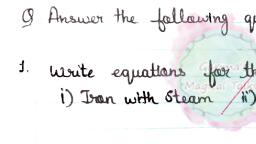Page 1 :
Chapter 3 - Metals and Non-metals, , Introduction, , There are 118 elements present in the periodic table, 92 of which are naturally occurring. Metals, and non-metals are characterized by distinctly different physical and chemical properties. At present, about 80 metals are known to us., , At room temperature, over half of the non-metals are gases, except bromine, which is a liquid., , The most abundant non-metal in the earth's crust is oxygen, which constitutes about 50% of the, earth's crust and along with nitrogen it forms the main constituents of air., , The next abundant nonmetal is silicon which constitutes about 26% of the earth’s crust. Oxygen and, silicon are the two major constituents of earth. Hydrogen and oxygen are the two major constituents, of the oceans., , Position of Metals and Non-metals in the Periodic Table, , Metalloids, Metals, Metals, , oan, i, , Metals occupy the groups on the left of the periodic table. Group IA consists of highly reactive, metals called the alkali metals, while group II A elements are called alkaline earth metals. Elements, between group ITA and IIIA are all called transition metals., , The non-metals are elements (with the exception of hydrogen) that are found to the right on the, Periodic Table i.e., groups IVA, VA, VIA &VIIA. The non-metallic character of these elements, increases from top to the bottom of the group. For example, in group VA the first and second, members are non-metals, the third and fourth are metalloids and the last member is a metal. The, metalloids are a group of elements which have properties similar to both the metals and non-metals., These metalloids are: Boron, silicon, germanium, arsenic, antimony, tellurium and astatine. The, non-metals are elements found to the right of these metalloids, including the element, hydrogen., , , , , , , , , , , , , , , , , , Group | Non Metals | Nitrogen,, VA Phosphorous,, Metalloids | Arsenic, Antimony, Metal Bismuth, Physical Properties of Metals, , Physical State - Metals are solids at room temperature with the exception of mercury and gallium,, which are liquids at room temperature., , Lustre - Metals have the quality of reflecting light from its surface and can be polished e.g., gold,, silver and copper., , Scanned with CamScanner
Page 2 :
Malleability - Metals have the ability to withstand hammering and can be made into thin sheets, known as foils. Except Zine which is brittle., , Ductility - Metals can be drawn into wires. Except Zinc which is brittle., , Hardness - All metals are hard except sodium and potassium, which are soft and can be cut with a, knife., , Conduction - Metals are good conductors because they have free electrons. Silver and copper are, the two best conductors of heat and electricity. Lead is the poorest conductor of heat. Bismuth,, , mercury and iron are also poor conductors, , Density - Metals have high density and are very heavy. Iridium and osmium have the highest, densities whereas lithium has the lowest density., , Melting and Boiling Point - Metals have high melting and boiling point. Tungsten has the highest, melting point where as silver has low boiling point. Sodium and potassium have low melting points., , Alloy Formation - Metals form homogeneous mixture with each other called an alloy. ExampleBrass is an alloy of copper and zinc., , Sonorous - Metals are sonorous i.e. they produce sound when hit with some solid object., , Physical Properties of Non-metals, , Physical State - Most of the non-metals exist in two of the three states of matter at room, temperature: gases (oxygen) and solids (iodine, carbon, sulphur). These have no metallic lustre,, , (except iodine) and do not reflect light. (Except carbon in the form of diamond)., , Nature - Non-metals are very brittle, and cannot be rolled into wires or pounded into sheets., Except- diamond is the hardest substance known., , Conduction - They are poor conductors of heat and electricity. (Except graphite conducts heat, both, graphite & gas carbon conduct electricity.), , Electronegative Character - Non-metals have a tendency to gain or share electrons with other, atoms. They are electronegative in character., , Reactivity - They generally form acidic or neutral oxides with oxygen., Melting and Boiling Points — Non-metals have low melting and boiling points., , Comparative Properties of Metals and Non-Metals, , , , Property Metals Non-metals, , , , State of matter These are usually solid, except mercury, | These exist in all the three states., , , , , , , , , , , , , , Scanned with CamScanner
Page 3 :
