Page 1 :
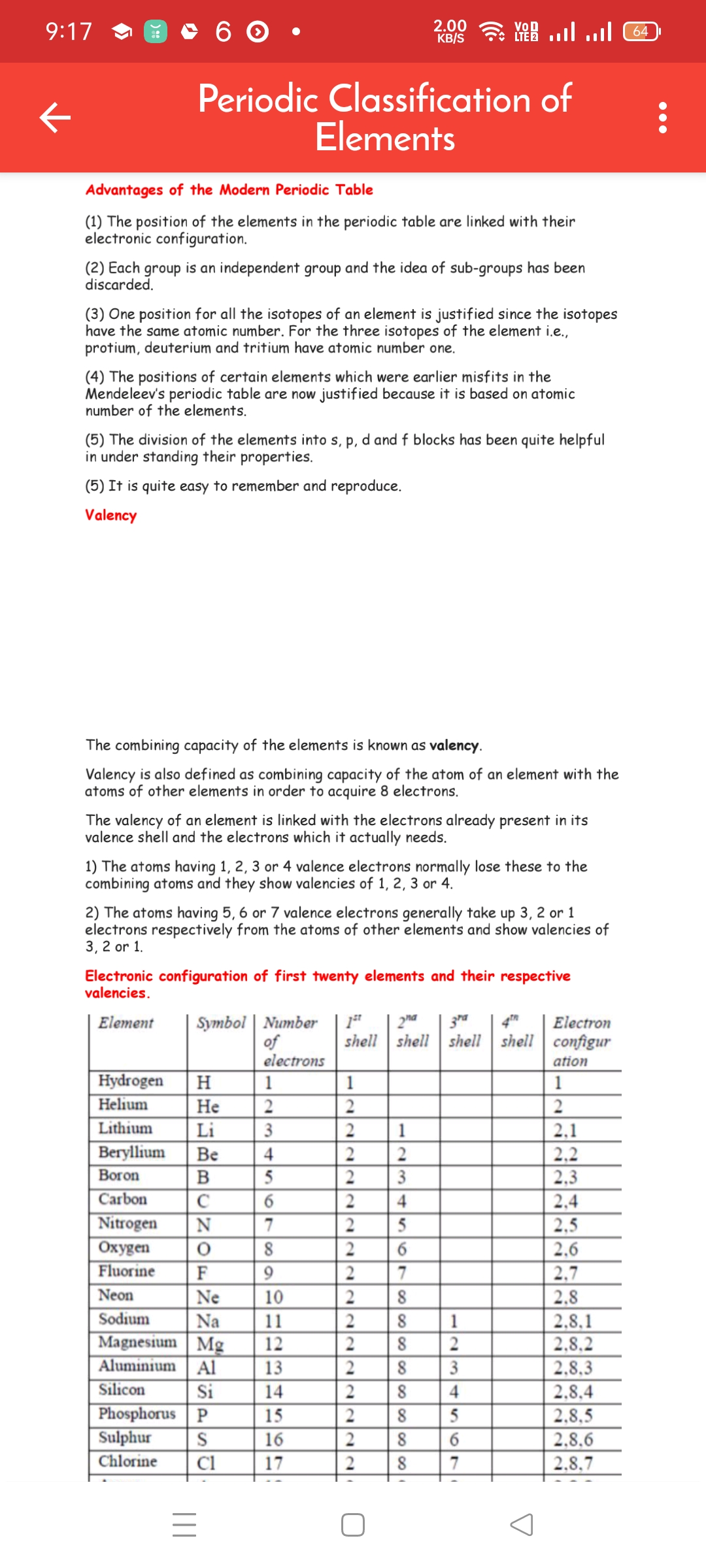
917 @Be 6 (O) 0 ae ane eT, , € Periodic Classification of, Elements, , , , Advantages of the Modern Periodic Table, , (1) The position of the elements in the periodic table are linked with their, electronic configuration., , (2) Each group is an independent group and the idea of sub-groups has been, discarded., , (3) One position for all the isotopes of an element is justified since the isotopes, have the same atomic number. For the three isotopes of the element i.,, protium, deuterium and tritium have atomic number one., , (4) The positions of certain elements which were earlier misfits in the, Mendeleev's periodic table are now justified because it is based on atomic, number of the elements., , (5) The division of the elements into s, p, d and f blocks has been quite helpful, in under standing their properties., , (5) It is quite easy to remember and reproduce., , Valency, , The combining capacity of the elements is known as valency., , Valency is also defined as combining capacity of the atom of an element with the, atoms of other elements in order to acquire 8 electrons., , The valency of an element is linked with the electrons already present in its, valence shell and the electrons which it actually needs., , 1) The atoms having 1, 2, 3 or 4 valence electrons normally lose these to the, combining atoms and they show valencies of 1, 2, 3 or 4., , 2) The atoms having 5, 6 or 7 valence electrons generally take up 3, 2 or 1, electrons respectively from the atoms of other elements and show valencies of, , 3,2or1., Electronic configuration of first twenty elements and their respective, valencies., Element Symbol | Number | 1* zy" | sF | Electron, of shell | shell | shell | shell | configur, electrons ation, , 8, 8, , :, :, , 2, A, , 2, g, , [Hooft, [He [2, [Li [3, [Be |4 |, [Bos, a, [N77], a, [Fo, [Ne] 10, , AEE, aang, 2 2, 5, z|z, 2, sls, , MM] lto [be bol hol bo) bolt lol, , POLO DODO) 3 Al ml S| witli, , », :, z, ARSE, ii, , 3, 3, S|, 2, , , , bel bole, min, , , , , , , , Chlorine | Cl 17, , ile, , |e, iin, , , , II, O, A