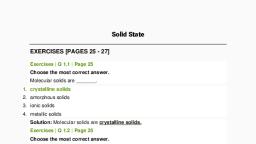
Page 1 :
Synopsis, 1.1 Introduction :, i., , Note : Ionic solids consist of cations and anions at the, regular sites of the crystal lattice. The packing, in ionic solids depends on the ratio of cation and, anion radii. Ionic solids are closely packed as, compared to metallic solids. Ionic crystals are, quite hard and brittle, have fairly high melting, points., , In solid satate, the constituent particles are, closely packed and thus, can only vibrate about, their mean position, because the intermolecular, forces of attraction are very strong., , ii., , iii., , A true solid has a shape which it maintains, against mild distorting forces, while, pseudo solid has a shape which can be, more easily distorted by bending or, compressing forces. Pseudo solids are, actually regarded as supercooled liquids., , E.g. KNO3 , NaCl and Na2SO4., , Crystalline Solids : Crystalline solids have, definite shape and are true solids and are, anisotropic., Amorphous Solids : Amorphous solids, have irregular shape and are pseudo solids, and are isotropic., Classification of Crystalline Solids :, a. Molecular solids :, 1. Polar molecular solids : HCl , SO2 etc, 2. Non polar molecular solids : CO2 ,, H2 , Cl2 , CH4 , CO, etc, 3. Hydrogen bonded moelcular solids:, H – O, H – N, H – F, b. Ionic Solids : Ionic salts are formed by, molecules containing positively charged, smaller in size cations and negatively, charged relatively bigger anions., c. Metallic Solids : They are the, crystalline solids formed by atom of, same metallic elements., d. Covalent Solids : They are formed by, chemical bond. i.e. covalent bonds, between the neighbouring constituent, atoms of non-metallic solids., E.g. Diamond, graphite and Fullerence., , (1), , iv., , A regular repeating arrangement of points, in space is called space lattice. It is an array, of points showing how molecules or atoms, or ions are arranged in different sites in, three dimensional space., , v., , All crystals do not have simple lattices., There can be 14 different ways in which, similar points can be arranged in a three, dimensional space. These are called 14, Bravais lattice.
Page 2 :
2, , MHT-CET - Part I (Std. 12), , vi., No., , Types of unit cell and Examples., Crystal System, , 1., , Cubic, , 2., , Tetragonal, , 3., , Orthorhombic, , 4., , Monoclinic, , 5., , Triclinic, , Type, , Edge length, , Angle, , Examples, , Simple, , a=b=c, , D = E = J = 90o, , Polonium, , Body centred, , a=b=c, , D = E = J = 90, , Fe, Rb, Na, , Face centred, , a=b=c, , D = E = J = 90o, , Cu, Al, Ni, , Primitive, , a=b z c, , D = E = J = 90, , SnO2, , Body centred, , a=b z c, , D = E = J = 90o, , TiO2 , CaSO4, , Primitive, , a=b z c, , D = E = J = 90, , Rhombic Sulphur, , Body centred, , azbzc, , D = E = J = 90o, , KNO3, , Face centred, , azbzc, , D = E = J = 90, , BaSO4, , End centred, , azbzc, , D = E = J = 90o, , Primitive, , azbzc, , D = E = 90o �� �J z 90o, , Monoclinic Sulphur, , End centred, , azbzc, , D = E = 90o �� �J z 90o, , Na2SO4 . 10H2O, , Primitive, , azbzc, , D z J z E = 90o, , K2Cr2O7 . H3BO3, , o, , o, , o, , o, , MgSO4 . 7H2O, , 6., , Hexagonal, , Primitive, , a=bzc, , D = E = 90 �J = 120, , 7., , Rhombohedral, , Primitive, , a=b=c, , D = E = J z 90o, , o, , o, , ZnO, BeO, SnS, Calcite, HgS, NaNO3, , vii. Total number of spheres in Simple cubic unit = 1, Total number of spheres in Body centred unit = 2, Total number of spheres in Face centred unit = 4, Note : When X-rays falls on a crystal face, they penetrate into the crystal and strike the atoms in, different planes. X-rays are deflected from each of these planes. Bragg gave the following relation, between the wavelength of the X-rays and the distance between the planes (2d sin T = nO, O)., viii. For an atom or ion, coordination number is the number of its nearest neighbours. Coordination number, in case of closely-packed metal crystals goes up to 12, whereas in case of ionic solids, it does not exceed, 8., ix., , Crystal structure of some elements and their coordination number (CN) :, Crystal structure, , Examples, , BCC, , Li, Na, K, Rb, Cs, Ba, , 8, , FCC or CCP, , Ni, Cu, Ag, Au, Pt, , 12, , HCP (Hexagonal closed packed) Zn, Mo, Cd, V, Be, Mg, , Coordination number, , 12
