 − 𝟐 𝒄𝒎, 𝝀, 𝒏𝟏 𝒏𝟐, , 𝟏, , The spectral series of hydrogen spectrum are, Series, Spectral region n1, n2, Lyman series, Ultraviolet, 1, 2,3,…., Balmer series, Visible, 2, 3,4,…., Paschen series, Infrared, 3, 4,5,…., Brackett series, Infrared, 4, 5,6,……, Pfund series, Infrared, 5, 6,7,……., 17. What are the postulates of Bohr atom model?, (I), The electrons in an atom revolve around the nucleus in circular paths called orbits. These orbits have, definite energies called energy shells or energy levels. These are numbered 1,2,3,4,… or designated as, K,L,M,N,…., (II), As long as electrons remain in a particular orbit, it does not lose or gain energy. Therefore these, orbits are called stationary states., (III), Only those orbits are permitted in which the angular momentum of the electron is a whole number, multiple of h/2π . i.e. Angular momentum, mvr =nh/2π n = 1,2,3,……, (IV), Energy is emitted or absorbed by an atom only when an electron in it moves from one orbit to other., The difference in energy , ∆EE = E2 - E1 = hν, 18. What are the merits of Bohr atom model?, (I), Bohr’s model can explain the stability of an atom., (II), Bohr’s model can explain the atomic spectrum of hydrogen, (III), Bohr’s theory helped in calculating energy of an electron in a particular orbit of hydrogen atom., 19. What are the draw backs (Limitations) of Bohr atom model?, (I), It cannot explain the line spectra of multi electron atoms., (II), It cannot explain Zeeman effect and Stark effect., (III), It cannot explain de Broglie concept of dual nature of matter, matter., (IV), It cannot explain Heisenberg’s uncertainty principle., (V), It cannot explain the ability of atoms to form molecules by chemical bonds., 𝟐..𝟏𝟖 𝑿 𝟏𝟎 𝟏𝟖 𝒁𝟐, 𝒏𝟐, 𝟐, 𝟓𝟐.𝟗, 𝟗𝒏, 𝒑𝒎, 𝒁, , 20. 𝑬𝒏𝒆𝒓𝒈𝒚 𝒐𝒇 𝒆𝒂𝒄𝒉 𝒐𝒓𝒃𝒊𝒕, 𝑬𝒏 =, 𝑹𝒂𝒅𝒊𝒖𝒔 𝒐𝒇 𝒆𝒂𝒄𝒉 𝒐𝒓𝒃𝒊𝒕 , 𝒓𝒏 =, , 𝑱/𝒂𝒕𝒐𝒎, Z = Atomic number ,, , n = Number, umber of orbits
Page 11 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 21. What is Dual nature of matter? Give de Broglie equation., According to de Broglie, all microscopic particles in motion (e.g., Electron) has particle character and wave, character., 𝐡, 𝐡, de Broglie equation is, 𝛌 =, =, 𝐦𝐯, 𝐩, Wavelength ( λ) → Wave character., Momentum (p) → Particle character., For electron, wave character is significant. It is against Bohr model., 22. State Heisenberg’s uncertainty principle. Give its mathematical forms and its significance., It is not possible to determine simultaneously both the position and velocity (or momentum) of a, microscopic moving particle such as electron with absolute accuracy., Mathematical form is, , ∆𝒙. ∆𝒑 ≥, , 𝒉, 𝟒𝛑, , ∆x = uncertainty in position. ∆p = uncertainty in momentum. h= plank’s constant =6.626 x 10 -34 Js, Momentum, p = mass x velocity , p =mv, ∆p= m∆v, , ∆𝐱. 𝐦∆𝐯 ≥, , 𝐡, 𝟒𝛑, , ∆v = uncertainty in velocity, , Significance:-This principle rules out the existence of definite paths or trajectories for moving, microscopic particles such as electrons., 23. Write a short note on Quantum mechanical model of atom., It is developed based on de Broglie concept of Dual nature of matter and Heisenberg’s uncertainty, principle., Its basic equation is Schrodinger equation is, , 𝑯𝛙=𝐄𝛙, , 𝑯, , = 𝐇𝐚𝐦 𝐥𝐭𝐨𝐧 𝐚𝐧 𝐨𝐩𝐞𝐫𝐚𝐭𝐨𝐫 (𝐦𝐚𝐭𝐡𝐞𝐦𝐚𝐭 𝐜𝐚𝐥 𝐨𝐩𝐞𝐫𝐚𝐭𝐨𝐫) , Ψ = wave function, , E = energy., The wave function ψ has no physical significance. It represents the amplitude of the electron wave., However ψ2 represent the probability density of electron cloud (Orbital)., 24. What are orbitals?, An orbital is the region in space around the nucleus where there is maximum probability of finding an, electron having a specific energy., The concept of orbital is in accordance with the wave nature of electrons and Heisenberg’s uncertainty, principle. Different orbitals have different shape., Orbitals are directional (except s-orbital) and can explain shapes of molecules., An orbital can accommodate maximum two electrons., 25. What are Quantum numbers? Which are four Quantum numbers? Explain each., Quantum numbers are a set of four numbers with the help of which we can get complete information, about the electron in an atom. It is address of an atom., , There are four quantum numbers., (I), (II), (III), (IV), (I), , Principal quantum number (n)., Azimuthal or Angular momentum quantum number (l)., Magnetic quantum number (m)., Spin quantum number(s), , Principal quantum number (n):-, , It represents the main energy level or shell in which the electron is located. It also determines the, average distance of the orbital or electron from the nucleus. n= 1, 2, 3, 4, ………., n=1, first energy level, n=2, second energy level, (II), , Azimuthal or Angular momentum quantum number (l) :-, , It determines the magnitude of the orbital angular momentum of an electron. It denotes the sub, shell in which electron is located in a shell., Its value determines the shape of the orbital. For a given value of n, l = 0 to n-1
Page 12 :
Join Telegram Channel: https://t.me/hsslive, , l, Subshell, Shape of the orbital, For 1st shell (n =1), For 2nd shell (n=2), For 3rd shell (n =3), For 4th shell (n =4), , 0, s, Spherical, , 1, p, D, Dumb-bell, , l =0, l =0,1, l =0,1,2, l =0,1,2,3, , Downloaded from www.Hsslive.in ®, , 2, d, Double dumb-bell, , one value, two values, three values, four values, , 3, f, complex, , one sub shell, two sub shells, three sub shells, four sub shells, , 1s, 2s 2p, 3s,3p,3d, 4s,4p,4d,4f, , Magnetic quantum number (m) ::-It describes the behavior of the electrons in an external, , (III), , magnetic field. It refers to the different orientations (orbitals) in a sub shell., For a given value of l, m= -l …..0……..+l, For s Sub shell( l=0), , m =0, , one value, , One s orbital, , For p Sub shell( l=1), , m = -1,0,+1, , Three values, , Three p orbitals, , For d Sub shell( l=2), For f Sub shell( l=3), , m ==-2,-1,0,+1,+2, m = -3,-2,-1,0,+1,+2,+3, , Five values, Seven values, , Five d orbitals, Seven f orbitals, , Spin quantum number(s):, number(s):- It describes the spin orientation of electrons., , (IV), , Spin orientation in two ways – clockwise (+1/2) or anti clockwise (-1/2), 26. Draw the plots of probability density (ψ 2 ) against distance from the nucleus for 1s and 2s orbitals, , 27. Draw the shapes of s- orbitals, p- orbitals, and d, d- orbitals., , ., , S orbital→ Spherical shape, , P orbital→ Dumb, umb bell shape
Page 15 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , An orbital can accommodate maximum two electrons., , (III), , Hund’s rule of maximum multiplicity, , This rule states that electron pairing in orbitals of same sub shell will not takes place until each, available orbital of that sub shell is singly occupied (with parallel spin)., N → 1s2 2s2 2px1 2py1 2pz1, , 32. Reason for exceptional configuration of chromium and copper., , Chromium : 1s2 2s2 2p6 3s2 3p6 3d5 4s1, Copper : 1s2 2s2 2p6 3s2 3p6 3d10 4s1, , Half filled and completely filled orbitals have more stability due to symmetrical arrangement and, greater exchange energy., , PROBLEMS, 1. What is the total number of orbitals associated with the principal quantum number n = 3 ?, Total number of orbitals = n2 = 32 = 9, 2. Represent the orbitals given below:, i) n = 1, l = 0 ii) n = 2, l = 1. iii) n = 4, l = 0 iv) n = 3, l = 2 v) n=4 , l=3, Solution : (i) 1s (ii) 2p (iii) 4s (iv) 3d (v) 4 f, 3. An electron is in 4f orbital. What possible values for the quantum numbers n, l, m and s can it have?, For 4f , n=4 , l=3 ,, m = -3 ,-2 ,-1, 0 , +1 , +2 , +3 , s = +1/2 or -1/2, 4. An electron is in 3d orbital. What possible values for the quantum numbers n, l, m and s can it have?, For 3d , n=3 , l=2 ,, m = -2 ,-1, 0 , +1 , +2 , s = +1/2 or -1/2, 5. Find the number of electrons in the subshell with azimuthal quantum number l =2., Azimuthal quantum number l =2 means d subshell. d sub shell contains maximum 10 electrons, 6. Find the spherical nodes of 4s , 2p and 4d orbitals., No. of spherical nodes = n-l-1, For 4s , No. of spherical nodes = n-l-1 = 4 – 0 - 1 = 3, For 2p , No. of spherical nodes = n-l-1 = 2 – 1 - 1 = 0, For 4d , No. of spherical nodes = n-l-1 = 4 – 2 - 1 = 1, 7. Which is higher in energy 3d or 4s? Why?, For 3d , n+l = 3 + 2 = 5, For 4s , n+l = 4 + 0 = 4, 3d has higher (n+ l) value and so it has higher energy., 8. How many orbitals are possible for (i) the main energy level with n=4 (ii) the subshell with n=5, l=3 ., Solution: (i) For n=4 , Total number of orbitals = n2 = 42 = 16, (ii) 5f . f subshell has 7 orbitals. Number of orbitals in a subshell = (2l +1 ) = (2 X 3 )+ 1 =7, 9. The number of unpaired electrons present in Ni is …2……. (Atomic number of Ni = 28) 3d 8 4s2, , ഈ NOTES ന്െട വീഡിേയാ, SUBSCRIBE െച, , ുക, , ാസുകൾ കാണാൻ CHEM DSM എ, , YOUTUBE ചാനൽ കാണുക.
Page 16 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 3 CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL : CHEM DSM, =======================================================, 1. Why do we need to classify elements?, It is very difficult to study chemistry of elements and their compounds individually. To avoid this, periodic, table is developed., 2. Law of triads →proposed by Dobereiner, The middle element of each triad had an atomic weight about half way between the atomic weights of, other two. Example for triads Li, Na, K, 3. Law of octaves → proposed by Newlands., Newland arranged the elements in increasing order of their atomic weights and noted that every eighth, element had properties similar to the first element., 4. State Mendeleev’s periodic law ., The properties of elements are periodic function of their atomic masses (atomic weights)., Mendeleev introduced the periodic law of elements for the first time., 5. Mendeleev’s periodic table :-Based on Mendeleev’s periodic law ,Mendeleev’s periodic table is formed., It consists of nine vertical columns called groups. There are 7 horizontal rows called periods., 6. What are the merits of Mendeleev’s periodic table?, (I), Mendeleev’s periodic table made the study of chemistry elements easier and systematic., (II), He predicted the properties of some undiscovered elements and left vacant places in his periodic, table. Example Eka aluminium (Gallium) and Eka silicon(Germanium)., (III), He placed elements with similar properties together by ignoring their atomic weights., (IV), He corrected the atomic masses of some elements. For example the atomic mass of Beryllium from, 13.5 to 9, 7. What are the demerits of Mendeleev’s periodic table?, (I), There is no proper position for hydrogen., (II), Isotopes of elements cannot be properly placed., (III), In certain pair of elements, the increasing order of atomic masses was not followed., 8. State Modern periodic law, The properties of elements are periodic function of their atomic numbers., Modern periodic law is proposed by Henry Moseley., 9. Long form of periodic table (Modern periodic table), It is based on modern periodic law., The elements are arranged in horizontal rows are called periods and vertical columns are called groups., There are seven periods and 18 groups in the periodic table., Each group constitutes a family of elements with similar properties., In the modern periodic table, the period indicates the value of principal quantum number (n)., There are 4 blocks in the periodic table -s-block, p-block, d-block, and f-block.
Page 17 :
Join Telegram Channel: https://t.me/hsslive, , 10. Periods of modern periodic table, Periods, Length of periods, 1, Very short period, 2, Short period, 3, Short period, 4, Long period, 5, Long period, 6, Monster period, 7, Incomplete period, 11. Blocks of periodic table, Blocks, , Groups, , s-block, , 1&2, , p-block, , 13 to 18, , d-block, , 3 to 12, , f-block, , Downloaded from www.Hsslive.in ®, , No. of elements, 2, 8, 8, 18, 18, 32, Maximum 32, , General electronic, configuration, , Electron filling sub shells, 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, , Main properties, , ns1-2, , They are all reactive soft metals with low ionization, energies. They form mainly ionic compounds, 2, 1-6, Includes metals, nonmetal and metalloids., ns np, They form ionic and covalent compounds., 1-10, 1-2, All are hard metals., (n-1)d ns, They form coloured compounds., They shows variable oxidation states., They show catalytic properties and paramagnetism., 1-14, 0-1, 2 Within each series properties are similar., (n-2)f (n-1)d ns, Most of the actinoids are radioactive and man made, , Lanthanoids, and, actinoids, 12. s and p block elements (except noble gases ) are called representative elements., 13. d-block elements are called transition elements. Why?, Since d-block elements show transition (change) from highly electropositive s-block elements to highly, electronegative p-block elements., 14. f-block elements are called inner transition elements., 15. How will you predict the position of elements in periodic table?, (I), Write electronic configuration, (II), The principal quantum number of valence shell = period of the element., (III), The sub shell in which the last electron is filled = block of the element, (IV), Group of the element :, (a) For s -block; group number = number of ‘s’ electrons, (b) For p -block; group number = 12 + number of ‘p’ electrons, (c) For d -block; group number =2 + number of ‘d’ electrons, (d) For f- block; group number = 3, 16. Which are periodic properties?, , (i)Atomic radius, (ii) Ionization energy, (iii) Electron gain enthalpy, (iv) Electronegativity, , (v) Valency, , 17. What is Atomic radius? Explain its variation along a period and in a group., Atomic radius is defined as the distance from the centre of the nucleus of the atom to the outer most shell, of electrons.
Page 18 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , In a period, from left to right, atomic radii decrease., From left to right atomic number increases, nuclear charge, increases. But the electrons are added in the same shell. As a, result electrons are attracted closer to the nucleus by increased, effective nuclear charge. This leads to decrease of atomic size., In a group, from top to bottom, atomic radii increase due to, increase in number of shells., , 18. Atomic radii of noble gases are higher than that of halogens. Why?, This is because noble gases are mono atomic. So Vander Waal’s radius is used to express the atomic radius, which is greater than metallic radius or covalent radius., 19. Why cation is smaller and anion is larger than parent atom?, Cation is formed by the loss of electrons. Nuclear charge remains same. So effective nuclear charge, increases, attraction increases and size decreases., Anion is formed by the gain of electrons. Nuclear charge remains same. So effective nuclear charge, decreases, attraction decreases and size increases., 20. What are isoelectronic species? Give examples. Arrange them in the increasing order of size., Atoms and ions which contain same number of electrons are called isoelectronic species., N3- ,O2- , F- , Na+ , Mg2+ , Al3+, (These have different nuclear charge , But contain 10 electrons each), The order of decreasing size is N3- > O2- > F- >Na+ > Mg2+ > Al3+, Among the isoelectronic species, greater the nuclear charge , smaller the size., 21. What is Ionization energy ? Explain its variation along a period and in a group., The amount of energy required to remove the most loosely bound electron from an isolated gaseous atom, is called ionization energy (ionization enthalpy)., In a period, from left to right ionization, enthalpy increases due to increase in nuclear, charge and so valence electrons are more, tightly held by the nucleus., But some irregularities are observed., Beryllium has higher ionization energy than, Boron due to stable electronic configuration, 2 2, ( 1s 2s ) . Similarly nitrogen has higher ionization energy than oxygen due to half filled stable electronic, configuration ( 1s22s22p3)., In a group, Ionization enthalpy decreases from top to bottom due to increase in atomic size., 22. What are the factors affecting ionization energy?, (i) Atomic size (ii) Nuclear charge (iii) shielding effect or screening effect of inner electrons, (iv) penetration effects of electrons (s>p>d>f) (v) electronic configuration., 23. First ionization enthalpy of sodium is lower than that of magnesium but its second ionization energy is, higher than that of magnesium. Explain., For sodium (1S22S22P63S1) , first electron is removed from 3s orbital , that is easy , because removal of, electron can form stable electronic configuration. So first ionization energy is low (Na +→1S22S22P6 )
Page 19 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , For Magnesium (1S22S22P63S2) , first electron is removed from completely filled 3s orbital , that is difficult ,, because removal of electron is from stable electronic configuration. So first ionization energy is high., high, +, But for Na , its second ionization energy is high because of stable electronic configuration., 24. What is electron gain enthalpy ( Electron affinity)? Explain its variation along a period and in a group., The amount of energy released when an electron is added to isolated gaseous atom is called electron gain, enthalpy., X (g) +e− → X(g) − ∆H = ∆egH, In a period, from left to right electron gain enthalpy becomes more and more negative., negative, It is due to increase in nuclear charge., In a group, Electron gain enthalpy decreases from top to bottom due to increase in atomic size., 25. Electron gain enthalpy of noble gases are zero or positive .Why?, Due to completely filled electronic configuration( ns2np6)., 26. Electron gain enthalpy of fluorine is less than that of chlorine .Why?, This is due to the very small size of fluorine atom. As a result, inter electronic repulsion in the 2p sub shell, of F is more than that in the relatively larger 3p sub shell in chlorine atom., 27. What are Factors affecting electron gain enthalpy ?, (i)Atomic size (ii) Nuclearr charge, (iii) electronic configuration, 28. What is Eectro negativity?? Explain its variation along a period and in a group., Electro negativity is the ability of an atom in a molecule to attract the shared pair of electrons towards it., Halogens have highest electro negativity in their periods., Fluorine is the most electro negative element., Electro negativity increases from left to right in a period due to increasing nuclear charge., Electro negativity decreasess from top to bottom in a group due to increase in atomic size., 29. What are Anomalous properties?, The first element of each group in s and p blocks differs from the rest of the elements in many properties, are called anomalous properties., It is due to the following reasons. (i) Small size (ii)High charge/radius ratio.(iii)High electro negativity, (iv) Non availability of d orbitals., 30. What is Diagonal relationship? What are the reasons for Diagonal relationship?, First element in any group shows similarities with second element in next group. This is called diagonal, relationship., For example, Lithium shows resemblance with Magnesium due to diagonal, iagonal relationship, Beryllium resembles aluminium due to diagonal relationship., , Reasons, :- (i) similar size, (ii)similar ionization energy , (iii) similar electronegativity, (iv)similar charge/radius, ratio, 31. What is Valency?, Valency is the combining capacity of an element. It is determined by valence electrons., , Valency = Number of valence electrons, OR 8 − No. of valence electrons, , In a group,, valency is same, since valence electrons are same.