Non-metals, , , , , , , , , , , , , , , , , , , , , , Property Metals, which is a liquid at room temperature. | Bromine is the only liquid. Solids, Gallium and Caesium melt below 30°C. | — iodine, carbon, sulphur., So if room temperature is around 30°C,, they may also be in liquid state, , Density They usually have high density, except | Their densities are usually low., , for sodium, potassium, calcium etc., , Melting point They usually have a high melting point | Their melting points are low., , except mercury, cesium, gallium, tin,, lead., , Boiling point Their boiling points are usually high. Their boiling points are low., , Hardness They are usually hard, except mercury, | They are usually not hard. But the, , sodium, calcium, potassium, lead ete. exception is the non-metal, diamond, the hardest substance., , Malleability They can be beaten into thin sheets. They are generally brittle., , Ductility They can be drawn into thin wires, | They cannot be drawn into thin, , except sodium, potassium, calcium etc. | wires., , Conduction of heat | They are good conductors of heat. They are poor conductors of heat., (exception- carbon in the form of, graphite), , Conduction of | They are good conductors of electricity. | They are non-conductors, except, , electricity for carbon in the form of graphite, and the gas carbon., , Lustre Newly cut metals have high lustre. | Usually not lustrous, except, , Some get tarnished immediately., , iodine and diamond - the most, lustrous of all the substances., , , , Alloy formation, , They form alloys., , Generally, they do not form, alloys. However, carbon,, phosphorus, sulphur etc. can be, , present in some alloys., , , , , , , , , , , , Tenacity These usually have high tensile strength | These have low tensile strength., except sodium, potassium, calcium,, lead etc., , Brittleness They are hard but not brittle, except | They are generally brittle., zinc at room temperature., , Electronic They usually have 1, 2 or 3 electrons in | They usually have 4, 5, 6 or 7, , , , their valence shell. The greater the, , , , electrons in the valence shell. If it, , , , Scanned with CamScanner
Page 4 :
Property Metals Non-metals, configuration number of shells and lesser the number | has 8 electrons, it is called a, of valence electrons, the greater is the | noble gas. Lesser the number of, reactivity of the metal. shells and greater the number of, valence electrons, greater is the, reactivity of the non-metal., Ionization They always ionize by losing electrons: | They always ionize by gaining, Na® - e~ Nat electrons: CI? + & >, Charge of ions Positively charged. Negatively charged., Type of valency Metals always exhibit electrovalency. Non-metal exhibit both, electrovalency or covalency., Deposition during | They are always deposited at the | They are always deposited at the, electrolysis cathode. anode., Redox reaction These lose electrons and hence get | These gain electrons and hence, , oxidized., , get reduced., , , , Redox agents, , They are reducing agents., , They are oxidizing agents., , , , , , , , , , , , , , Nature of oxides They generally form basic oxides, some | They generally form acidic, of which are also amphoteric, such as | oxides., aluminum oxide, zinc oxide, lead oxide ., ae: Neutral oxides are nitrous oxide,, nitric oxide, carbon monoxide, water etc., Hydrides They do form hydrides except some | They do form hydrides, e.g. NH3,, transition elements. PH, HCl, HBr, HI, H2S, H20 etc., Atomicity These are always monatomic. These can be mono, di, tri, or, polyatomic., Solubility They do not dissolve in solvents except | They dissolve in solvents and can, by chemical action. be re-obtained by evaporation., Example: Sulphur in carbon, disulphide., Action with | They produce chlorides, which are | They produce chlorides, which, chlorine electrovalent. are covalent., , , , Action with dilute, acids, , , , On reaction with dilute acids they give, respective salt and hydrogen., , , , They do not react with dilute, acids., , , , , , Scanned with CamScanner
Page 5 :
Chemical Properties of Metals, Metals are Electropositive Elements, , Metals are very reactive. Metals tend to lose electrons easily and form positively charged ions;, therefore, metals are called electropositive elements. Sodium metal forms sodium ions Na*. The, electropositive nature allows metals to form compounds with other elements easily., , Reaction of Metals with Oxygen, Metals like sodium (Na) and potassium (K) are some of the most reactive metals. Potassium,, sodium, lithium, calcium and magnesium react with oxygen and burn in air., Metals from aluminum to copper in the activity series of metals react slowly when heated in air to, form the metal oxides. Aluminum is the fastest and copper is the slowest of them., ¢ Sodium metal reacts with the oxygen of the air at room temperature to form sodium oxide., Hence, sodium is stored under kerosene to prevent its reaction with oxygen, moisture and, carbon dioxide., 4Na + Op —_—«o2Na20, , Sodium Oxygen Sodium oxide, , ¢ Sodium oxide is a basic oxide which reacts with water to form sodium hydroxide., Naz + HO —— > 2NaOH, , Sodium oxide Sodium hydroxide, , © Mg does not react with oxygen at room temperature. On heating, Mg burns in air with intense, light and heat to form MgO., , 2Mg + 0, —4-5 2Mgo, , e Zinc metal burns in air only on strong heating to form zinc oxide., , Zinc + Oxygen —4., Zine oxide, , 2zn + O02. —4 + 2zn0, , ¢ In moist air, iron is oxidized to give rust., Iron + Moist air —— Iron (Hl, II) oxide, , 3Fe + 202 + xH,O ——» Fe304.xH20, , ¢ On heating in air it burns with a brilliant flame forming triferric tetroxide., 3Fe +20, —4 5 Fes0,, , ¢ Copper is the least reactive metal and does not burn in air even on heating. However, on, prolonged strong heating copper reacts with oxygen and forms copper (II) oxide (CuO) outside, and copper (I) oxide (Cu20) inside., Copper + Oxygen _4 , Copper (II) oxide, , 2cu + o, —4, 2cuo, , Scanned with CamScanner