Page 3 :
Solid State, , x., , 3, , Radius of atom, edge length of atom, number of particles, volume of sphere and packing fraction, Radius of, Unit cell, , Edge length of, , atom (r), , unit cell (a), , Total no. of, , Volume of, , Packing, , % Packing, , particles,, per unit, , sphere, , fraction, , fraction, , S, Simple Cube, , xi., , 2r, , 01, , BCC, , 02, , FCC, , 04, , Packing in Solids :, a. Stage I :, Linear packing in one direction, b. Stage II :, Planar packing arrangement of, spheres., 1. AAAA type, 2. ABAB type, c. Three dimensional packing :, 1. AAAA type = Simple cubic structure, 2. ABAB type = Hexagonal close, packing structure, , xii. Density of Solid, d, , u, , =, , where,, , =, , Z = No. of atom per unit cells, a = Edge length, V = Volume of unit cell = a3, NA = Avogadro’s number, , MW = Atomic mass or molecular, mass, xiii. The space occupied by the spheres in, different packings is called the packing, fraction or packing density., Packing density =, , S, , =, , S, , 52.4, , S, , S, u, , S, , S, , S, , S, , 68.04, , 74, , xiv. The empty space present in a closely packed, structure is called the void or interstitial, site., A tetrahedral void is surrounded by 4, spheres while an octahedral void is, surrounded by 6 spheres., Radius of the voids are related with radius, of spheres (R) as:, Radius of the tetrahedral void = 0.225R, Radius of the octahedral void = 0.414 R, In case of ionic solids generally bigger ions, i.e. the anions occupy lattice sites. The, number of octahedral voids is always equal, to the number of anions and number of, tetrahedral voids are double the number, of anions., a. Voids : The empty spaces inside a, spheres are called “voids”. The size, and shape of voids depends upon the, type of unit cell and packing., b. Radius Ratio : The size of void is, expressed in terms of radius ratio of a, sphere that can be exactly fit in the, void. This expressed as :, �, , Radius ratio =
Page 4 :
4, , MHT-CET - Part I (Std. 12), , c. Types of voids, , xv., , Limiting value, , Coordination, , It is clear from above details that, Trigonal < Tetrahedral < Octahedral < Cubic, void, void, void, void, , of Radius Ratio, x < 0.155, , 2, , Classification of Ionic Structures :, , 0.155 < x < 0.225, , 3, , Ionic compounds are formed by the, simultaneous arrangement of cations and, anions in lattice/unit cell., The larger of two species occupies major positions, in a unit cell and the smaller ones occupy voids, according to their size. Which is decided on the, basis of radius ratio (r+/r–). The various ratios are, listed below., , Shape, , Example, , Number, Linear, , BeF2, , Planar, , AlCl3, , Triangular, 0.225 < x < 0.414, , 4, , Tetrahedron, , ZnS, , 0.414 < x < 0.732, , 6, , Octahedron, , NaCl, , 0.732 < x < 0.999, , 8, , Body Centred CsCl, Cubic
Page 5 :
Solid State, , Based on these radius ratio range, ionic crystal are classified into 5 categories which are as follows:, , 5
Page 6 :