Page 20 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , In a period , valency increases up to 4 and then decreases with respect to hydrogen and with respect to, oxygen, valency increases up to seven., 32. Explain the behavior of different type Oxides?, The normal oxide formed by the element on extreme left is the most basic (e.g. Na 2O, CaO)., The normal oxide formed by the element on extreme right is acidic ( e.g. Cl 2O7) ., Oxides of the elements in the centre are amphoteric (e.g. Al 2O3 , As2O3 ) or neutral (e.g. CO, NO, N2O), 33. Show by chemical reaction with water that Na2O is basic and Cl2O7 is acidic., Na2O with water forms a strong base whereas Cl2O7 forms a strong acid., , Na2O + H2O → 2 NaOH, Cl2O7 + H2O → 2 HClO4, , ,, , 34. Notation for IUPAC nomenclature of elements, digit name abbreviation, digit, name, abbreviation, 0, nil, n, 5, pent, p, 1, un, u, 6, hex, h, 2, bi, b, 7, sept, s, 3, tri, t, 8, oct, o, 4, quad q, 9, enn, e, 35., A, B, Most electronegative element, Fluorine (F), Most electron gain enthalpy element, Chlorine (Cl), Most electropositive element, Francium (Fr), Most abundant element in the universe, Hydrogen, Most abundant element in the earth crust, Oxygen, Most abundant element in the atmosphere, Nitrogen, PROBLEMS AND THEIR SOLUTIONS, 1. Justify the presence of 18 elements in the 5 th period of the periodic table., Fifth period , orbitals filled up = 5s , 4d , 5p . So No. of elements = 2 + 10 + 6 = 18, 2. What is the maximum number of elements that can be accommodated in the seventh period of the periodic, table?, Seventh period , orbitals filled up = 7s, 5f, 6d, 7p, So maximum number of elements = 2 + 14 + 10 + 6 = 32, 3. Write the electronic configuration and predict their position in periodic table, (I) A (atomic number 14) → 1s2 2s2 2p6 3s2 3p2 Period = 3 , Group = 4 + 10 = 14 , Block = p, (II) B (atomic number 29) → 1s2 2s2 2p6 3s2 3p6 3d10 4s1 Period = 4 , Group = 1 +10 = 11 , Block = d, (III) C (atomic number 117) → [Rn] 7s2 5f14 6d10 7p5 Period = 7 , Group = 7 + 10 = 17 , Block = p, (IV) D ( atomic number 120) → [Rn] 7s25f14 6d10 7p6 8 s2 Period = 8 , Group =2 , Block = s, 4. Predict the formula formed by the combination of following elements, (I), Lithium and oxygen → ( Valency of Li = 1 , O = 2 ) → Formula : Li 2O, (II), Magnesium and nitrogen →( Valency of Mg= 2 , N = 3 ) → Formula :Mg 3N2, (III), Aluminium and iodine → ( Valency of Al = 3 , I = 1 ) → Formula : AlI 3, (IV), Silicon and oxygen →( Valency of Si = 4 , O = 2 ) → Si2O4 Formula : SiO2, (V), Phosphorus and chlorine →( Valency of P = 3, 5 , Cl = 1 ) → Formula : PF3 OR PF5, 5. Write the IUPAC nomenclature of elements with atomic number from 101 , 120 , 109, 101 → Unnilunium (Unu) ,, 120 →Unbinilium ( Ubn) , 109 →Unnilennium ( Une), 6. The atomic number of element with IUPAC name Ununbium is 112, ഈ NOTES ന്െട വീഡിേയാ, SUBSCRIBE െച, , ുക, , ാസുകൾ കാണാൻ CHEM DSM എ, , YOUTUBE ചാനൽ കാണുക.
Page 21 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , CHAPTER 4 CHEMICAL BONDING AND MOLECULAR STRUCTURE, PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL : CHEM DSM, =======================================================, 1. State Octet rule, Atoms of various elements enter into chemical combination to attain eight electrons (octet of electrons) in, their valence shell ( outer most shell)., 2. What is Chemical bond?, The attractive force between the atoms in a molecule is called chemical bond., Chemical bonds are, (I), Covalent bond :- Formed byy the sharing of electrons, (II), Ionic bond :- Formed byy the transfer of electrons, 3. What is Covalent bond?, The bond formed by mutual sharing, ring of electrons between combining atoms., (I), H2 molecule :- H - H, Single bond between hydrogen atoms, (II), , Cl2 molecule :-, , Cl-Cl, Cl, , SSingle, ingle bond is formed by sharing of one electron each., each, , (III), , O2 molecule :-, , O=O, , Double, ouble bond is formed by sharing of two electrons each., , (IV), , N2 molecule :-, , N≡N, ≡N, , TTriple, riple bond is formed by the sharing of three electrons each., , (V), , CO2 molecule :-, , Two dou, double bonds with two oxygen atoms, , (VI), , H2O molecule :-, , Two single bonds with two hydrogen atoms and two lone pairs, , (VII), , CCl4 molecule :- Four single bonds with four chlorine atoms
Page 22 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 4. What are the limitations of octet rule, rule?, (I), It cannot explain the formation of mole, molecules with incomplete octet (eg: BeF2 , BF3), (II), It cannot explain the formation of molecules with expanded (super) octet (eg:, (eg PCl5, SF6), (III), It cannot explain the formation of compounds by Xe and Kr( eg :, XeF2 , XeF6), (IV), It cannot explain the formation of odd electron molecules (eg: NO, NO2), 5. Define ionic bond. How is it formed? Give examples., Ionic bond or electrovalent bond is formed by the, complete transfer of one or more electrons from, one atom to another atom., Positive ion called cation and is formed by the loss of electrons., Negative ion called anion and is formed by the gain of electrons., The attractive force between opposite charged ions is called ionic bond. e.g. NaCl , CaF2 , CaO, 6. What are the factors favouring ionic bond?, (i), Low, ow ionization energy of the electropositive atom (i.e., metal atom), (ii), High negative electron gain enthalpy of the electronegative atom., (iii), High lattice enthalpy of the ionic compound formed., 7. What is lattice energy and its importance?, The lattice enthalpy of an ionic solid is defined as the energy required to completely separate one mole of, the ionic compound in to its gaseous ions., The higher the lattice energy, higher the stability of the ionic compound formed., 8. What is Formal Charge?, The formal charge is the charge, harge assigned to some atoms in the lewis structure of certain compounds., , FC = V-N- B/2, FC = Formal Charge ,, V= Number of valence electrons in free atom,, N= Number of non bonding electrons ,, B= Number of bonding electrons, 9. What is resonance? Draw the resonance structure of ozone., The properties of some compounds cannot be explained by single lewis structure. Such, Suc compound exist as, a combination of two or more structures. This phenomenon is called resonance. Its Characteristics are, (I), Resonance stabilizes the molecule., (II), Resonance averages the bond characteristics as a whole., Resonance structure of ozone are, , 10. Polar molecules and non polar molecules, It is a polar molecule
Page 23 :
Join Telegram Channel: https://t.me/hsslive, , Polar molecules, , Downloaded from www.Hsslive.in ®, , Non polar molecules, , Covalent bonded molecules having partial, Covalent bonded molecules having nocharge, positive charge and negative charge are called, are called nonpolar molecules., polar molecules., Hetero nuclear diatomic molecules( HCl, HBr ), Homo nuclear diatomic molecules (O2 ,H2 N2), Irregular geometry molecules (H2O , NH3), Regular geometry molecules (BeF2, BF3 ,CH4), 11. What is Dipole moment?? Give its unit, unit., Dipole moment is defined as the product of the magnitude of charge and the distance between the centre, of charges. Its unit is Debye (D)., , Dipole moment ( µ) = charge (q) x distance (r), 12. The dipole moment of CO2 is zero. Why?, CO2 is linear molecule and the two equal bond dipoles are in opposite directions, and cancel each other. So the dipole moment of CO2 is zero., 13. The dipole moment of BeF2 is zero. Why?, BeF2 is linear molecule and the two equal bond dipoles are in opposite directions, and cancel each other. So the dipole moment of BeF 2 is zero., , 14. The dipole moment of H2O is not zero. Why?, Water, ter molecule has bent structure, structure. Two O-H, H bonds are oriented at an angle of, 0, 104.5 . The bond dipoles of two O, O-H bonds do not cancel each other., other So water, molecule has net dipole moment., 15., , The dipole moment of BF3 zero. Why?, , BF3 has trigonal planar structure in which three B-F, B bonds are, oriented at an angle of 1200 to one another. The, T three bonds lie, in one plane. Here the resultant of any two bond dipole is equal, and opposite to third and the dipole moments of these bonds, cancel one another giving net dipole moment equal to zero., 16. Ammonia (NH3) has higher dipole moment than NF3, even though F is more electronegative than hydrogen., Why?, Both have pyramidal structure. The individual, dipole moments do not cancel each other. So, they have net dipole moment., , But ammonia has higher dipole moment . It is due to the orbital dipole due to the lone pair is in the same, direction of three N-H bonds., But in nitrogen tri fluoride,, the resultant dipole of three N, N-FF bonds is in opposite direction to the orbital, orbit, dipole of lone pair. So partiallyy cancelled and dipole moment is low.
Page 24 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 17. State Fajan’s rule regarding the partial covalent character of an ionic bond., Covalent character of ionic bond increases with (i) Small, mall size of cation and large size of anion, (ii) Large, arge charge on both the cation and anion., Covalent character increases, reases in the order :, (1), , LiCl > NaCl > KCl (Here Lithium is small size cation), (2) NaCl < MgCl2 < AlCl3 (Here Alumin, Aluminium is small size cation), (3) NaF < NaCl < NaBr < NaI (Here iodine is large size anion), 18. What are the main postulates of valence shell electron pair repulsion theory (VSEPR), (I), The shape of the molecule depends on the number of valence electron pairs of the central atom., (II), The electron pairs repel each other, other.. As a result, the electron pairs try to stay as far apart to acquire a, state of minimum energy or maximum stability., (III), A multiple bond is treated as if it is a single electron pair., (IV), The repulsive interaction decreases in the order., Lone pair-Lone, Lone pair > Lone pair, pair-Bond pair > Bond pair-Bond pair, Type, No. of electron pairs, Shape of the molecule, Examples, Bond angle, AB2, 2 (bp), Linear, BeF2, BeCl2, 180 0, AB3, 3(bp), Trigonal planar, BF3, 120 0, AB4, 4(bp), Tetrahedral, CH4, 109.5 0, AB5, 5(bp), Trigonal bi pyramid, PCl5, 120 0, 90 0, AB6, 6(bp), octahedral, SF6, 90 0, AB3E, 3(bp), 1 (lp), Trigonal pyramidal, NH3, 107 0, AB2 E2, 2(bp), 2 (lp), Bent or inverted V shape H2O, 104.5 0, AB4E, 4(bp), 1 (lp), See- saw, SF4, AB3 E2, 3(bp), 2 (lp), T shape, ClF3, AB2 E3, 2 (bp), 3(lp), Linear, XeF2, AB5E, 5 (bp), 1 (lp), Square Pyramid, BrF5, AB4 E2, 4(bp), 2 (lp), Square planar, XeF4, 19. Explain the shape of following molecules on the basis of VSEPR theory., , BeCl2, Be →atomic number 4 , electronic configura on 2,2 . Be, Beryllium has two valance, electrons. Bonded, nded with two chlorine atoms. SSo Beryllium has two bond pairs around it., To minimize repulsion, linear geometry. Bond angle 180 0, , BF3, B→ atomic number 5 , Electronic, lectronic configuration 2,3 . B, Boron has three valance, electrons. Bonded with threee fluorine atoms. So Boron has three bond pairs around, it. To minimize repulsion, trigonal planar geometry. Bond angle 120 0, , CH4, C→ atomic number 6 , Electronic, lectronic configuration 2,4 ., Carbon has four valance electrons., Bonded with four hydrogen atoms, atoms. So Carbon has four bond pairs around it., To minimize repulsion, tetrahedral geometry. Bond angle 109.5 0
Page 25 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , PCl5, P→ atomic number 15, electronic configura on 2,8,5 ., Phosphorus has five valance electrons. Bonded with five chlorine, atoms. So Phosphorus has five bond pairs around it., To minimize repulsion, trigonal bipyramid geometry. Bond angle 120 0, and 90 0, , SF6, S→ atomic number 16, electronic configura on 2,8 ,6 ., Sulphur has six valance electrons. Bonded with six fluorine atoms ., So Sulphur has six bond pairs around it., To minimize repulsion, octahedral geometry. Bond angle 90 0, , NH3, N →atomic number 7 , electronic configura on 2,5 ., Nitrogen has five valance electrons. Bonded with three hydrogen atoms ., So Nitrogen has three bond pairs and one lone pair., There are two type repulsions., Bond pair-bond pair repulsion and bond pair- lone pair repulsion., Bond pair-lone pair repulsion is greater and bond angle is slightly reduced from tetrahedral angle to 107 0 ., Geometry is trigonal pyramidal., , H 2O, O → atomic number 8 , Electronic configuration 2,6 ., Oxygen has six valance electrons. Bonded with two hydrogen atoms., So Oxygen has two bond pairs and two lone pairs around it., There are three type repulsions., Bond pair-bond pair repulsion < bond pair- lone pair repulsion < lone pair – lone pair repulsion., Due to these repulsions bond angle is reduced from tetrahedral angle to 104.5 0., Geometry is bent shape or inverted V shape., , XeF4, It has square planar geometry, Four bond pairs and two lone pairs
Page 26 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 20. Draw the potential energy curve for the formation of a hydrogen molecule on the basis of inter nuclear, distance of the hydrogen atoms., , 21. The orbital overlap concept of covalent bond formation.(VALENCE BOND THEORY), (I), Covalent bonds are formed by the overlapp, overlapping, ing of half filled atomic orbitals present in the valence, shell of the combining atoms., (II), The orbitals undergoing overlapping must have electrons with opposite spins., (III), The greater the overlapping , the stronger the bond formed., 22. What are the difference between, n sigma bond and pi bond?, , Sigma bond (σ bond), , Pi bond (π bond), , Sigma bond is formed by the end to end (or axial ), Pi bond is formed by the side wise, overlap of atomic orbitals, (or lateral ) overlap of atomic orbitals, This can be formed by overlap of s-ss ,s, ,s-p ,p-p orbitals This can be formed mainly by overlap of p-p, p orbitals, Sigma bond is strong bond, Pi bond is weak bond, Free rotation of atoms around sigma bond is, Free rotation, on of atoms around pi bond is not possible, possible, Pz + Pz → sigma bond (σ), , Px + Px →pi bond (π) ,, Py + Py →pi bond (π), 23. What is Hybridization? Give their Characteristics, Inter mixing of atomic orbitals of same element with slightly different energies and different shape to get, orbitals of same energy and shape is called hybridisation., Characteristics, (I), The number of hybridised orbitals formed is equal to the number of orbitals that get hybridized., (II), Hybridized orbitals have same energy and shape and so more effective in forming stable bonds., (III), The hybrid orbitals are directed in some directions, and give geometry to the molecules., 24. Explain sp3 hybridisation using CH4 as example., , Here one s orbital and three p orbitals undergo hybridisation, an, and four sp3 hybridized orbitals are formed., 0, Tetrahedral geometry. Bond angle is 109.5 ., , One s orbital + Three p orbitals → Four sp3 hybridized orbitals
Page 27 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 25. Explain sp2 hybridisation using BF3 as example, , Here one s orbital and two p orbitals undergo hybridisation, and three sp2, hybridized orbitals are formed., , One s orbital + Two p orbitals → Three sp2 hybridized orbitals, , Trigonal planar geometry. Bond angle is 1200, 26. Explain sp hybridisation using BeCl2 as example, , Here one s orbital and one p orbital undergo, hybridisation, and two sp hybridized orbitals are, formed., , One s orbital + One p orbital → Two sp hybridized orbitals, , Linear geometry. Bond angle is 1800, 27. Explain sp3d hybridisation using PCl5 as example., , Here one s orbital , three p orbitals and one d orbital undergo hybridisation, and five sp 3d hybridized, orbitals are formed., , One s orbital + Three p orbitals + One d orbital → Five sp3d hybridized orbitals, , Trigonal bipyramid geometry. Bond angle is 120 0 and 90 0, , 28. Explain the geometry of PCl5 molecule and account for its high reactivity., Hybridisation is sp3d . Trigonal bipyramid geometry., There are three equatorial bonds and two axial bonds., The axial, al bonds are slightly longer than equatorial bonds due to greater repulsion from equatorial bonds., Due to different bond lengths, it unsymmetric and highly reactive., 29. Explain sp3d2 hybridisation using SF6 as example.
Page 29 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 32. What are the postulates of Molecular orbital theory(MOT)?, (I), In molecules, electrons are present in molecular orbitals., (II), Molecular orbitals are formed by the combination of atomic orbitals of same energy and proper, geometry., (III), The number of molecular orbitals formed is equal to the number of combining atomic orbitals., (IV), Molecular orbitals are associated with the nuclei of all the bonded atoms in a molecule., (V), In molecular orbitals electrons are filled according to Aufbau principle, Pauli’s exclusion principle and, Hund’s rule., 33. What are the differences between bonding molecular orbital and anti bonding molecular orbital?, , BMO, , ψA + ψ B, ABMO, ψA - ψ B, BMO is formed by the addition (attraction) of atomic ABMO is formed by the substraction (repulsion) of atomic, orbitals, orbitals, It has greater electron density between the nuclei of It has less electron density between the nuclei of bonded, bonded atoms, atoms, Its energy is less than the energy of atomic orbitals, Its energy is more than the energy of atomic orbitals, 34. Define bond order . How is bond order related to bond length and bond strength?, Bond order is defined as half of the difference between the numbe, numberr of electrons in the bonding molecular, orbitals and the number of electrons in the anti bonding molecular orbitals ., , Bond order = ½ [Nb - Na ], , If the bond order is positive , molecule is stable., If the bond order is zero,, molecule is unstable. Such molecule will not exist., Bond order is directly proportional to bond strength and bond dissociation energy., Bond order is inversely proportional to bond length., Bond order = 1 , single bond ,Bond, Bond order = 2 , double bond , Bond order = 3 , triple bond, 35. Explain the stability and magnetic property of H 2 molecule (2 electrons), σ1s2, Bond order = ½ [Nb - Na ] = ½ [2 - 0 ] = 1, Here the bond order is positive , molecule is stable., Bond order = 1 , single bond,, No unpaired electrons, diamagnetic., , 36. Why He2 molecule will not exist? (4 electrons), σ 1s2 σ*1s2, Bond order = ½ [Nb - Na ] = ½ [2 - 2 ] = 0, Here the bond order is zero , molecule is unstable. So it will, not exist.