6, , MHT-CET - Part I (Std. 12), , xvi. Imperfections in solids, Sometimes some defects or imperfections, occur in crystal structure., Classification of defects :, , Frenkel defect :, , Vacancies : These are defect that occur, when positions that should contain atoms, or ions are vacant., Interstitial sites : These are sites located, between regular positions, sometimes, atoms or ions may occupy these positions., , In ionic solids the smaller ion is dislocated, from its normal position to an interstitial, site. It creates a vacancy defect at its, original site and interstitial defect at new, location. It is also called as dislocation, defect. It does not change the density of, solid., This type of defect is shown by ionic, substances in which there is a large, difference in size of ions. eq.ZnS, AgCl,, AgBr etc., , Stoichiometric Defects :, These defects do not disturb stoichiometry, of solid substance., Schottky defects :, It is a vacancy defect in ionic solids., Number of missing cations and anions are, equal so electrical neutrality is maintained., This defect decreases the density of the, subtance. The defect is shown by ionic, substances in which cation and anion are, of almost similar sizes. eq.KCl, NaCl, AgBr, etc., , Non Stoichiometric Defects :, The compounds having these defects, contain combining elements in a ratio, different from required by their, stoichiometric formulae.
Page 7 :
Solid State, , a., , 7, , Metal Excess Defect :, , Metal Deficiency Defect :, , Due to anionic vacancies : The anion may, be missing from its lattice site leaving an e–, behind so that charge remains balanced., The site containing electron is called F, centre. They impart colour to the crystal, (F stands for Farbenzenter meaning, colour). This defect is similar to schottky, defect and is found is crystals having, schottky defect., , This defect occurs when metal shows, variable valency., , Example : NaCl, KCl etc., , Example : FeO, FeO mostly found is varying compositions, between Fe0.93O to Fe0.96O. In crystals of, FeO some Fe+2 cations are missing and the, loss of positive charge is made up by the, presence of required number of Fe+3 ions., xvii. Electrical properties :, a. The conductivity of metals in due to, the presence of mobile electrons., b. The conductivity of a semiconductor, is due to the presence of holes. Doping, is process in which a small amount of, foregin impurity is added to a crystal., Doping of group 14 elements with, group 15 elements gives rise to excess, electrons whereas, doping with group, 13 elements gives rise to holes. Both, makes group 14 elements semiconductors. The former are called ntype and latter are called p-type., , b., , Metal excess defect due to extra cation in, interstitial site :, An extra cation may be present in, interstitial site and an electron is present, in another interstitial site so that electrical, neturality is maintained. This is similar to, Frenkel defect and is found in crystal, having Frenkel defect., , xviii. Magnetic properties :, a. Paramagnetic solids are weakly, attracted by the magnetic field., Paramagnetism is due to the presence, of unpaired electron. It is as temporary, effect. They lose their magnetism in, absence of magnetic field., Example : TiO, VO2 and CuO., b. Ferromagnetic solids have a net, magnetic moment but the magnetic, moment is arranged in parallel and, antiparallel direction., nnpnnnpnp, Example : Fe3O4, They are strongly attracted by the, magnetic field. e.g. Fe, CO, Ni and CrO2., c. The, temperature, at, which, ferromagnetic, antiferromagentic, solids becomes paramagnetic due to, randomisation of magnetic dipoles is, called Curie temperature (Tc ).
Page 8 :
8, , MHT-CET - Part I (Std. 12), , d. Whe n a sub sta nce of fe rs n o, re sistance to the pas sage of, ele ctiricty. The phenomemon is, ca lled supercon ductiv ity. For, example, mercury at 4 K becomes, superconductor, alloy of Niobium, at, 23 K becomes superconductor., e. Alignment of magnetic moments in, opposite direction in a compensatory, mann er and resulting in a zero, magnetic moment (due to equal, number of parallel and antiparallel, magnetic dipoles) gives rise to anti, ferromagnetism., Example : MnO, Mn 2 O 3 and MnO 2 ., f . S u bs t a n c e s w h i c h a re w e a k l y, re p e ll e d by m a g n e t ic f i e ld a r e, called diamagnetic substances., Example : TiO 2 , H 2 O and NaCl., Diamagnetic substances have all, their electrons paired.
Page 9 :
Solid State, , 9, , CLASS WORK, , 1.2, , Classification of solid :, , (6), , A crystalline solid, , Multiple Choice Questions, 1.1, , Introduction to solids :, , (1), , The characteristic features of solids are, , (a) Changes abrupty from solid to liquid when, heated, (b) Has no define melting point, (c), , (a) Definite shape, , (d) Has an irregular 3-dimensional arrangements, , (b) Definite size, (c), , Definite shape and size, , (7), , (B) Short range order, , Select the incorrect statement, , (C) Long range order, , (a) Solid water is lighter than liquid water., , (D) Have no sharp melting point, , (b) Stronger the intermolecular forces of, attraction, higher is the melting point of the, solid., (c), , (3), , (a) A and C are correct, (b) B and C are correct, , Mercury has relatively very low density., , (d) Internmolecular distance of separation, between neighbouring molecules is shorter in, solid., , (c), , (8), , (b) Crystalline solid, (c), , (b) High pressure and high temperature, , (4), , (5), , (9), , Mercury has relatively high, (b) Fluidity, , (c), , (d) Rigidity, , Compressibility, , (10), , _______ of a crystal is reflected in the magnitude, of its melting point., (a) Reactivity, , (b) Dissociation, , (c), , (d) Solubiltiy, , Stability, , Amorphous solid, , (d) both a and c, , Low temperature and high pressure, , (a) Density, , Glass is _________, (a) Supercooled liquid, , (a) Low pressure and high temperature, , (d) High pressure and low temperature, , C and D are correct, , (d) B and D are correct, , o liquid, conversion is possible at, Gas , , (c), , Amorphous substances show, (A) Short and long range order, , (d) Definite shape, size and rigidity, (2), , Undergoes deformation of its geometry easily, , (11), , (12), , Which solid will have weakest intermolecular, forces ?, (a) Ice, , (b) Phosphorus, , (c), , (d) Sodium fluoride, , Naphthalene, , Which of the following is a molecular crystal ?, (a) SiC, , (b) NaCl, , (c), , (d) Ice, , Graphite, , Among solids the highest melting point is, established by, (a) Covalent solids, , (b) Ionic solids, , (c), , (d) Molecular solids, , Pseudo solids, , The ability of a given substances to assume two or, more crystalline structure is called, (a) Amorphism, , (b) Isomorphism, , (c), , (d) Isomerism, , Polymorphism
Page 10 :
10, , (13), , MHT-CET - Part I (Std. 12), , The major binding force of diamond is, , (20), , (a) ionic bond, (b) covalent bond, (c), , (14), , (a) AB2, , dipole-dipole attraction, , (d) A2B, , (a) 8, , (b) 4, , (c), , 2, , (d) 6, , (a) ionic bond, , 1.4, , Packing in Solids :, , (b) covalent bond, , (22), , Hexagonal close packed arrangement of ions is, described, , dipole-dipole attraction, , (d) induced dipole-dipole attraction, , (a) ABC ABA, , (b) ABC ABC, , Most crystal show good cleavage because their, atoms, ions or molecules are, , (c), , (d) ABBAB, , ABABA, , (a) weakly bonded together, , In the closest packed structure of a metallic lattice, the, number of nearest neighbour of a metallic atom is, , (b) strongly bonded together, , (a) Twelve, , (b) Four, , (c), , (c), , (d) Six, , (23), , spherically symmetrical, (24), , Unit cell, two and three dimensional lattice :, , (16), , The general formula of an ionic compound, crystallizing in the body centred cubic structure is, _________., (a) AB3, , (b) A2B, , (c), , AB2, , (b) 1, , (c), , (d), , An ionic compound AxBy occurs in fcc type crystal, structure with