Page 32 :
Join Telegram Channel: https://t.me/hsslive, , 44., , Downloaded from www.Hsslive.in ®, , What is hydrogen bond? Which are different type hydrogen bonds? Explain each, Hydrogen bond is defined as the attractive force between hydrogen atom bonded tto, o fluorine, oxygen or, nitrogen and an electronegative atom of the same or adjacent molecule., There are two types of hydrogen bonds, (I), Inter, ter molecular hydrogen bond ::- Hydrogen, ydrogen bond between different molecules of same type or, different type., It increases the boiling point., e.g., H bonding in HF, …….H, …….H-F……H-F…….H-F……..H-F….., (II), , Intra molecular hydrogen bond, bond:- Hydrogen bond within the same molecule., It decreases the boiling point., e.g., Hydrogen bonding in Ortho nitr, nitro phenol, , 45. Ortho nitro phenol and para nitro phenol can be separated by steam distillation. Explain, , In ortho nitro phenol intra molecular hydrogen bond is possible and so its boiling point is low and steam, volatile., But in para nitro phenol, inter molecular hydrogen bonding and so boiling point is high and so it is not, steam volatile. So these can be separated by steam dist, distillation., 46. H2O is liquid ,H2S is gas at room temperature. Give reason, , In water , molecules are associated by inter molecular hydrogen bonds. So it exist, exists as liquid at room, temperature., But in hydrogen sulphide , no hydrogen bond is possible. So it exists as alone and so gas., 47. HF is liquid while HCl is gas at room temperature. Give reason, In between HF molecules, inter molecular hydrogen bonds are possible and so liquid., But not in HCl, …….H-F……H-F…….H-F……..H-F…..
Page 33 :
Join Telegram Channel: https://t.me/hsslive, , CHAPTER, , Downloaded from www.Hsslive.in ®, , 5, , STATES OF MATTER, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL : CHEM DSM, =============================, =======================================================, ==========================, 1. Name the different types vander Waal’s forces (attractive inter molecular forces), (I) Dispersion, on forces or London forces: In atoms and non polar molecules., (II) Dipole – Dipole forces: Between polar molecules., (III) Dipole-induced dipole forces: B, Between polar and non polar molecules., 2. State Boyle’s law and give its mathematical forms., The law states that at constant temperature, the volume of a given mass of gas is inversely proportional, to its pressure., Mathematically,, 𝑽= 𝒌, , 𝑽∝, , 𝟏, 𝑷, , (at constant T and n), , 𝟏, 𝑷, , PV = constant, OR, Pressure x volume = constant, P1V1 = P2V2, 3. Draw the graphical representations of Boyle’law (isotherm), , 4. The size of weather balloons become larger and larger as it ascends to higher altitudes. Give reason., At higher altitudes, atmospheric pressure is low., When pressure decreases volume increases (Based on Boyle’s law), 5. At constant temperature , for a given mass of a gas , density is directly proportional to pressure. Prove., A relationship, p between density and pressure of a gas is derived from Boyle’s law., Density = mass/volume, Volume = mass/density , 𝑽 =, , 𝒎, 𝒅, , According to Boyle’s law , PV = k, Substituting the value of V, 𝑷, There fore 𝒅 = 𝑷, d ∝ P, , 𝒎, 𝒌, , 𝒎, 𝒅, , = 𝒌, , = P k’, , ie. , density is directly proportional to pressure.
Page 34 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 6. State Charle’s law and gives its mathematical forms., The law states that at constant pressure, the volume of a given mass of gas is directly proportional to the, temperature on Kelvin scale., Mathematically,, OR, OR, , OR, , 𝑽, 𝑻, , V∝ T, , (at constant P and n), , =𝒌, 𝑽𝒐𝒍𝒖𝒎𝒆, , 𝑻𝒆𝒎𝒑𝒆𝒓𝒂𝒕𝒖𝒓𝒆, 𝑽𝟏, 𝑻𝟏, , =, , = 𝒄𝒐𝒏𝒔𝒕𝒂𝒏𝒕, , 𝑽𝟐, 𝑻𝟐, , 7. Draw the graphical representations of charle’s law (isobar), , The, he lowest hypothetical or theoretical, temperature of – 273.15 0C (0 K) at which all, gases are supposed to have zero volume is, called absolute zero. A scale of temperature, based upon this is called the absolute scale of, temperature., , T K = t 0C +273, 8. State Gay Lussa’s law,, give its mathematical forms and graph, The law states that at constant volume, the pressure of a given mass of gas is directly proportional to the, temperature on Kelvin scale., Mathematically, P ∝ T (at constant V and n), P = kT, OR, 𝑷, 𝑻, 𝑷𝒓𝒆𝒔𝒔𝒖𝒓𝒆, 𝑻𝒆𝒎𝒑𝒆𝒓𝒂𝒕𝒖𝒓𝒆, , OR, , OR, , =𝒌, , = 𝒄𝒐𝒏𝒔𝒕𝒂𝒏𝒕, , 𝑷𝟏, 𝑻𝟏, , =, , 𝑷𝟐, 𝑻𝟐, , 9. The tyres of automobiles are inflated to lesser pressure during summers and to higher pressure during, winter. Why?, On hot summer days, the air inside the tyre gets heated up. TThe pressure inside the tube increases. This, may cause a tyre burst., During winter, the pressure inside the tyre decreases considerably., 10. State Avogadro’s law and gives its mathematical form., Avogadro’s law states that equal volume of all gases under similar conditions of temperature and, pressure contain equal number of molecules., , V ∝ n (at constant T and P )
Page 35 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 11. Derive ideal gas equation (equation of state)., A gas that follows Boyle’s law, Charles’ law and Avogadro law strictly is called an ideal gas., Such a gas is hypothetical., 𝟏, , Boyle’s law, 𝑽 ∝, , 𝑷, , (at constant T and n), , Charle’s law, V ∝ T (at constant P and n), Avogadro’s law, V ∝ n (at constant T and P ), Combining the above three laws, 𝑽 ∝, 𝑽=, , 𝒏𝑻, 𝑷, , OR, , 𝑹𝒏𝑻, 𝑷, , PV = nRT, This equation is called ideal gas equation (equation of state)., R = universal gas constant. R = 8.314 JK-1mol-1 R = 0.0831 L bar K-1mol-1 R = 0.0821 L atm K-1mol-1, 12. Prove that the density of a gas is directly proportional to molar mass., Ideal gas equation, PV = nRT, n =w/M, ∴ 𝑷𝑽 =, 𝑷=, , 𝒘𝑹𝑻, 𝑴, , 𝒘𝑹𝑻, 𝑽𝑴, , ∴𝑷=, , ,, , d = w/V , density = mass/volume, , 𝒅𝑹𝑻, 𝑴, , ∴𝒅=, , 𝑷𝑴, 𝑹𝑻, , i.e. density is directly proportional to molar mass., 13. Combined gas law is, , 𝑷𝟏 𝑽𝟏, 𝑻𝟏, , =, , 𝑷𝟐 𝑽𝟐, 𝑻𝟐, , 14. State Dalton’s law of partial pressure .Give its equation and application., The total pressure exerted by the two or more non reacting gases is equal to the sum of their partial, pressures., If p1 , p2 , p3....... are the partial pressures of the component gases.,, , Then total pressure P = p1 + p2 +p3+ ….., This law is used to calculate the pressure of dry gas collected over water in laboratory., , P observed = Pgas + Pwater vapour, P observed = Pgas + aqueous tension, Pgas = P observed - aqueous tension, 15. Can we use Dalton’s law of partial pressure to calculate the pressure of NH 3 and HCl. Why?, No. Dalton’s law of partial pressure is applicable only to a mixture of two or more non reacting gases ., NH3 and HCl react to form NH4Cl. So Dalton’s law is not applicable ., NH3+ HCl →NH4Cl.
Page 36 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 16. Prove Pi = Xi Ptotal., , Total pressure P = P1 + P2, PV = nRT, 𝑷𝟏 =, , 𝒏𝟏 𝑹𝑻, 𝑽, , ∴𝑷=, ,, , 𝑷𝟐 =, , 𝑷𝟏, 𝑷𝒕𝒐𝒕𝒂𝒍, , =, , =, , 𝒏𝟏, 𝒏𝟏 𝒏𝟐, , 𝑽, , 𝒏𝟐 𝑹𝑻, 𝑽, , Total pressure, Ptotal = P1 + P2 =, 𝒏𝟏 𝑹𝑻, 𝑽, (𝒏𝟏 𝒏𝟐 )𝑹𝑻, 𝑽, , 𝒏𝑹𝑻, , 𝒏𝟏 𝑹𝑻, 𝑽, , +, , 𝒏𝟐 𝑹𝑻, 𝑽, , =, , (𝒏𝟏 𝒏𝟐 )𝑹𝑻, 𝑽, , = X1 ( mole fraction of first gas), , P1 = X1 Ptotal. , Similarly P2 = X2 Ptotal. ,, Therefore Pi = Xi Ptotal., Partial pressure of individual gas= Molefraction of individual gas X Total pressure, 17. What are the postulates of Kinetic molecular theory of gases?, (I), All the gases are made up of extremely small particles called molecules., (II), The molecules are separated by large distance and so there is no attractive force between, the gas molecules., (III), The volume of the gas molecule is negligible as compared to the total volume of the gas., (IV), The molecules are in random and rapid motion. During their motion, they collide with each, other and on the walls of the container., (V), The pressure of the gas is due to the collision of molecules on the walls of the container., (VI), Molecular collisions are perfectly elastic ie. There is no net loss or gain energy in their, collisions. However, there may be redistribution of energy during such collisions., (VII) Different molecules possess different speed and hence different energies. However, the, average kinetic energy of the molecules is directly proportional to its absolute temperature., 18. Which are the ways of expressing molecular speeds? Explain each, (I), Average speed ( Uav), (II), Most probable speed ( Ump), (III), Root mean square speed ( Urms), Average speed ( Uav) :- It is the mean or average of the speeds of the different molecules of the gas., If there are n number of molecules in a sample and their individual speeds are u 1, u2, …….un, then, average speed of molecules,, 𝒖𝟏 + 𝒖𝟐 + ⋯ 𝒖𝒏, 𝑼𝒂𝒗 =, 𝒏, Most probable speed ( Ump) :- The speed possessed by maximum number of molecules is called most, probable speed., Root mean square speed ( Urms) :- It is the square root of the mean of the squares of the speeds of the, molecules ., 𝑼𝒓𝒎𝒔 =, , 𝒖𝟐𝟏 + 𝒖𝟐𝟐 + … . 𝒖𝟐𝒏, 𝒏
Page 37 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 19. Relationships between different types of speed( Root mean square speed, average speed and most, probable speed), urms > uav > ump, The ratio between the three speeds is given below :, , u mp: uav : urms : : 1 : 1.128 : 1.224, , 20. Which are the factors that depends on molecular speeds, (I) Temperature : When temperature increases , most probable speed increases., (II) Mass of the molecules :- Higher the mass of the molecules, lower the speed ., For example: Lighter nitrogen molecules move faster than heavier chlorine molecules., 21. Draw the graphs of P vs PV graph and Z vs P graph of real gases and ideal gas., , Compressibility factor,, , 𝒁=, , 𝑷𝑽, 𝒏𝑹𝑻, , For ideal gas Z =1 ., For Hydrogen, Nitrogen etc , Z value is greater than that for ideal gas., At low pressure and high temperature, real gases show ideal behavior., 22. Why do real gases deviate from ideal behavior?, It is due to the two faulty assumptions of kinetic theory of gases., (I), There is no attractive force between the gas molecules., (II), The volume of the gas molecules is negligible as compared to the total volume the gas., But at high pressure and low temperature, total volume of the gas is low. So the gas molecules are, closer, there is attractive force between the gas molecules and the volume of the gas molecules, cannot be neglected., 23. What is Boyle temperature or Boyle point?, The temperature at which a real gas behaves like an ideal gas over an appreciable range of pressure is, called Boyle temperature or Boyle point., Boyle temperature or Boyle point of a gas depends upon its nature., 24. Write Vander Waals equation ( Modified ideal gas equation) and explain each term., , 𝒏𝟐 𝒂, 𝑷 + 𝟐 (𝑽 − 𝒏𝒃) = 𝒏𝑹𝑻, 𝑽
Page 38 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , P= pressure , V = volume ,, n2a/V2 = pressure correction ,, nb = volume correction, ‘a’ and ‘b’ are vander waal’s constant and depends on the nature of the gas., The value of ‘a’ is a measure of the magnitude of attractive force between the gas molecules., The value of ‘b’ is a measure of the effective size of the gas molecules., 25. What is Critical temperature ?, Critical temperature is the temperature above which it cannot be liquefied by application of pressure., Critical temperature of CO2 is 30.98 0C. while that of NH3 is 132.5 0C., Both CO2 and NH3 are liquefiable at room temperature., NH3 is easily liquefiable. Because its critical temperature is above room temperature., Higher the critical temperature of the gas, the more easily can it be liquefied., H2 and He cannot be liquefied at room temperature on applying very high pressure., Because its critical temperature is very low, 26. What is Vapour pressure?, The pressure exerted by the vapours over the surface of the liquid at equilibrium is called vapour, pressure., The factors affecting vapour pressure are, (I), Nature of liquid:- If the inter molecular force are weak, the molecules can easily leave the liquid and, come to the vapour phase and hence vapour pressure will be high. For example, the vapour, pressures of acetone,ether, alcohols are higher than that of water at the same temperature., (II), Temperature of the liquid:- The vapour pressure increases with increase of temperature because, more vapours are produced., 27. What is Boiling point?, Boiling point is the temperature at which vapour pressure of the liquid is equal to the external pressure., At 1 atm pressure boiling temperature is normal boiling point., If pressure is 1 bar then boiling point is called standard boiling point., The normal boiling point of water is 100 0C., The standard boiling point of water is 99.6 0C., 28. Pressure cooker is used at hill stations for cooking food. Why?, The normal boiling point of water is 100 0C. Boiling point depends on external pressure., If the external pressure is low, the liquid boils at low temperature., At high altitudes (on the top of a mountain) external pressure is low., Therefore Pressure cooker is used at hill stations for cooking food., 29. What is Surface tension? What happens when temperature increases?, A molecule in the bulk of liquid experiences equal intermolecular forces from all sides. The molecule,, therefore does not experience any net force. But for the molecule on the surface of liquid, net attractive, force is towards the interior of the liquid , due to the molecules below it. As a result of this inward pull on, all molecules lying at the surface , the surface behaves as if it were under tension (like a stretched, membrane). This effect is called surface tension ., As a result of this , the molecules at the surface are pulled inwards and tend to make the surface area of the, liquid as small as possible., Surface tension of a liquid is defined as the force acting per unit length on the surface perpendicular to, the surface of the liquid., Its unit is newton per metre. (Nm-1), Surface tension decreases with rise in temperature. At high temperature, kinetic energy of the molecules, increases and inter molecular force decreases.
Page 39 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 30. Give any consequences of surface tension., (I), The spherical shape of liquid drops (water) is due to surface tension because a sphere has a, minimum surface area for a given volume of a liquid., (II), Particles of soil at the bottom of a river separated, but they stick together, when taken out. It is, due to surface tension., (III), Fire polishing of glass is due to surface tension., (IV), Rise and fall of liquids in capillary tubes (capillary action) is due to surface tension., 31. Explain Viscosity of a liquid., The internal resistance to flow of a liquid is called viscosity., The stronger the intermolecular force , the higher is the viscosity., Honey and glycerine are highly viscous liquids and so flow slowly., Water and kerosene are low viscous liquids and so flow rapidly., Viscosity decreases with rise in temperature., At high temperature, inter molecular force decreases and kinetic energy of the molecules increases., 32. What is laminar flow, The flow in which there is a regular gradation of velocity in passing from one layer to the next is called, laminar flow., A force is required to maintain the flow of layers. This force is proportional to the area of contact of layers, (A) and velocity gradient (du/dz), Viscous force , F ∝ A.du/dz, F =η. A.du/dz, η = coefficient of viscosity, In CGS system , the unit of coefficient of viscosity is poise., In SI system the unit is Nsm-2 ( Newton second per square metre), 33. The window panes of old buildings are thicker at the bottom. Why?, Glass is highly viscous liquid and it flows very slowly. So the window panes of old buildings are thicker, at the bottom., 34. The lowest hypothetical temperature at which gases are supposed to occupy zero volume is called, ………………, Ans: Absolute zero of temperature ( 0 K , -273.15 0C ), 35. How are the densities of O2(g) and CH4(g) related, if they are kept at the same temperature and, pressure?, Ans:, Molar mass of O2 = 32, Molar mass of CH4 = 16., So O2 is two times denser than CH4., 36. Which property of liquids is associated with fire polishing of glass?, Ans: Surface Tension, 37. Give the reason behind the following: Sharp glass edges are heated for making them smooth., On heating, the glass melts and the surface of the liquid tends to take the rounded shape at the edges,, due to surface tension.