B ion at the centre of each face and, A ion occupying corners of the cube. Give the, formula AxBy., (a) AB3, , (b) AB4, , (c), , A3B, , (a) XY2Z 3 (b) XYZ3, , (25), , (c) X2Y2Z3 (d) X8YZ6, , The number of atoms in the HCP unit cells is, , (26), , (b) 6, , S, , (d) 17, , (b), , S, , S, , (c), , (d), , S, , (b) 47.6%, , (c), , 32%, , (d) 26%, , Total number of voids in 0.5 moles of a compound, forming hexagonal closed packed structure are, (a) 6.022 u 1023, , (b) 3.011 u 1023, , 9.033 u 1023, , (d) 4.516 u 1023, , (c), (28), , 12, , The empty space in the HCP unit cell is, (a) 74%, , (27), , (c), , Total volume of atoms present in face-centred, cubic unit cell of a metal is (r is atomic radius), (a), , (d) A4B, , A solid compound contains X, Y and Z atoms in a, cubic lattice with X atoms occupying the corners,, Y atoms in the body centred positions and Z atoms, at the centres of faces of the unit cell. What is the, empirical formula of the compound., , Eight, , (a) 4, , (d) AB, , If an atom is present in the centre of the cube, the, participation of that atom per unit cell is, (a), , (19), , A2B5, , The major binding force in silicon is, , 1.3, , (18), , (c), , In a face-centred cubic lattice, a unit cell is shared, equally by how many unit cells, , (21), , (d) arranged in planes, , (17), , (b) A2B3, , (d) induced dipole-dipole attraction, , (c), , (15), , In a face centred cubic lattice, atom A occupies, the corner positions and atom B occupies the face, centre positions except one face, , Vacant spaces in two dimensional close packing, is called, (a) Tetrahedral voids (b) Octahedral voids, (c), , (29), , Hexagonal voids, , (d) Triangular voids, , Number of neighbouring spheres in both hcp and, ccp methods of stacking is, (a) Three, , (b) Six, , (c), , (d) Six in its own layer, , Twelve
Page 11 :
Solid State, , (30), , 11, , Berylium crystallizes in, , (37), , (a) BCC structure, , (a) 6 u 1020, , (b) HCP structure, (c), , (c), , Simple cubic structure, (38), , (d) CCP structure, (31), , The packing efficiency for a body centred cubic, structure is, (a) 0.42, , (32), , 0.68, , (b) 28%, , (c), , 30%, , (b) 2 Z, , (c), , Z/2, , (a), , S, , (b), , S, , (c), , S, , Density of unit cell :, , (35), , If ‘a’ stands for the edge length of the cubic, systems; simple cubic, body centred cubic and face, centred, then the ratio of radii of the spheres in, these systems will be respectively, (b), , (c), , (d), , (c), , 42.5, , g/cm3, , (b) 4.25 g/cm3, (d) 0.425, , g/cm3, , Sodium metal crystallises as a body centred cubic, lattice with the cell edge 4.29 A. What is the radius, of sodium atom., (a) 1.857 u 10–8 cm, , (b) 2.371 u 10–7 cm, , 3.817 u 10–8 cm, , (d) 9.312 u 10–7 cm, , (c), (40), , (42), , CsBr crystallises in a body centred cubic lattice., The unit cell length is 436.6 pm. Given that the, atomic mass of Cs = 133 and that of Br = 80 amu, and Avogadro number being = 6.02 u 1023 mol1., CsBr the density of CsBr is, (a) 8.25 g/cm3, , (39), , (41), , (a), , 1.38 u 1021 unit cell, , (d) 1.71 u 1024 unit cell, , S, , 1.5, , (36), , (c), , (d) Z 4, , (d), , How many unit cells are present in a cube shaped, ideal crystal of NaCl of mass 1.00 g., , (b) 5.14 u 1024 unit cell, , (d) 32%, , The fraction of total volume occupied by the atoms, present in a simple cube is, , (d) 0.5 u 1024, , (a) 2.57 u 1021 unit cell, , (d) 0.82, , If ‘Z’ is the number of atoms in the unit cell that, represents the closest packing