Page 40 :
Join Telegram Channel: https://t.me/hsslive, , CHAPTER, , Downloaded from www.Hsslive.in ®, , 6, , THERMODYNAMICS, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL : CHEM DSM, =======================================================, 1. Define system and surrounding., System :- The part of universe under study, Surroundings :- The remaining part of the universe that interact with system, Universe = System + Surrounding, 2. Explain open system, closed system and isolated system with example., (I), Open system :- A system which can exchange both energy and matter with the surroundings., e.g. Hot water in a cup, (II), Closed system :- A system can exchange only energy but not matter with the surroundings., e.g. Hot water in a closed steel tumbler., (III), Isolated system :- A system which can neither exchange matter nor energy with the surroundings., e.g. Hot water in a perfectly insulated thermos flask., 3. What are state functions and path functions? Give examples for each., A function or property that depends only on the initial state and final state of the system and not on, the path followed is called state function., Examples :- Temperature(T) , Pressure (P), Volume (V) , Internal energy (U) , Enthalpy (H) , Entropy (S) ,, Gibbs free energy (G), A function or property that depends on the initial state and final state of the system and on the path, followed also is called path function., Examples:- Heat(q) , work (w), 4. What are extensive and intensive properties? Give examples for each., (I), Extensive properties :- These are properties which depend on the amount of matter present in, the system., Examples: - Mass (m) , Volume (V) , Length (l), Internal energy (U) , Enthalpy (H) , Entropy (S) ,, Gibbs free energy (G), heat capacity etc, (II), Intensive properties:- These are properties which are independent on the amount of matter, present in the system., Examples : - Temperature ,pressure, density , refractive index , viscosity , surface tension ,, specific heat , molar heat capacity, 5. Explain (a) isothermal process (b) isobaric process (c) isochoric process (d) adiabatic process., Types of process, Explanation, Condition, Isothermal process, A process which takes place at constant temperature, ∆T= 0, Isobaric process, A process which takes place at constant pressure, ∆P= 0, Isochoric process, A process which takes place at constant volume, ∆V= 0, Adiabatic process, A process which takes place at constant heat, d q= 0
Page 41 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 6. What are reversible and irreversible processes, Reversible process:- A process is thermodynamically reversible if it can be reversed at any stage by a very, small change in some conditions such as temperature, pressure or concentration. At every stage the, process is in thermodynamic equilibrium and the process will take place infinitesimally slowly., Irreversible process:- A process which cannot be reversed by a small change in any one of the controlling, properties is called an irreversible process., An irreversible process proceeds to one direction and it does not remain in equilibrium, 7. What is Internal energy (U) ? How internal energy can be changed?, Internal energy is the total energy present within a substance., Internal energy is the sum of all types of molecular energies like translational energy, rotational energy,, vibrational energy, electronic energy , nuclear energy etc., It is a state function and extensive property. It can be changed by the following ways:, (i), By allowing heat to flow in to the system or out of the system, (ii), By doing work on the system or by the system., 8. State First law of thermo dynamics and give its mathematical form., It is law of conservation of energy. It states that energy can neither be created nor destroyed., , Mathematical form is ∆U = q + w, ∆U = change in internal energy, q = heat, w = work, For expansion work ( w = - P∆V) , ∆U = q − P∆V, Work done on the system, w = +ve,, Work done by the system, w = −ve,, Heat absorbed by the system, q = +ve ,, Heat liberated by the system , q = −ve, 9. Write the expression for, (i), Work done by the gas in irreversible expansion or compression, W = −Pext∆V = −Pext(Vf-Vi), (ii), Work done in isothermal reversible expansion or compression of an ideal gas., Wrev = −2.303 nRT log Vf/Vi, OR, , Wrev = − 2.303 nRT log Pi/Pf, 10. What is the the significance of ∆U ?, Change in internal energy (∆U) is the heat absorbed or evolved at constant volume., ∆U = q − P∆V, For a process taking place at constant volume (∆V = 0 )., So, ∆U = qv, 11. Define Enthalpy (H), Enthalpy is the heat content of the system., Enthalpy is the sum of the internal energy and pressure volume energy ., H = U + PV, , It is a state function and extensive property., 12. Give the relation connecting ∆H and ∆U., , ∆H = ∆U + P ∆V, ∆H = ∆U + ∆n RT, , OR, , Where, ∆n= nP − nR, 13. What is the significance of ∆H?, Change in enthaly (∆H) is the heat absorbed or evolved at constant pressure., ∆H = ∆U + P ∆V ( at constant pressure ), From first law of thermodynamics , ∆U = q − P∆V, ∆U + P∆V = q, Therefore ∆H = qp ., , 14., , The relation connecting q p and q v is q p, , = q v + ∆n RT
Page 42 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 15. What are exothermic and endothermic reactions? Give its sign of ∆H., Exothermic reactions, Endothermic reactions, The reactions which takes by the liberation of, The reactions which takes by the absorption of, heat is called exothermic reactions, heat is called endothermic reactions, Eg. C+O2→CO2 ∆H = −393.5 kJ, Eg. N2+O2→ 2 NO ∆H = 180.5 kJ, For exothermic reactions, ∆H = −ve., For endothermic reactions, ∆H = +ve., , 16. Define Heat capacity, Specific heat capacity (specific heat), Molar heat capacity ?, Heat capacity of a substance is defined as the amount of heat required to raise its temperature through 1 0C, It is an extensive property., Specific heat capacity (specific heat) :-It is the amount of heat required to raise the temperature of 1 gram, of the substance through 10C ., Molar heat capacity :- It is the amount of heat required to raise the temperature of 1 mol substance, through 10C ., Specific heat capacity and molar heat capacity are intensive properties., 17. Derive the relation between Cp and Cv for an ideal gas., Cp → Molar heat capacity at constant pressure. C v →Molar heat capacity at constant volume., , q =C∆T, qv = Cv∆T = ∆U and qp = Cp∆T = ∆H, H = U + PV, ∆H = ∆U + ∆(PV) OR, ∆H = ∆U + ∆(RT) (For 1 mol of ideal gas), OR, ∆H = ∆U + R∆T, Substituting the value of ∆H and ∆U in the above equation, Cp∆T = Cv∆T + R∆T, Cp = C v + R, OR, , Cp − Cv = R, 18. What is Thermo chemical equation?, A chemical equation which indicates the enthalpy change occurring during the reaction is called thermo, chemical equation., Eg. C(s) +O2(g)→CO2 (g) ∆H = −393.5 kJ, (I), For exothermic reactions, ∆H = −ve, For endothermic reactions, ∆H = +ve., (II), Physical states of reactants and products should be specified., (III), When the coefficients in the chemical equations are multiplied or divided, the value of ∆H must, be multiplied or divided., (IV), When a chemical equation is reversed, the sign of ∆H is reversed. (magnitude remain same)
Page 43 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 19. Define each of the following., (a) Enthalpy of reaction:The enthalpy change during a chemical reaction., , ∆rH = Sum of enthalpies of products − Sum of enthalpies of reactants, The enthalpy change during a chemical reaction when all participating substances are in standard state is, called standard enthalpy of reaction. (standard state: pure forms, 1 bar pressure, 298 K temperature), (b) Standard enthalpy of combustion :-It is defined as the enthalpy change when one mole of a substance is, completely burned in the presence of air or oxygen when all their reactants and products are in their, standard state., Enthalpy combustion is always negative., (c) Standard enthalpy of formation :The enthalpy change when one mole of a compound is formed from its, elements in their stable states., (d) Enthalpy of solution:The enthalpy change when one mole of a substance is dissolved in a specified amount, of solvent., (e) Standard enthalpy of fusion :The enthalpy change when one mole of a solid is converted to its liquid state, at its melting point under standard pressure 1 bar., (f) Standard enthalpy of vapourisation: The enthalpy change when one mole of a liquid is converted to its, gaseous state at its boiling point under standard pressure 1 bar., (g) Standard enthalpy of sublimation:The enthalpy change when one mole of a solid is directly converted to, its gaseous state at a constant temperature and 1 bar pressure., 20. Importance of standard enthalpy of formation:-It is useful for calculating standard enthalpies of a reaction, , Standard enthalpy change of a reaction =, Standard enthalpies of formation of products - Standard enthalpies of formation of reactants., The standard enthalpy of formation of all elements in their standard state is taken as zero., O2→ 0, H2→ 0, N2→ 0, Cgraphite→ 0, Srhombic→ 0, 21. State and illustrate Hess’s Law of Constant Heat of Summation., , It states that the enthalpy change in a chemical reaction is the same whether the, reaction takes place in one step or several steps., ∆Hr = ∆H1 +∆H2 + ∆H3, , 22. Give some applications of Hess’s law., (I), It is used to determine the enthalpies of reaction., (II), It is used to determine the enthalpy of formation, (III), It is used to determine the enthalpy of transition of allotropic forms., (IV), It is used to determine the bond enthalpy and lattice enthalpy., 23. Define Bond enthalpy, The amount of heat required to break one mole of covalent bonds to gaseous products is called bond, dissociation enthalpy., For diatomic molecules, bond dissociation enthalpy and bond enthalpy are same.
Page 44 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , In the case of poly atomic molecules, bond dissociation enthalpy is different for different bonds within, the same molecule. In such cases, bond enthalpy is the average of bond dissociation enthalpies of, various similar bonds., 24. How will you calculate enthalpy change of a reaction from bond enthalpies?, , ∆𝑯𝟎𝒓 = 𝑺𝒖𝒎 𝒐𝒇 𝒃𝒐𝒏𝒅 𝒆𝒏𝒆𝒓𝒈𝒊𝒆𝒔 𝒐𝒇 𝒓𝒆𝒂𝒄𝒕𝒂𝒏𝒕𝒔 − 𝑺𝒖𝒎 𝒐𝒇 𝒃𝒐𝒏𝒅 𝒆𝒏𝒆𝒓𝒈𝒊𝒆𝒔 𝒐𝒇 𝒑𝒓𝒐𝒅𝒖𝒄𝒕𝒔, 25. What is Lattice enthalpy ?, The lattice enthalpy of an ionic compound is the enthalpy change when one mole of the ionic compound, dissociates in to its gaseous ions., We cannot calculate lattice enthalpy directly by experiment. So Born Haber cycle is used to calculate it., 26. Draw Born Haber cycle for the determination of lattice enthalpy., (Construct an enthalpy diagram for the determination of lattice enthalpy of sodium chloride), , ∆𝑯𝟎𝒇 = ∆𝑯𝒔𝒖𝒃 + 𝑰𝑬 + ∆𝑯𝒅𝒊𝒔𝒔 + 𝑬𝑨 + 𝑼, 𝑼 = ∆𝑯𝟎𝒇 − (∆𝑯𝒔𝒖𝒃 + 𝑰𝑬 + ∆𝑯𝒅𝒊𝒔𝒔 + 𝑬𝑨), 27. What is spontaneous process and non spontaneous process?, Spontaneous process is a process that takes place without the help of any external agency., e.g. Flow of water from high level to low level, flow of heat from hot body to cold body, Non spontaneous process is a process that takes place with the help of an external agency., E.g. flow of water from low level to high level., 28. Which are driving forces for spontaneous process?, (a) Decrease in energy (b) increase in disorder or randomness of the system, 29. Define Entropy (S) ?, Entropy (S) is a measure of degree of disorder or randomness of the system., Its unit is J/K/mol, , 𝐪𝐫𝐞𝐯𝐞𝐫𝐬𝐢𝐛𝐥𝐞, 𝐓, 30. Predict in which of the following entropy increases(∆S = +ve) entropy decreases(∆S = -ve), (a) Melting of ice → entropy increases (∆S = +ve), (b) Adding a drop of ink in water→ entropy increases (∆S = +ve), (c) 2 N2O5(g) → 4 NO2(g) + O2(g) → entropy increases (∆S = +ve), (d) Condensation of steam in to water→ entropy decreases (∆S = −ve)
Page 45 :
Join Telegram Channel: https://t.me/hsslive, , (e), (f), (g), (h), (i), (j), (k), (l), , Downloaded from www.Hsslive.in ®, , Freezing of water in to ice→ entropy decreases (∆S = −ve), Solid camphor converted to camphor vapour→ entropy increases (∆S = +ve), 2 NaHCO3 (s) → Na2CO3 (s) + CO2 (g) + H2O (g) → entropy increases (∆S = +ve), 4 Fe + O2 (g) →2 Fe2O3 (s) → entropy decreases (∆S = −ve), Graphite to diamond→ entropy decreases (∆S = −ve), H2 (273 K)→ H2 (300 K) → entropy increases (∆S = +ve), I 2 (s)→ I 2 (g) → entropy increases (∆S = +ve), NaCl (s) + H2O → NaCl (aq) → entropy increases (∆S = +ve), , 31. State Second law of thermo dynamics and gives equation, It states that the entropy of the universe increases in the course of every spontaneous (natural) change., , ∆Stotal = ∆Ssystem + ∆Ssurrounding > 0, ∆Suniverse > 0, 32. State third law of thermo dynamics, The entropy of any pure crystalline substance approaches zero as the temperature approaches absolute, zero. This is called third law of thermodynamics, 33. Define Gibb’s free energy (G), Gibbs energy is defined as the maximum amount of available energy that can be converted to useful work., , G =H – TS, 34. The relation connecting ∆G, ∆H, and ∆S is, , ∆G= ∆H −T ∆S., 35. Explain Gibb’s energy and spontaneity., (i), If ∆G is negative, the process will be spontaneous., (ii), If ∆G is zero, the process is in equilibrium., (iii), If ∆G is positive, the process will be non spontaneous., 36. What are the conditions for ∆G to be negative (spontaneous process), (I), Exothermic process (∆H = −ve) and ∆S = +ve , ∆G will be always negative and the reaction is, spontaneous., (II), Exothermic process (∆H = −ve) and ∆S = −ve , ∆G will be negative only when, ∆H > T ∆S and the reaction is spontaneous only at low temperature., (III), Endothermic process (∆H = +ve) and ∆S = +ve , ∆G will be negative only when, ∆H < T ∆S and the reaction is spontaneous only at high temperature., (IV), Endothermic process (∆H = +ve) and ∆S = − ve , ∆G will be always positive and the reaction is always, non spontaneous., 37. The relation connecting standard Gibb’s energy (∆G0 ) and equilibrium constant(K) is, 0, , ∆G = − 2.303 RT logK, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, CHEM DSM, ( ഈ NOTES ന്െട വീഡിേയാ, SUBSCRIBE െച, , ാസുകൾ കാണാൻ CHEM DSM എ, , YOUTUBE ചാനൽ കാണുക., , ുക, , =========================================================================================
Page 46 :
Join Telegram Channel: https://t.me/hsslive, , CHAPTER, , Downloaded from www.Hsslive.in ®, , 7, , EQUILIBRIUM, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL : CHEM DSM, =======================================================, 1. State Henry’s law and give its one application., Henry’s law states that the mass of a gas dissolved in a given mass of solvent at a particular temperature is, directly proportional to the pressure of the gas above the solvent., i.e., 𝒎 ∝ 𝒑, or, m=kp, Example: Soda water., In soda water bottle, there is an equilibrium between the carbon dioxide molecule in the gaseous state and, the molecules dissolved in the liquid., When soda bottle is opened, pressure decreases, solubility decreases, dissolved gases escape out rapidly, with a sound fizz., 2. What are the general characteristics of physical equilibrium?, (I), Equilibrium can be established only in closed systems., (II), The measurable properties remain constant., (III), The equilibrium is always dynamic. This means that two opposite processes occur at the same rate., 3. What are reversible and irreversible reactions ., A reaction which takes place both in forward and backward directions under the same conditions is called, reversible reactions., N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g) ,, 2SO 2 (g) + O2 (g) ⇌ 2 SO3 (g), A reaction in which the products do not react to give back the reactant is called irreversible reactions., AgNO3 + NaCl → AgCl + NaNO3, 4. Explain the concept of chemical equilibrium., Consider a general reversible reaction, 𝑨 + 𝑩 ⇌ 𝑪 + 𝑫, Suppose the reaction is carried out in a closed container. In the, beginning, the concentration of A and B are maximum and, concentration of C and D are minimum (zero). As the reaction, proceeds, the concentration of A and B decreases and, concentration of C and D increases. Hence the forward reaction, will be high in the beginning and it will decrease gradually, because of the fall in concentration of A and B. On the other, hand, the velocity of backward reaction increases due to, increase in concentration of C and D. Finally a stage is reached in, which the rate of forward reaction will become equal to the rate, of backward reaction. This stage is called chemical equilibrium.
Page 47 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 5. Chemical equilibrium is dynamic in nature. Explain., At equilibrium, the reaction will be still going on in the forward and backward reaction., But the rate of forward reaction will become equal to the rate of backward reaction., To understand dynamic nature of equilibrium, synthesis of ammonia by Haber process is carried out in two, closed systems under identical conditions., In the first system N2 and H2 are heated and at equilibrium N2 ,H2 and NH3 will be present., In the second system N2 and D2 are heated and at equilibrium N2 ,D2 and ND3 will be present., After this , both systems are mixed and analysed., It contain N2 ,H2,, D2 , NH3 , NH2D, NHD2 and ND3, Here mixing of isotopes takes place. This shows that at equilibrium the reaction is still going on in both, forward and backward directions. But the rate is same., 6. What are the characteristics of chemical equilibrium?, (I), Chemical equilibrium is dynamic in nature., (II), The observable properties of the system such as pressure, concentration, colour etc. become, constant at equilibrium and remain unchanged thereafter., (III), Chemical equilibrium can be attained from either direction. i.e., from the direction of reactants and, from the direction of products., (IV), Chemical equilibrium can be attained only in closed systems., 7. What is law of chemical equilibrium and equilibrium constant?, Consider a general reversible reaction, 𝑨 + 𝑩 ⇌ 𝑪 + 𝑫 ,, , 𝑲𝒄 =, , [𝑪][𝑫], [𝑨][𝑩], , Consider a general reversible reaction,𝒂𝑨 + 𝒃𝑩 ⇌ 𝒄𝑪 + 𝒅𝑫, [𝑪]𝒄 [𝑫]𝒅, 𝑲𝒄 =, [𝑨]𝒂 [𝑩]𝒃, At a given temperature, the product of molar concentration of products divided by the product of molar, concentration of reactants with each concentration term raised to a power equal to its coefficients in the, balanced chemical equation is a constant. This constant is called equilibrium constant. This law is called law, of chemical equilibrium., In the case of gaseous reaction, it can be written in terms partial pressure also., , 𝑲𝒑 =, , 𝒑𝒄𝑪 𝒑𝒅, 𝑫, 𝒃, 𝒑𝒂, 𝑨 𝒑𝑩, , 8. Give the relation between Kp and Kc ., Kp = Kc (RT)∆n, Kp = Equilibrium constant in terms of pressure. Kc = Equilibrium constant in terms of concentration., ∆n = No. of mole of gaseous products - No. of mole of gaseous reactants., If ∆n = 0 , Kp = Kc, , If ∆n = 1 , Kp = Kc (RT), , Derivation:, Consider a general reversible reaction,𝒂𝑨 + 𝒃𝑩 ⇌ 𝒄𝑪 + 𝒅𝑫, [𝑪]𝒄 [𝑫]𝒅, 𝑲𝒄 =, [𝑨]𝒂 [𝑩]𝒃, 𝒑𝒄𝑪 𝒑𝒅𝑫, 𝑲𝒑 = 𝒂, 𝒑𝑨 𝒑𝒃𝑩
Page 48 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , According to ideal gas equation , PV = nRT, ∴𝑷=, , 𝒏𝑹𝑻, 𝑽, , = 𝑪𝑹𝑻 = [ ]𝑹𝑻, , PA = [A]RT, PB = [B]RT, PC = [C]RT, PD = [D]RT, 𝑲𝒑 =, , 𝒑𝒄𝑪 𝒑𝒅𝑫, 𝒑𝒂𝑨, , 𝒑𝒃𝑩, , =, , ([𝐂]𝐑𝐓)𝒄 ([𝐃]𝐑𝐓)𝒅 [𝑪]𝒄 [𝑫]𝒅, =, (𝑹𝑻)(𝒄, ([𝐀]𝐑𝐓)𝒂 ([𝐁]𝐑𝐓)𝒃 [𝑨]𝒂 [𝑩]𝒃, , 𝒅) (𝒂 𝒃), , = 𝑲𝒄 (𝑹𝑻)∆𝒏, , Kp = Kc (RT)∆n, 9. What are homogeneous equilibria and heterogeneous equilibria?, The equilibria in which all reactants and products are in same phase are known as homogeneous equilibria., , N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g), 2SO 2 (g) + O2 (g) ⇌ 2 SO3 (g), The equilibria in which reactants and products are in different phase are known as heterogeneous, equilibria., , CaCO3 (s) ⇌, C(s) + H 2O (g), , CaO (s), + CO2 (g), ⇌ CO (g) + H2 (g), , 10. What are the characteristics of equilibrium constant?, (I), The equilibrium constant has a definite value for every reversible reaction at a particular temperature., However, it varies with change in temperature., (II), The value of equilibrium constant is independent of initial concentration of reactants., (III), For a reversible reaction, the equilibrium constant for the reverse reaction will be the reciprocal of the, equilibrium constant for the forward reaction., (IV), The value of equilibrium constant is not affected by the addition of a catalyst to the reaction., This is because the catalyst increases the speed of both forward reaction and backward reaction to the, same extent., 11. What are the applications of equilibrium constant?, (I), Prediction of extent of reaction:- Larger the value of equilibrium constant , greater is the extent to, which the reactants are converted into the products., If Kc > 10 3 , products predominates over the reactants., If Kc < 10 -3 , reactants predominates over the products., If Kc is between 10 3 and 10 -3 , appreciable concentration of reactants and products., (II), Prediction of direction of reaction., If Qc > Kc , the reaction will proceed in the backward direction .(in the direction of reactants), If Qc < Kc , the reaction will proceed in the forward direction. .(in the direction of products), If Qc = Kc , the reaction will be in equilibrium., (III), Calculation of equilibrium concentration:- If the equilibrium constant of a reaction is known ,, equilibrium concentration of a substance in the equilibrium mixture can be calculated., 12., , State Le-chatelier principle., If a system in equilibrium is subjected to change in concentration, temperature or pressure, the equilibrium, shifts in the direction that tends to reduce the effect of the change.
Page 49 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 13. How do the effect of concentration, temperature and pressure affect of the rate of a chemical reaction?, , (I), , Effect of change of concentration:-, , If the concentration of any one of the reactants is increased, the equilibrium will shift in the forward, direction and more of the products will be formed., If the concentration of any one of the products is increased, the equilibrium will shift in the backward, direction and more of the reactants will be formed., Removal of a product equilibrium mixture will shift the equilibrium in the forward direction., (II), , Effect of change of temperature:If the forward reaction is exothermic, the backward reaction will be endothermic and vice versa., Increase of temperature will shift the equilibrium in the direction of endothermic reaction, (in the direction of absorption of heat)., Decrease of temperature will shift the equilibrium in the direction of exothermic reaction, (in the direction of heat is produced)., , (III), , Effect of change of pressure:-, , Pressure has effect only when the change of number of moles of gaseous substances., Increase of pressure will shift the equilibrium in the direction in which the pressure is reduced., ( decrease in the number of molecules)., Decrease of pressure will shift the equilibrium in the direction in which the pressure is increased, (increase in the number of molecules)., 14. What happens when catalyst is added to equilibrium?, The equilibrium is not affected by the addition of a catalyst to the reaction. This is because the catalyst, increases the speed of both forward reaction and backward reaction to the same extent., But it help to achieve the equilibrium quickly., 15. What happens when inert gas is added to equilibrium?, The equilibrium remains undisturbed by the addition of inert gas., It is because the addition of an inert gas at constant volume does not the molar concentration or partial, pressure of the substance involved in the reaction., 16. Explain the effect of concentration, temperature and pressure in the following reaction., , N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g),, , ∆H = -92.4 kJ., , (I), , Effect of concentration:- If we increase the concentration of N2 or H2, the equilibrium will shift in, forward direction and more NH3 is formed. If we remove NH3 formed by cooling, the equilibrium, will shift in forward direction and more NH3 is formed., (II), Effect of pressure:- Here forward reaction is accompanied by the decrease in the number of moles,, so high pressure will favour forward reaction and more NH3 is formed., (III), Effect of temperature:- Here forward reaction is exothermic and so low temperature will favour, forward reaction. But at very low temperature, N2 and H2 will be less reactive and so optimum, temperature is used (500 0C)., 17. Explain the effect of concentration, temperature and pressure in the following reaction., , 2SO 2 (g), (I), , + O2 (g) ⇌ 2 SO3 (g), , ∆H = -92.4 kJ., , Effect of concentration:- If we increase the concentration of SO 2 or O2 , the equilibrium will shift in, forward direction and more SO3 is formed. If we remove SO3 formed, the equilibrium will shift in, forward direction and more SO3 is formed.
Page 50 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , (II), , Effect of pressure :-Here forward reaction is accompanied by the decrease in the number of moles,, so high pressure will favour forward reaction and more SO3 is formed., (III), Effect of temperature:-Here forward reaction is exothermic and so low temperature will favour, forward reaction. But at very low temperature, SO2 and O2 will be less reactive and so optimum, temperature is used (450 0C)., 18. Explain the effect of pressure in the following reactions., , (I), , PCl5 (g), , ⇌ PCl3 (g) + Cl2 (g), , Here forward reaction is accompanied by the increase in the number of moles, so low pressure, will favour forward reaction. High pressure will favour backward reaction., , (II), , H2 (g) + I2 (g) ⇌ 2 HI (g), , Pressure has effect only when the change of number of moles of gaseous substances. Here no, change in number of molecules of reactants and products. So pressure has no effect., 19. Explain the effect of temperature in the following reactions., , N2 (g) + O2 (g) ⇌ 2 NO(g),, , ∆H = 180 kJ., , Here forward reaction is endothermic, and hence increase in temperature will favour forward reaction., 20. An aqueous solution of CuSO4 is acidic while that of Na2SO4 is neutral. Explain., CuSO4 +2 H2O →Cu(OH)2 + H2SO4, Here Cu(OH)2 is weak base and H2SO4 is strong acid. H2SO4 ionises completely. More H+ ions are formed, and so acidic solution., Na2SO4 +2 H2O →2 NaOH+ H2SO4 ( 2 Na+ + 2 OH- + 2 H+ + SO42-), Here NaOH and H2SO4 are formed. Both are strong and ionizes completely. So H+ and OH− concentrations, are equal and neutral solution., 21. Explain the effect of pressure in the following equilibrium, (I), CO(g) + 3 H2(g) ⇌CH4(g) + H2O(g), Number of moles of gaseous reactants= 1+3 =4, Number of moles of gaseous products = 1+1 =2, When pressure increases , the equilibrium will shift in the direction in which the number of, molecules are decreased. Here forward direction., (II), N2(g) + O2(g) ⇌ 2NO(g)., Number of moles of gaseous reactants= 1+1 =2, Number of moles of gaseous products =2, 22. Define Ionic equilibrium?, The equilibrium established between ionized molecules and unionized molecules in solution of weak, electrolyte is called ionic equilibrium., , CH3COOH (aq) + H2O(l) ⇌ H3O+ (aq) + CH3COO- (aq), 23. Explain Arrhenius concept of acids and bases with examples, Arrhenius acid is a substance which give hydrogen ion(H+) in aqueous solution., e.g., HCl, CH3COOH etc., Arrhenius base is a substance which give hydroxyl ion(OH-) in aqueous solution., e.g., NaOH, NH4OH etc., 24. Explain Bronsted Lowry concept of acids and bases with examples., Bronsted acid is a proton(H+) donor., Bronsted base is a proton(H+) acceptor., , CH3COOH, Acid, , + H2O(l) ⇌ H3O+, Base, , Acid, , + CH3COOBase
Page 51 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 25. What are conjugated acid base pairs with examples, Conjugated acid = Base + H+, Conjugated base = Acid - H+, The pairs of acids and bases which are formed by the loss or gain of proton are called conjugated acid, base pairs., , CH3COOH, , + H 2 O ⇌ H 3 O+, , + CH3COO-, , Acid, Base, Acid, Base, Here CH3COO is a conjugate base of acid CH3COOH, H3O+ is a conjugate acid of base H2O, , NH3, , + H2O ⇌ NH4+, , + OH-, , Base, Acid, Acid, Base, Here OH is a conjugate base of acid H2O., NH4+ is a conjugate acid of base NH3, Conjugate base of strong acid is weak and conjugate base of weak acid is strong., , Species, H2 O, HCO3 –, HSO4 –, NH3, , Conjugate acid, H3 O +, H2CO3, H2SO4, NH4+, , Conjugate base, OHCO3 2–, SO4 2–, NH2 –, , 26. Explain Lewis concept of acids and bases with examples., Lewis acids are electron pair acceptors., Lewis acids are electron deficient molecules or cations (Positive ions) e.g. , BF 3 , AlCl3 , Mg2+ , Co3+ , H+, Lewis bases are electron pair donors., Lewis bases are neutral molecules having lone pair or anions (Negative ions) e.g. , NH3 , H2O , OH- , Cl27. What are Amphoteric substances?, Substances which can act as both acid and base. E.g., H2O , HCO3- , HSO4-, , 28. Explain ionization constant of weak acid and its importance., , HX, , + H 2 O ⇌ H 3 O+, , + X-, , 𝑲𝒂 =, , [𝑯𝟑 𝑶 ][𝑿 ], , Ka = ionization constant of acid., Larger the value of Ka , higher the concentration of H3O+ , and stronger the acid., Degree of dissociation of weak acid ,, , 𝜶=, , 𝑲𝒂, 𝑪, , C = concentration., 29. Explain ionization constant of weak base and its importance., , MOH ⇌ M+, , + OH-, , Kb = ionization constant of base., , 𝑲𝒃 =, , [𝑴 ][𝑶𝑯 ], [𝑴𝑶𝑯], , [𝑯𝑿, 𝑯𝑿]
Page 52 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , Larger the value of Kb, higher the concentration of OH- , and stronger the base., Degree of dissociation of weak base, 𝜶, , =, , 𝑲𝒃, 𝑪, , 30. Define ionic product of water. What is its value at 298 K?, Water undergo self ionization as follows., H2O, , + H2O ⇌ H3O+, , + OH-, , 𝑲=, , K[H2O]2 = Kw = [H3O+ ] [ OH-], , [𝑯𝟑 𝑶 ][𝑶𝑯 ], [𝑯𝟐 𝑶]𝟐, , Ionic product of water is the product of concentration of hydronium ion and hydroxyl ion., Its value at 298 K is 1 x 10-14, In pure water , concentration of hydronium ion and hydroxyl ion is equal. [H 3O+ ] =1 x 10-7 , [ OH-]=1 x 10-7, 31. Define PH and calculate the PH of neutral solution., PH is defined as negative logarithm of hydronium concentration., , PH = −log[H3O+ ], For neutral solution, [H3O+ ] =1 x 10-7 , [OH-] =1 x 10-7, , PH = - log(1 x 10-7) = 7, , For acidic solution PH is less than 7., For basic solution PH is greater than 7, For neutral solution PH is 7, 32. The relation between PH and POH is, 33. The relation between Ka and Kb is, 34. The relation between pKa and pKb is, , PH + POH =14, Ka x K b = K w, P Ka + P Kb = P Kw =14, , 35. What is Buffer solution? Which are two types? Explain each, , Buffer solution is a solution which resists the change in pH value by the addition of small amount, of acid or base., Blood is an example of natural buffer., These are two types, (I), Acidic buffer:- Its pH is less than 7. It is mixture of weak acid and its salt with strong base., eg. , solution of acetic acid and sodium acetate., (II), Basic buffer:- Its pH is more than 7. It is mixture of weak base and its salt with strong acid., eg. , solution of ammonium hydroxide and ammonium chloride., 36. Give Henderson-Hasselbalch equation for acidic buffer and basic buffer., , 𝑭𝒐𝒓 𝒂𝒄𝒊𝒅𝒊𝒄 𝒃𝒖𝒇𝒇𝒆𝒓,, 𝑭𝒐𝒓 𝒃𝒂𝒔𝒊𝒄 𝒃𝒖𝒇𝒇𝒆𝒓,, , [𝒔𝒂𝒍𝒕], [𝒂𝒄𝒊𝒅], [𝒔𝒂𝒍𝒕], = 𝑷𝑲𝒃 + 𝒍𝒐𝒈, [𝑩𝒂𝒔𝒆], , 𝑷𝑯 = 𝑷𝑲𝒂 + 𝒍𝒐𝒈, 𝑷𝑶𝑯, , 37. Define Common ion effect and give examples., Common ion effect is defined as the suppression of dissociation of weak electrolyte by the addition of, strong electrolyte containing common ion., e.g. The ionisation of ammonium hydroxide is suppressed by adding ammonium chloride., The ionisation of acetic acid is suppressed by adding sodium acetate.
Page 54 :
Join Telegram Channel: https://t.me/hsslive, , UNIT 8, , Downloaded from www.Hsslive.in ®, , REDOX REACTIONS, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL: CHEM DSM, =====================================================, 1. Define Redox reactions, Reactions in which both oxidation and reduction takes place simultaneously are called redox reactions., Reduction + oxidation→ Redox reac on, Examples from our daily life : (I) Rusting of iron (II) Operation of cells and batteries (III) Combustion of, cooking gas , coal , wood etc., 2. Define oxidation and reduction on the basis of classical concept, Oxidation, Reduction, Addition of oxygen., C + O2 → CO2, Removal of oxygen., ZnO + C → Zn + CO, Removal of hydrogen, 2H2S + O2 → 2S + 2H2O, Addition of hydrogen. 2H2S + O2 → 2S + 2H2O, Addition of electronegative element., Removal of electronegative element., Mg + Cl2→ MgCl2, 2 FeCl3+ H2→ 2 FeCl2+ 2HCl, Removal of electropositive element., Addition of electropositive element., 𝟐𝑲𝟒 [𝑭𝒆(𝑪𝑵)𝟔 + 𝑯𝟐 𝑶𝟐, 𝟐 𝑯𝒈𝑪𝒍𝟐 + 𝑺𝒏𝑪𝒍𝟐 → 𝑯𝒈𝟐 𝑪𝒍𝟐 + 𝑺𝒏𝑪𝒍𝟒, → 𝟐𝑲𝟑 [𝑭𝒆(𝑪𝑵)𝟔 + 𝟐 𝑲𝑶𝑯, 3. Define oxidation and reduction on the basis of electronic concept, Oxidation: Removal of electrons. Zn→ Zn2+ + 2eReduction: Addition of electrons., Cu2+ + 2e-→ Cu, 4. Give examples for redox reactions that takes place in aqueous solution ., (I), When zinc metal is added to blue coloured copper sulphate solution, zinc displaces copper and, solution become colourless., Zn + Cu2+ → Zn2+ + Cu, Here Zn is oxidized to Zn2+ and Cu2+ is reduced to Cu., Here Zn is reducing agent (reductant), Cu2+ is oxidizing agent (oxidant)., (II), When copper metal is added to silver nitrate solution, copper displaces silver., Cu + 2 Ag+ → Cu2+ + 2 Ag, Here Cu is oxidized to Cu2+ and Ag+ is reduced to Ag., Here Cu is reducing agent (reductant), Ag+ is oxidizing agent (oxidant)., 5. Define the term Oxidation state (oxidation number), The charge that an atom would have in a compound or ion., 6. Rules assigning oxidation number., (I), In free state (O2, Cl2, N2, H2, Cu , Na , Fe , P4 , S8 ) → 0, (II), For mono atomic ion, charge is the oxidation state (Ag + → +1, Cu2+ →+2 , Cl - →-1, O 2- →-2), (III), Fluorine→ -1 (in compounds), (IV), Hydrogen → +1 (in compounds)(excep on in metal hydrides like NaH -1), (V), Oxygen → -2 (in compounds)(exception in peroxides like H 2O2 → -1, in OF2→+2), (VI), Alkali metals→+1 ,Alkaline earth metals→ +2 in their compounds, (VII), For neutral molecule total charge equal to zero, (VIII) For poly atomic ions, sum is equal to the charge of the ion.
Page 55 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 7. Define oxidation and reduction on the basis of oxidation number concept, Oxidation: Increase in oxidation number., Reduction: Decrease in oxidation number., -2, , 0, , -1, , 0, , H2S + Cl2→ 2HCl + S, Here the oxidation number of sulphur in H2S is -2, it is increased to zero (oxidation )., Here the oxidation number of chlorine in Cl 2 is 0, it is decreased to -1 (Reduction )., H2S is reducing agent (reductant), Cl2 is oxidizing agent (oxidant)., 8. What is Oxidant and reductant?, The substance undergoing oxidation is reducing agent(reductant) and the substance undergoing, reduction is oxidizing agent (oxidant), 9. Which is the substance undergoing oxidation, reduction, oxidant and reductant in the reactions., 0, , +4, , +2, , (I), , Pb + PbO2 +2 H2 SO4 → 2 PbSO4 + 2H2O, Pb undergo oxidation , PbO2 undergo reduction, PbO2 is oxidizing agent, Pb is reducing agent., +4, -1 +2, 0, (II), MnO2 +4 HCl→MnCl2+Cl2+2H2O, HCl undergo oxidation , MnO2 undergo reduction, MnO2 is oxidizing agent, HCl is reducing agent., -2, , +5, , +4, , 0, , (III), H2S +2 HNO3 → 2NO2+ 2H2O+S, H2S undergo oxidation, HNO3 undergo reduction, HNO3 is oxidizing agent, H2S is reducing agent., 10. Explain the different types of redox reactions with examples., (I), , Combination reactions:-A reaction in which one element combines with another element or, compound to form product is called combination reactions., 2 H2 + O2→ 2H2O ,, CH4 + 2 O2 → CO2 +2 H2O, , (II), , Decomposition reactions:- A reaction in which a compound breaks down to form two or, more components in which one of the product should be in the elemental state., 2KClO3→2KCl+3 O2 ,, 2 NaCl → 2 Na + Cl2, , (III), , Displacement reactions:-A reaction in which an atom or ion in a compound is replaced by, another atom or ion., CuSO4 + Zn →Cu + ZnsO4 (metal displaces a metal), 2 Na + 2 H2O→ 2 NaOH + H2 (metal displaces a non metal), , (IV), , Disproportionation reactions:-A reaction in which the same species undergo simultaneous, oxidation and reduction is called disproportionation reaction., The element should be in the intermediate oxidation state., -1, -2 0, (i) 2 H2O2→2H2O +O2, 0, , -3, , +1, , (ii) P4 + 3 NaOH + 3 H2O→ PH3 + 3 NaH2PO2, 11. All decomposition reactions are not redox reactions. Give example., Decomposition of calcium carbonate to calcium oxide and carbon di oxide is not a redox reaction. In this, reaction, no change in the oxidation number of any elements.