sequence., In ABCABC, the number of tetrahedral voids in, the unit cell is equal to, (a) Z, , (34), , (c), , 1.5 u 1023, , (b) 3 u 1022, , [Atomic masses : Na = 23 Cl = 35.5], , Percentage of free space in a body centred cubic, unit cell is, (a) 34%, , (33), , (b) 0.53, , The number of unit cells present in 58.5 g of cube, shaped ideal crystal of NaCl is nearly, , (43), , Copper crystallises in fcc with a unit cell length of, 361 pm. What is the radius of copper atom, (a) 108 pm, , (b) 127 pm, , (c), , (d) 181 pm, , 157 pm, , Ammonium chloride crystalizes in a body-centred, cubic lattice with edge length of unit cell equal to, 387 pm. If the size of Cl– ion is 181 pm, the size of, NH+4 ion would be, (a) 116 pm, , (b) 154 pm, , (c), , (d) 206 pm, , 174 pm, , AB crystallizes in a body centred cubic lattice with, edge length ‘a’ equal to 387 pm. The distance between, two oppositely charged ions in the lattice is, (a) 300 pm, , (b) 335 pm, , (c), , (d) 200 pm, , 250 pm, , Ice crystallises in a hexagonal lattice having a, volume of the unit cell as 132 u 10–24 cm3. If density, of ice at the given temperature is 0.92 g cm–3 , then, the number of H2O molecules per unit cell is, (a) 1, , (b) 2, , (c), , 3, , (d) 4
Page 12 :
12, , (44), , (45), , MHT-CET - Part I (Std. 12), , The cubic unit cell of Al (molar mass 27 g mol–1), has an edge length of 405 pm. Its density is 2.7 g, cm–3. Its density is 2.7 g cm–3. The cubic unit cell is, (a) face centred, , (b) body centred, , (c), , (d) edge centred, , The BCC crystal contains 4 u 1024 atoms. The, number of unit cell present are, (a) 8 u 1024, , (b) 2 u 1024, , 4 u 1024, , (d) 1 u 1024, , (c), (46), , primitive, , A metal X crystallises in a face-centred cubic, arrangement with the edge length 862 pm. What, is the shortest separation of any two nuclei of the, atom ?, (a) 406 pm, , (b) 707 pm, , (c), , (d) 609.6 pm, , 862 pm, , 1.6, , Packing in Ionic Solids and Radius Ratio rule :, , (47), , The maximum radius of sphere that can be fitted, in the octahedral hole of cubical closed packing of, sphere of radius r is, , (48), , (a) 0.732 r, , (b) 0.414 r, , (c), , (d) 0.155 r, , 0.225 r, , (c), , (c), , 3, , (b) 2, , (c), , 3, , Simple cubic, , (d) Fluorite structure, (53), , The number of octahedral voids in a unit cell of a, cubical closest packed structure is, (a) 1, , (54), , (a) X2Y3, (55), , (57), , (d) 8, , 4, , (d) 8, , (b) X2Y, , (c), , X 3Y 4, , (d) X4Y3, , (b) 50%, , (c), , 100% (d) 75%, , An ionic crystal lattice has r+/r– radius ratio of, 0.524. Its coordination number is, (b) 4, , (c), , 6, , (d) 8, , In the crystal of which of the following ionic, compounds would you expect maximum distance, between centres of cations and anions, (a) LiF, , (58), , (c), , If AgI crystallises in zinc blende structure with I, ions are lattice points. What fraction of tetrahedral, voids is occupied by Ag ions., (a) 25%, , (56), , (b) 2, , In a compound atoms of Y form ccp lattice and, those of element X occupy 2/3rd of tetrahedral, voids. The formula of the compound will be, , (a) 2, , (b) CsF, , (c), , CsI, , (d) Lil, , KCl crystallises in the same type of lattice as does, NaCl. Given that, , /, , = 0.55 and, , /, , = 0.74. Calculate the ratio of the side of the unit, cell for KCl to that of NaCl., , (d) 4, , The number of tetrahedral sites per sphere in CCP, structure is,, , (a) 1.123 (b) 0.891, , (c), , 1.414 (d) 0.414, , The structure of TiCl is similar to CsCl. What, would