Page 56 :
Join Telegram Channel: https://t.me/hsslive, +2 +4 -2, , +2 -2, , Downloaded from www.Hsslive.in ®, , +4 -2, , CaCO3→ CaO+ CO2, 12. ClO3− undergo disproportionation, but ClO4− does not. Explain., In ClO4− , the oxidation state of chlorine is +7. This is the highest oxidation state of chlorine., It can only decrease its oxidation number, but cannot increase it. So ClO 4- does not undergo, disproportionation reaction ., In ClO3- , the oxidation state of chlorine is +5. It can increase or decrease its oxidation number. So it, undergo disproportionation reaction., +5, , -1, , +7, , 4 ClO3- → Cl - + 3 ClO4−, 13. Balance the following equation using oxidation number method. Cu +NO 3− → NO2+ Cu2+, (I), Write skeleton equation and assign oxidation number., 0, , +5, , +4, , +2, , +NO3−, , Cu, → NO2+ Cu2+, (II), Find out increase and decrease of Oxidation Number and equalize Cu +2 NO 3− → NO2+ Cu2+, (III), Balance atoms other than hydrogen and oxygen., Cu +2 NO3− →2 NO2+ Cu2+, (IV), Equalize the charge on both side by adding H + ( since it is in acidic medium), Cu +2 NO3− + 4 H+ →2 NO2+ Cu2+, (V), Balancing hydrogen atom by adding H2O and check the number of oxygen atoms., Cu +2 NO3− + 4 H+ →2 NO2+ Cu2+ +2 H2O, 14. Balance the following equation using oxidation number method , Permanganate ion react with bromide, in basic medium to form manganese dioxide and bromate ion., (I), Write skeleton equation and assign oxidation number., +7, , -1, , MnO4−, , +4, , +5, , + Br →MnO2 + BrO3− ( Oxidation number of Mn decreases by 3 and oxidation, number of Br increased by 6), (II), Equalize increase and decrease in oxidation number. Multiply permanganate ion by 2 ., 2MnO4- + Br- →MnO2 + BrO3(III), Equalize atoms other than hydrogen and oxygen., 2MnO4- + Br- →2 MnO2 + BrO3(IV), Equalize the charge on both side by adding OH- ( since it is in basic medium), 2MnO4- + Br- →2 MnO2 + BrO3- +2 OH(V), Balancing hydrogen atom by adding H2O and check the number of oxygen atoms., 2MnO4- + Br- + H2O →2 MnO2 + BrO3- +2 OH15. Write the balanced chemical equation of K2Cr2O7 with Na2SO3 in acidic medium to form chromium (III), ion and sulphate ion., (I), Write skeleton equation and assign oxidation number., +6, , (II), (III), (IV), (V), , +4, , −, , +3, , +6, , Cr2O72- +SO32- →Cr3+ + SO42- - ( Oxidation number of Cr decreases by 6 ( 2 x 3 ) and oxidation number, of S increased by 2), Equalize increase and decrease in oxidation number. Here multiply SO 32- ion by 3 ., Cr2O72- +3 SO32- →Cr3+ + SO42Equalize atoms other than hydrogen and oxygen., Cr2O72- +3 SO32- →2Cr3+ + 3 SO42Equalize the charge on both side by adding H + ( since it is in acidic medium), Cr2O72- +3 SO32- + 8 H+ →2 Cr3+ +3 SO42Balancing hydrogen atom by adding H2O and check the number of oxygen atoms., Cr2O72- +3 SO32- + 8 H+ →2 Cr3+ +3 SO42- + 4 H2O
Page 59 :
Join Telegram Channel: https://t.me/hsslive, , UNIT : 9, , Downloaded from www.Hsslive.in ®, , HYDROGEN, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL: CHEM DSM, =====================================================, 1. Explain the position of Hydrogen in the periodic table., , Similarity with alkali metals:, (i), (ii), , Hydrogen→ Is1 , Alkali metals→ ns1, Hydrogen→ unipositive (+1), Alkali metals→ unipositive (+1), , Similarity with halogens:, Hydrogen(Is1 ) has one electron short to nearest noble gas helium (Is 2).Halogens (ns2np5) also, have one electron short to nearest noble gases(ns 2np6), (ii), Hydrogen→ uni negative (-1) , halogens → uni negative (-1), (iii), Hydrogen nonmetal, halogens nonmetals., (iv), Hydrogen is diatomic (H2), halogens are diatomic(F2,Cl2, Br2)., (v), Ionization enthalpy of hydrogen( 1312 kJmol-1) is similar to halogens( F , 1680 kJmol-1), So hydrogen can be placed above alkali metals of 1 st group and halogens of 17th group., 2. Which are the isotopes of hydrogen?, Isotopes of hydrogen, Relative, No. of neutrons, abundance, 1, Protium (hydrogen), 99.985 %, 0, No neutron, 1H, 2, Deuterium (D), 0.0156 %, 1, Known as heavy, 1H, hydrogen, -15, 3, Tritium (T), 10, %, 2, radioactive, 1H, 3. Give examples for the laboratory preparation of hydrogen., (i), Zinc react with acids give hydrogen., Zn + 2 HCl →ZnCl2 + H2, (ii), Zinc react with aqueous alkalies give hydrogen., Zn + 2 NaOH →Na2ZnO2 + H2, 4. Explain the commercial preparation of dihydrogen., (i), By the electrolysis of water using platinum electrodes., 2H2O → 2 H2 + O2, (ii), By the electrolysis of brine solution., 2 NaCl + 2 H2O → 2 NaOH + H2 + Cl2, (iii), From water gas: Water gas or syn gas is a mixture of carbon monoxide and hydrogen., It is produced by heating hydrocarbons or coke with steam., CH4 +H2O(g) → CO+ 3 H2, C+H2O(g) → CO+ H2, The process of producing water gas from coal is coal gasification., The carbon monoxide in water gas is converted to carbon dioxide by passing steam in the presence of, iron chromate catalyst. This is called water gas shift reaction., CO+ H2+H2O(g) → CO2+2 H2, CO2 is removed by dissolving in water and dihydrogen is produced., (i)
Page 60 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 5. Explain the chemical reactivity of dihydrogen, Hydrogen is smallest molecule and has high bond dissociation enthalpy. So it is inert at room, temperature. However, it is reactive at high temperature or in the presence of catalyst., (I), , 𝑭𝒆 ,𝟕𝟕𝟑 𝑲, , N2 + 3 H2 ⎯⎯⎯⎯⎯ 2 NH3, 𝑪𝒐𝒃𝒂𝒍𝒕,𝟕𝟕𝟎 𝑲, , (II), CO + 2 H2 ⎯⎯⎯⎯⎯⎯⎯⎯ CH3OH, 6. How does the atomic hydrogen torch function for cutting and welding purpose?, In atomic hydrogen torches, hydrogen atoms are produced by applying electric arc. These hydrogen, atoms are allowed to recombine on the surface to be welded to generate high temperature of 4000K., Hence these are used for cutting and welding purpose., 7. What is Hydrogenation of oil ?, Hydrogenation of vegetable oils using nickel a catalyst gives edible fat ( vanaspathi), Unsaturated oil + Hydrogen → vegetable fat, 8. Give some uses of hydrogen, (I), For the manufacture of ammonia , methanol, hydrochloric acid, vegetable fat etc., (II), In atomic hydrogen torches., (III), It is used as rocket fuel., 9. What are Hydrides? Which are different type hydrides? Explain each, The binary compounds of hydrogen with other elements are hydrides., These are three types (i) ionic hydride (ii) covalent hydride (iii) metallic hydride, (i), Ionic or saline hydride: With s block elements. They are crystalline. They conduct electricity in molten, state and in aqueous solution. E.g. NaH , KH , CaH2 (-1 oxidation state), (ii), Covalent or molecular hydride: With p block elements.The hydrides of group 13 are electron deficient, hydrides(B2H6). These are lewis acids. The hydrides of group 14 are electron precise hydrides (CH 4)., The hydrides of group 15,16,17 are electron rich hydrides(NH3, H2O,HF ). These are lewis bases., (iii), Metallic hydrides or interstitial hydrides: With d and f block elements. e.g. LaH2.87, VH0.56 . They are non, stoichiometric. Hydride gap is group 7,8,9., 10. Water has high boiling point. Why?, Due to inter molecular hydrogen bonding., 11. Why ice floats in water?, Ice has ordered three dimensional hydrogen bonded structure in which each oxygen atom is, surrounded by four hydrogen atoms tetrahedrally. The resulting structure has vacant spaces or holes., Therefore the density of ice is less than water. So ice floats in water., 12. Water is amphoteric substance. Explain, Water can act as both acid or base, HCl, + H2O ↔ H3O+ + ClBase, , NH3, , + H2O ↔ NH4+, , + OH-, , Acid, , 13. Which are Different types of hydrates ?, (I), Co-ordinated water : [Cr(H2O)6]Cl3, (II), Interstitial water : BaCl2.2 H2O, (III), Hydrogen bonded water: In CuSO4.5 H2O, one water is hydrogen bonded.
Page 61 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 14. What is Hard water and soft water ? Give the reason for hardness of water., The water which give lather with soap is called soft water., The water which does not give lather with soap is called hard water., Hardness of water is due to the presence of bicarbonates, chlorides and sulphates of calcium and, magnesium., The cations present in hard water form insoluble salt with soap and prevent the formation of lather., 𝟐𝑪𝟏𝟕 𝑯𝟑𝟓 𝑪𝑶𝑶𝑵𝒂 + 𝑴𝟐 → (𝑪𝟏𝟕 𝑯𝟑𝟓 𝑪𝑶𝑶)𝟐 𝑴 + 𝟐 𝐍𝐚 +, (M = Ca or Mg), 15. What is Temporary hardness?, Hardness can be removed by boiling is called temporary hardness., It is due to the presence of bicarbonates of calcium and magnesium., 16. Explain different methods used for the removal of temporary hardness, (i), Boiling: Insoluble magnesium hydroxide and calcium carbonates are produced. Filtered., M(HCO3)2 →MCO3+ H2O + CO2, , ( M= Mg, Ca), (ii), Clarks method: By adding lime , bicarbonates are converted as magnesium hydroxide and calcium, carbonates. Filtered., Mg(HCO3)2 + 2 Ca(OH)2 →2 CaCO3+ Mg(OH)2 + 2 H2O, 17. What is Permanent hardness?, Hardness can not be removed by boiling is called permanent hardness., It is due to the presence of chlorides and sulphates of calcium and magnesium., 18. Explain different methods used for the removal of permanent hardness., (i), By the treatment with washing soda ( sodium carbonate), CaCl2 +Na2CO3 →CaCO3+ 2 NaCl, (ii), By calgon’s method :-Sodium hexa meta phosphate is commercially known as calgon ., The calcium and magnesium ion form soluble complexes with calgon which remain in solution ,, but does not react with soap., Na6P6O18 → 2 Na+ + [Na4P6O18]2[Na4P6O18]2- + M2+ → [Na2MP6O18]2- + 2 Na+, (M2+ = Ca2+ ,Mg2+), (iii), By ion exchange method/ Zeolite method:-Zeolite is hydrated sodium aluminium silicate, (NaAlSiO4 .x H2O).It is represented as NaZ. It can exchange calcium and magnesium ion of hard, water., 2 NaZ + M2+ →MZ2 + 2 Na+, (iv), By synthetic resin method :- Hard water is passed through cation and anion exchange resins. Both, cations and anions are exchanged. To get pure deminerised water , the best method is synthetic, resin method., 19. Give some Disadvantages of hard water, (I), Hard water is not good for laundry purpose because it forms precipitate with soap and do not give, lather with soap., (II), Hard water is harmful for boilers because of scale formation. This reduces the efficiency of boilers., 20. Give the laboratory preparation of hydrogen peroxide., (i), By the action of dilute sulphuric acid on hydrated barium peroxide., BaO2. 8 H2O + H2SO4 → H2O2 +BaSO4 + 8H2O, (ii), By the hydrolysis of peroxo disulphuric acid which is obtained by the electrolytic oxidation of, acidified sulphate ion., 2 HSO4-→H2S2O8, H2S2O8 + 2H2O→ H2O2+ 2 H2SO4
Page 62 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 21. Give the industrial method for the preparation of hydrogen peroxide, By the auto oxidation of 2-ethyl, ethyl anthraquinol hydrogen peroxide is formed., , 2-ethyl anthraquinol., , 𝑶𝟐 (𝒂𝒊𝒓, 𝒂𝒊𝒓), , ⎯⎯⎯, ⎯, , 2-ethyl anthraquinone + H2O2, , 22. Draw the structure of hydrogen peroxide ., It has non planar open book type structure., , 23. Explain why hydrogen peroxide is not stored in glass vessels., Hydrogen peroxide decomposes in the presence of light or by rough surface. So it is kept in dark plastic, or wax lined glass bottles., 2H2O2 → 2H2O +O2, 24. Give examples for oxidizing and reducing character of hydrogen peroxide., (i), Oxidizing action in acidic medium., PbS + 4 H2O2 → PbSO4 + 4 H2O, (ii), Reducing action in acidic medium., 2MnO4- + 6H+ + 5H2O2 →2 Mn2+ + 8 H2O + 5 O2, (iii), Oxidizing action in basic medium., Mn2+ + H2O2 →Mn4+ + 2 OH(iv), Reducing action in basic, ic medium., 2MnO4 + 3H2O2 →2 MnO2 +3 O2 + 2 H2O + 2 OH25. Hydrogen peroxide re store the colour of the lead paintings. Give reason, It is due to the oxidizing action of H 2O2. It oxidizes black lead sulphide to white lead sulphate., 26. Hydrogen peroxide is a bleaching agent. Why?, Bleaching action of hydrogen peroxide is due to oxidation., H2O2 → H2O +[O], Coloured substance + [O] → Colourless substance., It bleaches ivory, silk,wood and paper pulp etc., 27. Give some uses, ses of hydrogen peroxide, (I), Hydrogen peroxide is used as antiseptic under the name of perhydrol., (II), It is used as hair bleach., (III), It is used to bleach textiles, paper pulp, leather, oils, fat etc., 28. What is heavy water( D2O) ? How is it produced? Give its uses., Heavy water( D2O) is deuterium oxide., It is produced by the prolonged electrolysis of water., It is used as moderator in nuclear reactors. It is used to study reaction mechanism., 29. What is Hydrogen economy ?, The principle of hydrogen economy is the transportation and storage of energy in the liquid or gaseous, dihydrogen. Energy is transmitted in tthe, he form of hydrogen and not as electric power.Hydrogen can produce, more energy. Pollution free. Dihydrogen is used as rocket fuel. But the di hydrogen gas is converted to, liquid state by cooling to 20 K. This would require expensive insulated tanks
Page 63 :
Join Telegram Channel: https://t.me/hsslive, , UNIT : 10, , Downloaded from www.Hsslive.in ®, , THE S- BLOCK ELEMENTS, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL: CHEM DSM, =============================================================, 1. Group 1 and 2 are s-block elements., Group No., , Members of the family, , 1 (Alkali metals), , Lithium (Li) → [He] 2s1, Sodium (Na) → [Ne] 3s1, Potassium(K) → [Ar] 4s1, Rubedium (Rb) → [Kr] 5s1, Cesium(Cs), → [Xe] 6s1, Francium(Fr) → [Rn] 7s1, Beryllium (Be), → [He] 2s2, Magnesium (Mg) → [Ne] 3s2, Calcium(Ca), → [Ar] 4s2, Strontium(Sr), → [Kr] 5s2, Barium(Cs), → [Xe] 6s2, Radium(Ra), → [Rn] 7s2, , 2 (alkaline earth metals), , General outer electronic, configuration, ns1, , Oxidation, state, +1, , ns2, , +2, , 2. Group 1 is called Alkali metals . Why?, Because they form hydroxides with water and are alkaline in nature., 3. Group 2 is called Alkaline earth metals . Why?, Because their oxides and hydroxides are alkaline in nature and they are present in the earth crust., 4. Alkali metals are kept in kerosene oil. Why?, Because of its high reactivity with air and water., 5. Alkali metals not found in nature. Why?, Because they are highly reactive and hence do not occur free, 6. Compare some properties of alkali metals and alkaline earth metals, Ionisation enthalpy of alkaline earth metals (due to small size and ns 2 stable electronic configuration) are, higher than alkali metals., Alkali metal oxides are more basic than the oxides of group 2 because alkali metals are more, electropositive than alkaline earth metals., The hydroxides of alkali metals are more soluble than those of alkaline earth metals., 7. Arrange alkali metal ions in the decreasing order of hydration enthalpy., , Li+ > Na+ > K+ > Rb+ > Cs+, Li+ has maximum degree of hydration and for this reason Lithium salts are mostly hydrated. LiCl .2 H 2O, 8. Alkali metals and their salts give characteristics colour to non luminous flame. Why?, This is because heat from the flame excites the outermost electrons to higher energy levels., When these electrons return to their original level, radiations are emitted in the visible region., Thus they appear coloured., Lithium →crimson red, Sodium → Golden yellow , Potassium→ Violet.
Page 64 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 9. How do alkali metals react with air?, , Alkali metals tarnish in air due to oxide formation., Lithium gives monoxide (Li2O) only with oxygen., Sodium forms monoxide and peroxide (Na2O, Na2O2 )., Others form monoxides, peroxides and super oxides ( K2O, K2O2, KO2 )., Lithium reacts with nitrogen gives lithium nitride. Others not., 10. Solutions of alkali metals in liquid ammonia are blue in colour. Why?, The blue colour is due to the presence of ammoniated electrons., , M + (x+y) NH3→ [M(NH3)x]+ + [e(NH3)y]−, 11. Which are the Anomalous properties of lithium ?, (I), Lithium gives monoxide (Li2O) only. Others form monoxides, peroxides and super oxides., (II), Lithium reacts with nitrogen gives lithium nitride. Others not., (III), Lithium chloride is deliquescent. Others not., (IV), Lithium is harder and has high melting and boiling point., , 12. Give some Diagonal relationships between lithium and magnesium (similarity in properties), (I), Both lithium and magnesium are harder., (II), Both lithium and magnesium form nitride., (III), Both lithium chloride and magnesium chloride are deliquescent., (IV), Both lithium and magnesium carbonate decompose easily, , 13.Explain the Manufacture of sodium carbonate(solvay process)., Raw materials : Lime stone(CaCO3), ammonia (NH3) and brine solution (NaCl)., In this process, carbon dioxide obtained by the decomposition of lime stone is passed through, brine solution saturated with ammonia., Sodium bicarbonate is precipitated. It is filtered and heated to get sodium carbonate., By product in this process is calcium chloride., , 2 NH3 + H2O + CO2 → (NH4)2 CO3, (NH4)2 CO3 + CO2 + H2O, →, 2 NH4HCO3, NH4HCO3 + NaCl → NaHCO3 + NH4Cl, 2 NaHCO3 → Na2 CO3 + CO2 +H2O, , 14. Why solvay process is not used for the preparation of potassium carbonate?, The intermediate Potassium bicarbonate is water soluble and cannot be precipitated., , 15.Explain Manufacture of sodium hydroxide in Kastner- Kellner cell, By the electrolysis of brine solution (NaCl) in Kastner- Kellner cell., Mercury cathode and carbon anode., 𝑯𝒈, , At cathode, Na+ + eNa/Hg, 2 Na/Hg + H2O → 2 NaOH + H2 +2 Hg, 16. Give the preparation of sodium hydrogen carbonate, , Sodium bi carbonate is prepared by passing carbon dioxide gas through sodium carbonate solution., , Na2 CO3 + CO2, , +H2O → 2 NaHCO3
Page 65 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 17. What are the major roles of Na+ and K+ in our body?, Sodium ions help in the transmiss, transmission, ion of nerve signals, regulation of water flow across cell, membrane., Potassium ions help in the oxidation of glucose to ATP and in tthe transmission, ion of nerve signals., 18. Arrange alkaline earth metal ions in the decreasing ord, order, er of hydration enthalpy, , Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+, 19. Which are the anomalous behavior of beryllium ?, (i), Beryllium does not react with water even at high temperature. while others react., (ii), Compounds of Beryllium are covalent. Others form ionic compounds., (iii), Beryllium oxide and Beryllium hydroxide is amphoteric. Others form basic oxides and, hydroxides., 20. Which are The diagonal relationship (similarity in properties) between Beryllium and Aluminium., (i), (ii), (iii), , Beryllium hydroxide ad aluminium hydroxide are amphoteric., Like Aluminium , Beryllium is not attacked by acids due to the formation of oxide film., Both beryllium chloride and aluminium chloride have bridged structure., , 21. How calcium oxide and calcium hydroxide is produced?, Calcium oxide (Quick lime) is formed by heating Lime stone (CaCO3)., , CaCO3 → CaO + CO2, , Calcium hydroxide (slaked lime) is produced by adding water to quicklime (CaO), , CaO +H2O → Ca(OH)2, , 22. What is Milk of lime and lime water ?, Slaked lime, me is white solid sparingly soluble in water., A suspension of slaked lime in water is called milk of lime., An aqueous solution of slaked lime is called lime water., 23. What is Bleaching powder ? How is it prepared?, Bleaching powder is a mixture of calcium chloride and calcium hypochlorite., It is prepared by passing chlorine gas through dry slaked lime., , 2 Ca(OH)2 + 2 Cl2 → Ca(OCl)2 + CaCl2 + 2 H2O, 24. What happens when carbon dioxide is passed through lime water?, When carbon dioxide is passed through lime w, water,, ater, it turns milky due to the formation of insoluble, calcium carbonate., When excess carbon dioxide is passed, milkiness disappears due to the formation of soluble, calcium bicarbonate., , Ca(OH)2 + CO2 → CaCO3+ H2O, CaCO3+ H2O + CO2 → Ca(HCO3)2, 25. Give the reaction between calcium carbonate and hydrochloric acid., , CaCO3+ 2HCl → CaCl2 + H2O + CO2
Page 66 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 26. What is Plaster of paris? How is it prepared?, Plaster of paris is calcium sulphate hemi hydrate., It is prepared by heating gypsum at 393 K, , CaSO4. 2 H2O → CaSO4. 1/2 H2O, , 27. Plaster of paris is used for moulding statues. Why?, On mixing with water , it forms a plastic mass which sets in to hard solid., 28. What is Dead burned plaster ?, When heated above 393 K, plaster of paris loses water of crystallization and becomes dead burnt, plaster (CaSO4 ) which does not set in the presence of water., 29. What is cement? How cement is manufactured?, The important constituents of cement are di calcium silicate, tri calcium silicate and tri calcium, aluminate., Raw materials : Lime stone , clay, Lime stone and clay are mixed and heated , cement clinker is formed. It is mixed with gypsum and, powdered. This is called Portland cement., Gypsum is added to slow down setting of cement., 30. What is Setting of cement?, When mixed with water, cement slowly set in to hard mass., Setting is due to hydration of molecules of cement. It is an exothermic process., , 31. Important compounds, formula and uses, Compound, Washing soda OR, sodium carbonate deca hydrate, Caustic soda OR, sodium hydroxide, , Formula, , Common salt OR, sodium chloride, , NaCl, , Baking soda OR, sodium hydrogen carbonate, Quick lime OR, calcium oxide, Slaked lime OR, calcium hydroxide, Calcium carbonate OR, Lime stone OR Marble, Plaster of paris OR, calcium sulphate hemi hydrate, , NaHCO3, , Gypsom OR, calcium sulphate dihydrate, Milk of magnesia OR, magnesium hydroxide, , CaSO4. 2 H2O, , Uses, Used for softening of hard water, In soap ,glass and paper industries, In the manufacture of soap, paper, and artificial silk, For the purification of Bauxite, Used as table salt for cooking, In the manufacture of NaOH,, Na2CO3 etc., For making cakes, Mild antiseptic, Used in building industry, Used as flux in metallurgy, Used in white wash, For softening of hard water, To make quick lime, cement etc., Marble is used as building material, For making models, statues etc., As plaster for setting fractured, bones, In dentistry, To slow down the setting of cement, , Mg(OH)2, , used as antacid, , Na2CO3. 10 H2O, NaOH, , CaO, Ca(OH)2, CaCO3, CaSO4. 1/2 H2O, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, CHEM DSM
Page 67 :
Join Telegram Channel: https://t.me/hsslive, , CHAPTER : 11, , Downloaded from www.Hsslive.in ®, , THE P-BLOCK ELEMENTS, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL: CHEM DSM, =====================================================, 1. Group 13 to 18 are p block elements., 2. Boron family and carbon family, Group No., , Members of the family and, General outer electronic, electronic configuration, configuration, 2, 1, 13 (Boron, Boron (B), → [He] 2s , 2p, ns2np1, 2, 1, family), Aluminium (Al) → [Ne] 3s , 3p, Gallium (Ga), → [Ar] 3 d10 ,4s2 , 4p1, Indium (In), → [Kr] 4 d10 ,5s2 , 5p1, Thalium (Tl), → [Xe] 4f14 ,5 d10 , 6s2 , 6p1, 14 (Carbon, Carbon (C), → [He] 2s2 , 2p2, ns2np2, 2, 2, family), Silicon (Si), → [Ne] 3s , 3p, Germanium (Ge) → [Ar] 3 d10 ,4s2 , 4p2, Tin(Sn), → [Kr] 4 d10 ,5s2 , 5p2, Lead(Pb), → [Xe] 4f14 ,5 d10 , 6s2 , 6p2, 3. What is Inert pair effect? Give its reason. What are its consequences?, The reluctance of s electrons to participate in chemical bonding is called inert pair effect., It is seen in group 13, group 14 ,and group 15 ., In these groups as the atomic size increases(i.e. down the group), oxidation state two units less than group, oxidation states is common ., Reason: Down the group, due to poor shielding effect of inner d and f electrons, the increased nuclear, charge holds ns2 electrons more tightly restricting their ability to participate in chemical bonding., So only p-electrons participate in chemical bonding., , Consequences:, In group 13, group oxidation state is +3, but for thalium +1 is more stable (eg. TlCl is more stable than TlCl 3), In group 14, group oxidation state is +4, but for Lead +2 is more stable (eg. PbCl 2 is more stable than PbCl4), 4. In group 13 elements, atomic radius increases down the group. But atomic radius of gallium, ( [Ar] 3d104s24p1) is less than that of aluminium. Why?, This is due to the presence of completely filled d-orbitals in Gallium. Due to this shielding effect is low and, greater attraction by the nucleus., 5. Explain the oxidation state of Boron family., Group oxidation state +3., Down the group +1 predominates due to inert pair effect., 6. What are electron deficient compounds ? Explain with examples., Molecules in which central atom has electrons less than 8 are called electron deficient compounds. They, act as lewis acids because they can accept electron pair., E.g. BCl3 ,AlCl3
Page 70 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 20. Explain the allotropes of carbon., , (i), , Diamond :In diamond, each carbon has sp3 hybridisation. Each carbon is tetrahedrally connected to four, other carbon atoms and three dimensional net work structure is formed. So it is hard. Due to the, absence of free electrons , it is not electrical conductive., , (ii), , Graphite :In graphite, each carbon has sp2 hybridisation. It has hexagonal layered structure. Different layes, are held by weak vander waals forces. So each layer can slide over one another. Therefore graphite, is soft and slippery. Due to the pre, presence of free electrons, it is a good conductor of electricity., Graphite is used as solid lubricant., , (iii), , Fullerenes:-, , Fullerenes, renes are synthetic allotropes of carbon. Most common is C60 . It is called buckminster, fullerene. It contain 20 six membered rings and 12 five membered rings. A six membered, membere ring is, fused with six or five membered rings . Five membered ring is fused with six membered rings only., Each carbon is sp2 hybridised. Each carbon forms three sigma bonds with three carbon atoms and, fourth electron is free. This electron is delocalized and so fullerene is aromatic., 21. Graphite is the thermodynamically most stable form of carbon, 22. What are water gas and producer gas? Give their preparation and use., Water gas is a mixture of carbon monoxide and hydrogen. It is produced by passing steam through, throug coke., , C + H2O (g), , 𝟒𝟕𝟑 𝟏𝟐𝟕𝟑 𝑲, , ⎯⎯⎯⎯⎯⎯⎯, , CO, , (g), , + H2 (g), , Producer gas is a mixture of carbon monoxide and nitrogen. It is produced by passing hot air through coke., 𝟏𝟐𝟕𝟑 𝑲, , 2 C + O2 (g) + 4 N2 (g) ⎯⎯⎯, , 2CO, , (g), , + 4 N2 (g), , Both are industrial fuels., 23. Draw the structure of Carbon monoxide., In CO , there is one sigma bond and two pi bonds between carbon and oxygen atom., 24. Draw the structure of Carbon dioxide., oxide., , Carbon dioxide is a linear molecule with sp hybridisation. It has no dipole moment., Carbon forms two sigma bonds and two pi bonds with two oxygen atoms., 25. CO2 is gas, while SiO2(silicon di oxide or silica) is solid. Explain, In CO2 molecule, C atom undergoes undergoes sp, hybridization. So it has linear shape. It exist as discrete, (separate) molecules, cules and there is only weak attractive, between the different CO2 molecules.. So CO2 is gas, But in silica, each silicon atom undergoes sp3 hybridisation ., Here each silicon atom is tetrahedrally surrounded by four, oxygen atoms. So it has three dimensional net work, structure and hence it is solid.
Page 71 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 26. What are silicons? Give their preparation and uses., Silicons are organo silicon polymers with repeating unit (-R2SiO-)., It is produced by the hydrolysis of dichloro dialkyl silane followed by polymerization., They are used as sealant, greases, electrical insulator, and for water proofing of fabrics., Being biocompatible they are also used in surgical instruments and in cosmetics., , 𝑪𝒖 𝑷𝒐𝒘𝒅𝒆𝒓 ,𝟓𝟕𝟑 𝑲, , 2 RCl + Si ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯, , 𝟐 𝑯𝟐 𝑶, , R2SiCl2 ⎯⎯, , 𝑷𝒐𝒍𝒚𝒎𝒆𝒓𝒊𝒔𝒂𝒕𝒊𝒐𝒏 , 𝑯𝟐 𝑶, , R2Si(OH)2 ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯, , 27. What are silicates?, The basic units silicates are SiO44- tetrahedral. E.g. Feldspar,, zeolites, mica, asbestose.etc., Glass and cement are man made silicates., In silicates SiO44- units exist as separate or several such units, are joined together by oxygen atoms.., There are chain silicates, ring silicates, and three, dimensional silicates., 28. What are zeolites? Give its uses., Zeolites are alumino silicates of certain metals., Zeolites are used for cracking and isomerisation in petrochemical industries., ZSM-5 is used for conversion of alcohol in to gasoline., It is also used for softening of hard water., 29. Diamond ,graphite and fullerene, , Diamond, , 3, , sp hybridisation, Tetrahedral and three dimensional net, work structure, Not electrical conductor, Hardest in nature, Used in ornaments and in cutting tools, , Graphite, , 2, , Fullerene, , 2, , Sp hybridisation, Hexagonal layered structure, , Sp hybridisation, C60 soccer ball shape, , Electrical conductor, Soft, Thermodynamically most stable, Used as solid lubricant, , Not electrical conductor, Hard (Synthetic), Aromatic, Use in tumor treatment
Page 72 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , CHAPTER :12, ORGANIC CHEMISTRY- SOME BASIC PRINCIPLES AND TECHNIQUES, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL: CHEM DSM, ================================================================, 1. Organic chemistry is the study of hydrocarbons and their derivatives., 2. Urea:- First synthesized organic compound in the laboratory by Frederic Wohler., 3. Why organic compounds are larger in number?, (a) Due to tetra valency of carbon (b) Due to the ability to form single bond and multiple bonds, (c) Due to catenation (d) Due to isomerism., 4. Catenation:- Self linking property of an element to form long chains and rings is called Catenation, Carbon has highest catenation property., 5. What is Functional group?, Functional group is an atom or group of atoms that determine the properties of an organic compound., Functional group, Name of functional group, Name of organic compounds, -OH, Hydroxyl group, Alcohols, -NH2, Amino group, Amines, -COOH, Carboxyl group, Carboxylic acids, -COCarbonyl group, Ketones, -CHO, Aldehydic group, Aldehydes, -X (-F , -Cl , -Br , -I ), Halo group, Halo compounds, 6. What is homologous series ?, A series or group of organic compounds in which adjacent members are differed by –CH 2 group is called, homologous series. They contain same functional groups. They have similar chemical properties and, show gradual change in physical properties. They can be prepared by some general methods of, preparation., Eg:- alkanes , alkenes, alkynes, alcohols, amines, carboxylic acids etc., , 7. Nomenclaturre of organic compounds, Alkanes → Root word + ane, Alkenes → Root word + ene, Alkynes → Root word + yne, Number of, Root word, carbon atoms, 1, Meth2, Eth3, Prop4, But4, Pent-, , Number of carbon atoms, , Root word, , 6, 7, 8, 9, 10, , HexHeptOctNonDec-
Page 77 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , Electrophiles:- Electron, lectron loving species. A reagent that takes away an electron pair is called an, electrophile. Eg:- H+ , Cl+ , carbocations (positive ions), BF3, AlCl3 (electron deficient, ent molecules), 20. Explain the different types of electron displacement effects in covalent bonds., (I), , Inductive effect:It is the permanent polarization of a sigma bond due to the presence of polar group. It is a permanent, effect., Positive inductive effect (+I) :- By electron releasing groups . e.g. alkyl groups, , Negative inductive effect (--I) : By electron withdrawing groups e.g. –NO2 , -CN, CN , -COOH , -F, -Cl, (II), , Electromeric effect:It is a temporary effect .It is complete transfer of pi electrons of mu, multiple, ltiple bond in the presence of, attacking reagent. When attacking reagent is removed, shift back to its original condition., Positive electromeric effect (+E) ::- pi electron shifting towards attacking reagent, , Negative electromeric effect ((-E) : -pi electron, ectron shifting away from the attacking reagent, , (III), , Resonance effect (Mesomeric effect): It is the charge produced in the molecule by the interaction of, two π- bonds or between a π- bond and lone pair of electrons present on an adjacent atom., Positive, sitive mesomeric effect (+M) : EElectron shifting towards conjugated system., –OH , -OR, –NH2 , halogens, , Negative, gative mesomeric effect ((-M) : Electron, lectron shifting away from the conjugated system., –NO2 , -CN , -COOH, , (IV), , Hyper conjugation:, It is a permanent effect. Here the sigma electrons of C, C-H, H bond of alkyl group enter in to partial, conjugation with the unsaturated system or with the unshared p orbital.
Page 78 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , The hyper conjugative structures of propene are, , The hyper conjugative structures of ethyl carbocations are, , 21. Purification techniques:, Purification techniques, Sublimation, Crystallisation, , Distillation, , When it is used, It is used to separate sublimable, compounds from non sublimable, impurities, It is based on the difference in the, solubilities of the compound and the, impurities in a suitable solvent., , Examples, Naphthalene, iodine, camphor, A mixture of benzoic acid and, naphthalene can be separated, from hot water in which benzoic, acid dissolves but naphthalene, does not, Chloroform (bp. 334K) and, Aniline(bp. 457 K), , To separate (1) volatile liquids from, non volatile impurities (2)the liquids, having sufficient difference in their, boiling points, Fractional distillation, To separate liquids having close, Crude oil, toluene and, boiling points, cyclohexane, ethanol and water, Distillation under reduced To purify liquids which decompose at, Glycerol can be separated from, pressure, or below its boiling points, spent lye., Steam distillation, To separate substances which are, Aniline water mixture, steam volatile and are immiscible with, water, Differential extraction, When an organic compound is present, in aqueous solution, it is separated by Benzoic acid from water is, shaking it with an organic solvent in, extracted by using benzene, which it is more soluble than in water, 22. What is chromatography? Which are different types of chromatography?, Chromatography means colour writing. This method is used to separate mixtures into their, components, purify components and also to test the purity of compounds., These are two types, (I) adsorption chromatography (II) partition chromatography( Paper chromatography)., 23. Name two types of chromatographic techniques based on adsorption., (I)Column chromatography (II) Thin layer chromatography, 24. Explain column chromatography and Thin layer chromatography., (I), Column chromatography :-The compound to be separated is converted in to movable phase and, passed through a column of adsorbent packed in a glass tube ( Stationary phase). Silica gel or, alumina gel is used as adsorbent. The components are adsorbed at different places depending
Page 79 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , on their degree of adsorption. The most readily adsorbed component is retained near the top, and other come down to various distances in the column., (II), Thin layer chromatography :-Thin layer of adsorbent is coated on a glass plate is called, chromatography plate. The mixture is to be separated is spotted 2 cm above the plate. It is, placed in jar containing eluant. As the eluant rises up the plate, the components are separated., The relative adsorption of components are expressed in terms of retardation factor (R f factor), 𝑫𝒊𝒔𝒕𝒂𝒏𝒄𝒆 𝒕𝒓𝒂𝒗𝒆𝒍𝒍𝒆𝒅 𝒃𝒚 𝒕𝒉𝒆 𝒔𝒖𝒃𝒔𝒕𝒂𝒏𝒄𝒆 𝒇𝒓𝒐𝒎 𝒕𝒉𝒆 𝒃𝒂𝒔𝒆 𝒍𝒊𝒏𝒆, 𝑹𝒇 =, 𝑫𝒊𝒔𝒕𝒂𝒏𝒄𝒆 𝒕𝒓𝒂𝒗𝒆𝒍𝒍𝒆𝒅 𝒃𝒚 𝒕𝒉𝒆 𝒔𝒐𝒍𝒗𝒆𝒏𝒕 𝒇𝒓𝒐𝒎 𝒕𝒉𝒆 𝒃𝒂𝒔𝒆 𝒍𝒊𝒏𝒆, Colorless spots are converted to coloured spots by putting the plate in ultraviolet light or placing in, iodine jar., 25. Explain partition chromatography (e.g. paper chromatography)., It is based on differential partitioning of components of a mixture between stationary phase and mobile, phase. Chromatographic paper trapped water is stationary phase. Mixture is spotted at the base of the, paper. The paper is suspended in solvent. As the solvent rises up , the components are partitioned., 26. Detection of carbon and hydrogen, Organic compound is heated with cupric oxide. Carbon is converted to carbon dioxide which turns lime, water milky. Hydrogen is converted to water which turns anhydrous copper sulphate to blue., 27. Detection of nitrogen, sulphur and halogen: Lassaigne’ s test, It is done using sodium fusion extract (Lassaigne’ s extract) .It is prepared by heating organic compound, with sodium in a fusion tube. When red hot, it is plunged in to water taken in a china dish. The solution, is boiled and filtered. Filtrate is called sodium fusion extract (Lassaigne’ s extract) ., It is prepared to convert covalent bonded organic compounds into water soluble ionic compounds., No. Experiment, Observation, Inference, 1, Extract is treated with ferrous sulphate Prussian blue colour, Presence of nitrogen, and concentrated sulphuric acid, 2, To extract sodium nitro prusside is, Violet colour, Presence of sulphur, added, 3, To extract acetic acid and lead acetate, Black precipitate (PbS), Presence of sulphur, is added, 4., To extract nitric acid and silver nitrate, White precipitate (AgCl), Presence of chlorine, is added, soluble in ammonium, hydroxide, Pale yellow (AgBr) precipitate, Presence of bromine, slightly soluble in ammonium, hydroxide, Yellow precipitate(AgI), insoluble in ammonium, hydroxide, , Presence of iodine, , 28. Ferriferrocyanide, Fe4[Fe(CN)6]3:- The blue coloured compound in the Lassaigne’s test for nitrogen., 29. In the Lassaigne’s test for halogen, they are precipitated as silver halide( AgX ), 30. Explain the detection of phosphorous in an organic compound., The organic compound is heated with sodium peroxide, phosphate is formed. This solution is boiled, with nitric acid ammonium molybdate . Yellow colour or yellow precipitate is formed. The yellow, precipitate is ammonium phosphomolybdate
Page 83 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , Normal alkanes having six orr more carbon atoms on heating at high temperature and pressure in the, presence of catalyst like chromium oxide, benzene and its homologues are formed., 9. Explain pyrolysis or cracking with example, The decomposition of bigger hydrocarbons in to simple hydrocarbons, ydrocarbons by heating at high temperature is, called cracking or pyrolysis., 𝑪𝟔 𝑯𝟏𝟒, 𝑪𝟔 𝑯𝟏𝟒, 𝑪𝟔 𝑯𝟏𝟒, , 𝟕𝟕𝟑 𝑲, , ⎯⎯, , 𝟕𝟕𝟑 𝑲, , ⎯⎯, , 𝟕𝟕𝟑 𝑲, , ⎯⎯, , 𝑪𝟔 𝑯𝟏𝟐 + 𝑯𝟐, 𝑪𝟒 𝑯𝟖 + 𝑪𝟐 𝑯𝟐, 𝑪𝟑 𝑯𝟔 + 𝑪𝑯𝟒, , 10. What are Conformations ?, The different spatial arrangements of atoms arising due to free rotation around a carbon-carbon, carbon, single bond are called conformations., 11. What are different types of conformations of ethane? Compa, Compare their stability., Sawhorse, whorse projections of ethane, Newman projections of ethane, , Ethane has staggered ,eclipsed and skew conformations., Staggered conformation is more stable. This is because hydrogen atoms of two carbon atoms are at, maximum distance and repulsion is minimum and stability is maximum., Eclipsed conformation has hydrogen atoms closed together , repulsion maximum, stability minimum., The intermediate conformations are called skew conformatio, conformations, 12. What is meant by geometrical isomerism?, The isomerism arises due to restricted rotation about carbon, carbon- carbon double bond is called, geometrical isomerism., For geometrical isomerism, the groups attached to each carbon must be different., If the same group same side is called cis isomer., If the same group opposite side is called trans isomer., Cis isomer is more polar., Trans isomer is more stable, 13. Draw the geometrical isomers of 2--butene and but-2-enoic acid.
Page 87 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , (v), , Kekuke structure indica, indicates, tes the possibility of two types of disubstituted derivatives. However, benzene form only one type disubstituted derivative., , (vi), , Benzene has resonance structures, , (vii), , Even though benzene contain three double bonds, benzene is stable., , In benzene, each carbon is in sp2 hybridised state., Each carbon has one unhybridised p orbital. So, continuous overlap is possible. So pi electrons are, delocalized. These delocalized pi electrons are, responsible for the stability of benzene., , 32. State huckel’s rule aromaticity., A cyclic , conjugated , planar system is aromatic if it contains (4n +2) pi electrons in the ring., Where n = 1,2,3 etc, , Benzene, Cyclopentadienyl anion Cyclohepta trienyl cation, These are planar molecules, complete delocalization of pi electrons in the ring is possible,, Huckel rule obeys. So these are aromatic. 4n +2 = 6 (here n=1), 33. With the help of huckel’s explain 1,4, 1,4- hexadiene and 1,3-butadiene, butadiene are not aromatic., , 1,3-butadiene, No. of pi electrons = 4 (2 π bonds x 2 e -1 ), 4n +2 = 4 ( n = 0.5), Hence the value of n is 0.5. So it does not obey Huckel rule and is not aromatic.
Page 88 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 34. Naphthalene is aromatic. Explain using Huckel rule., Naphthalene is planar, complete delocalization of pi electrons are possible., No. of pi electrons = 10 (5 π bonds x 2 e -1 ), 4n +2 = 10 ( n = 2), Hence the value of n is 2. So it obeys Huckel rule and is aromatic., 35. Both anthracene and phenanthrene are aromatic. Explain using Huckel rule., Both anthracene and phenanthracene are planar, complete, delocalization of pi electrons are possible. No. of pi electrons, = 14 (7 π bonds x 2 e -1 ), 4n +2 = 14 ( n = 3) Hence the value of n is 3. So it obeys, Huckel rule and is aromatic., 36. Cyclo octatetraene is not aromatic . Explain, No. of pi electrons = 8 (4 π bonds x 2 e -1 ), 4n +2 = 8 ( n = 1.5) Hence the value of n is 1.5., So it does not obeys Huckel rule and is not aromatic., 37. How will you prepare benzene ?, , (I), , From acetylene (ethyne) :When acetylene (ethyne) is passed through red hot iron tube, benzene is formed., , (II), , From sodium benzoate :Benzene is formed by the decarboxylation of sodium benzoate with soda lime., , (III), , From Phenol :- When phenol is heated with zinc dust benzene is formed
Page 89 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 38. Electrophilic substitution reaction :-Positively charged electrophile replaces hydrogen of the benzene ring., (i), Halogenation: Benzene is converted to chlorobenzene when chlorine is added in the presence, anhydrous aluminium chloride., , (ii), , Nitration: Benzene is converted to nitrobenzene in the presence of nitrating mixture(Mixture of, concentrated nitric acid and concentrated sulphuric acid), , (iii), , Sulphonation : Benzene is converted to benzene sulphonic acid by adding fuming sulphuric acid, , (iv), , Friedel –Craft alkylation(benzene to toluene):- When benzene is treated with methyl chloride in the, presence of anhydrous aluminium chloride, toluene is formed. It is called Fridel-Craft alkylation, , (v), , Fridel-Craft acylation (convert benzene to aceto phenone), , When benzene is treated with acetyl, chloride in the presence of anhydrous, aluminium chloride, acetophenone is, formed., 39. What happens when excess chlorine is added to benzene in the presence of anhydrous aluminium chloride?, , Hexachlorobenzene (C6Cl6 ) is formed.
Page 91 :
Join Telegram Channel: https://t.me/hsslive, , CHAPTER 14, , Downloaded from www.Hsslive.in ®, , ENVIRONMENTAL CHEMISTRY, , PREPARED BY: YOOSAFALI T K , GHSS VARAVOOR (8040) ,9947444175, YOUTUBE CHANNEL: CHEM DSM, ================================================================, 1. What is Environmental pollution?, It is the undesirable changes in our surroundings that have harmful effects on plants, animals and, human beings., 2. Which are responsible for tropospheric pollution?, (i), Gaseous air pollutants like oxides of nitrogen, sulphur and carbon., (ii), Particulate pollutants like dust, fumes smoke etc., 3. How Gaseous air pollutants are formed in the atmosphere?, Sulphur dioxide is formed in the atmosphere due to the burning of fossil fuels and by volcanic, eruptions., Nitrogen dioxide form in the atmosphere during lightning and by the burning of fossil fuels in, automobiles., Carbon monoxide is formed by the incomplete combustion of fuels. Carbon dioxide is formed by the, complete combustion of fuels., 4. Sulphur dioxide is poisonous to both plants and animals. Justify the statement., (i), It leads to respiratory diseases like asthma, bronchitis etc., (ii), It can cause cough, irritation of eyes etc., (iii), It damages vegetable crops and affects plant growth., 5. What are the harmful effects of NO2 ?, (i), It damages leaves and decreases the rate of photosynthesis., (ii), It affects textile fibers and metals., (iii), It irritates lungs and cause respiratory diseases., (iv), It leads to photochemical smog., 6. Carbon monoxide is highly poisonous. Explain., It binds with haemoglobin to form carboxy haemoglobin. This reduces the oxygen carrying capacity of, haemoglobin. The low oxygen content in blood results in head ache, weak eye sight, cardiovascular, disorder and finally leads to death., 7. Explain Green house effect and global warming, Green house effect is the phenomenon in which earth’s atmosphere traps the heat from the sun and, prevents it from escaping into outer space resulting in the rise of atmospheric temperature. When more, infrared radiations are trapped, the atmosphere becomes hotter and the global temperature rises up., This is called global warming., 8. Which are the green house gases? ( gases responsible for global warming), Carbon dioxide , methane ,ozone ,Chlorofluorocarbons, water vapour, 9. What are the adverse effects of green house effect and global warming?, (i), Increases global temperature and leads to infectious diseases such as yellow fever, dengue fever etc., (ii), It leads to melting of polar ice caps and flood in low level areas., 10. What can we do to reduce global warming?, (i), Reduce the burning of fossil fuels by minimizing the use of automobiles., (ii), Plant trees and encourage afforestation, (iii), Avoid burning of dry leaves, wood etc., (iv), Aware the public about the bad effects of global warming.
Page 92 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , 11. What is Acid rain? How is it formed?, When the PH of rain water falls below 5.6, it is called acid rain., Sulphur dioxide and nitrogen dioxide present in polluted air is responsible for acid rain., , 2 SO2 + O2 + 2 H2O→ 2H2SO4, 4 NO2 + O2 + 2 H2O→ 4HNO3, , 12. What are harmful effects of acid rain?, (i), It is harmful to vegetation and aquatic life., (ii), It corrodes water pipes and dissolves heavy metals such as copper, lead and iron from soil and, rocks., (iii), It damages building materials. (e.g Tajmahal is affected by acid rain), 13. What are Particulate air pollutants ?, These are tiny solid or liquid particles in air. These are two types., (i), Viable particulates: By minute living organisms such as bacteria, fungi, mould, algae etc, (ii), Non viable particulates: Non living things like mist, fumes, smoke, dust., 14. What is Smog? Explain each., Smog is a mixture of smoke and fog. These are two types.(i) Classical smog and (ii) Photochemical smog, , Classical smog, , Photochemical smog, , It is due to sulphur dioxide, , It is mainly due to the presence of nitrogen dioxide, ozone, and some other oxidants., It occurs warm, dry and sunny climate, It is oxidizing smog, , It occurs in cool humid climate, It is called reducing smog, 15. How is photochemical smog is formed?, At high temperature present in petrol and diesel engines, N 2 and O2 combines to form nitric oxide(NO), and emit into atmosphere along with unburned hydrocarbons. Nitric oxide then oxidized to nitrogen, dioxide(NO2). This NO2 the absorb energy from light and breaks up into NO and oxygen atom. The, oxygen atom is very reactive and combines with oxygen gas form ozone., Ozone so formed combines with NO forms nitrogen dioxide. NO2 and ozone are strong oxidizing agents, and react with unburned hydrocarbons to form substances such as formaldehyde, acrolein, peroxy, acetyl nitrate (PAN) etc., 16. What are the adverse effects of photochemical smog?, (i), Both ozone and PAN (peroxy acetyl nitrate) are eye irritants., (ii), Ozone and NO irritates nose and throats and in high concentration causes head ache chest pain etc., (iii), Damages plants., (iv), It causes corrosion metals , building materials, painted surfaces., 17. How can we control photochemical smog?, (i), Use catalytic convertors in auto mobiles to prevent the release of oxides of nitrogen and, hydrocarbons etc., (ii), Planting certain plants such as Pinus, Juniparus, etc which can metabolize nitrogen oxides., 18. Explain how is ozone formed in the stratosphere?, Ozone is formed in the stratosphere by the effect of ultraviolet radiation on dioxygen. Some oxygen, molecule split into oxygen atoms which combine with other oxygen molecules to form ozone., 𝑼𝑽, , 𝑶𝟐 𝑶 + 𝑶, O + O2 → O 3, , 19. Explain ozone layer depletion (ozone hole formation) by chlorofluorocarbons (CFC)., Freons reach stratosphere and release chlorine free radicals under the influence ultraviolet radiation., CF2Cl2 → Cl. + .CF2Cl, The chlorine free radical react with ozone to form chlorine monoxide free radical and O 2, Cl. + O3 → ClO. + O2
Page 93 :
Join Telegram Channel: https://t.me/hsslive, , Downloaded from www.Hsslive.in ®, , The chlorine monoxide free radical reacts with atomic oxygen and chlorine free radical., ClO. + O → Cl. + O2, These chlorine free radical again react with ozone and continuous depletion occurs., 20. What are the adverse effects of ozone layer depletion?, (I), More ultraviolet radiation enters atmosphere and leads to cataract, sunburn and skin cancer., (II), Ultraviolet radiation increases the evaporation of water from leaves of plants and decreases, moisture content of the soil., (III), UV radiations damage paints and fibers., 21. What are the major causes of water pollution?, (i), Pathogens like bacteria and other micro organism that enter water from domestic sewage and, animal excreta., (ii), Organic wastes like leaves, grass etc., (iii), Chemical pollutants like metals such as cadmium, mercury and pesticides and fertilizers., 22. What is Biological oxygen demand (BOD)?, It is the amount of oxygen required by micro organism to oxidize organic matter present in the polluted, water. It is expressed in ppm (parts per million)., Clean water has BOD less than 5 ppm., Highly contaminated water has BOD 17 ppm or more., 23. What is Eutrophication, Pollution of water by nutrients such as phosphate from detergents and fertilizers accelerate the growth, of algae and other plants in river water. This reduces the dissolved oxygen and adversely affect aquatic, life. This phenomenon is known as eutrophication., 24. What are the international standards for drinking water?, (i), Fluoride ion up to 1 ppm, (ii), Nitrate ion up to 50 ppm, (iii), Lead up to 50 ppb (parts per billion), (iv), PH between 5.5 and 9.5, (v), Cadmium up to .005 ppm, Excess nitrate in drinking water can cause blue baby syndrome., Excess lead can damage kidney, liver and reproductive system., Fluoride prevents tooth decay. However fluoride concentration above 2ppm causes brown mottling of, teeth., 25. What are the reasons for soil pollution?, Indiscriminate use of fertilizers, pesticides (DDT, aldrin, Dieldrin) and Herbicides [sodium chlorate, (NaClO3) , sodium arsenite ( Na3AsO3)] etc. ,Dumping of waste materials, Deforestation., 26. How can we control environmental pollution?, Recycling of industrial wastes., Sewage water is filtered to remove large solids and then allowed to settle., Convert biodegradable wastes into compost., 27. What is green chemistry?, Green chemistry is the programme of developing new chemical processes or making improvements in, the already existing processes so as to make less harmful to human health and environment. It does not, employ any toxic reagents or solvents or severe reaction conditions. It would bring minimum pollution., 28. Give some applications of green chemistry in day to day life., (i), For dry cleaning of cloths liquid carbon dioxide is used., (ii), For bleaching of paper hydrogen peroxide is used., (iii), Ethanol is commercially prepared by one step oxidation of ethene in the presence of ionic, catalyst in aqueous solution., 29. Abbreviations and their full forms, (i), PCB→ Poly chlorinated biphenyls ( Pollu on Control Board), (ii), DDT → dichloro dipheny l trichloro ethane