be the radius ratio in TiCl, , A solid has a structure in which ‘W’ atoms are, located at the corners of a cubic lattice ‘O’ atoms, at the centre of edges and ‘Na’ atoms at the centre, of the cube. The formula for the compound is, , (a) 0.155 - 0.225, , (b) 0.225 - 0.414, , (a) NaWO2, , (b) NaWO3, , (c), , (d) 0.732 - 1.000, , (c), , (d) NaWO4, , (a) 1, (51), , (b) Face centred cubic, , The number of octahedral sites per sphere in CCP, structure is,, (a) 1, , (50), , (b) 4, , Structure of ZnS is, (a) Body centred cubic, , How many molecules are there in the unit cell of, sodium chloride ?, (a) 2, , (49), , (52), , (b) 2, , 0.414 - 0.732, , (c), , 3, , (59), , (d) 4, , (60), , Na2WO3, , In zinc blende structure the coordination number, of Zn2+ ion is, (a) 2, , (b) 4, , (c), , 6, , (d) 8
Page 13 :
Solid State, , 13, , 1.7, , Defects :, , (61), , Due to Frenkel defect the density of ionic solid, , (62), , (66), , (a) increases, , (b) decreases, , (c), , (d) fluctuates, , remains same, , (a) all metals, (b) alkali metals only, (c), , The correct statement(s) regarding defects in solids, is (are), (a) Frenkel defect is usually favoured by a very, small difference in the sizes of cation and, anion., , (67), , (b) equal number of cations and anions are, missing from the lattice, , Trapping of an electron in the lattice leads to, the formation F-centre., , (c), , (68), , Positive ions and negative ions are of different size, , (b) 1.3, , Positive ions are small and negative ions are big, , (c), , (d) Positive ions are big and negative ions are small, (64), , In AgBr crystal, the ion size lies in the order, Ag+ << Br–. The AgBr crystal should have the, following characteristics, , (69), , Which of the following is correct ?, (a) Schottky defect lowers the density, (b) Frenkel defect increases the dielectric, constant of the crystals, , (b) schottky defect, , (c), , frenkel defect, , (d) both schottky and frenkel defect, (65), , 1.5, , (d) slightly less than unity, , (a) defect less (perfect) crystal, , (c), , Frenkel defect is found in crystals in which the, radius ratio is, (a) low, , (b) Positive ions and negative ions are of same size, (c), , some lattice sites are occupied by electrons, , (d) some imurity is present in the lattice, , Schottky defects occurs mainly in electrovalent, compounds where, (a), , Schottky defect is observed in crystals when, (a) some cations move from their lattice site to, interstitial sites, , (d) Both b and c, (63), , alkaline earth metals only, , (d) transition metals only, , (b) Frenkel defect is a dislocation defect., (c), , Non-stoichiometric metal deficiency is shown in, the salts of, , What type of crystal defect is indicated in the, diagram below, , Stoichiometric defects make the crystals good, electrical conductors., , (d) All the above., (70), , When carbon are trapped into the crystal of iron,, the defect is known as :, (a) Schottky defect, , �, , �, , (b) Frenkel defect, (c), , Stoichiometric defect, , (d) Interstitial defect, (a) Frenkel defect, (b) Frenkel and Schotty defects, (c), , Interstitial defect, , (d) Schottky defect, , (71), , When NaCl crystal is doped with MgCl2 , the, nature of defect produced is, (a) interstitial defect, , (b) Schottky defect, , (c), , (d) None of these, , Frenkel defect
Page 14 :
14, , (72), , (73), , MHT-CET - Part I (Std. 12), , Stainless steel, an interstitial alloy is formed when, iron is mixed with, (a) Zinc atoms, , (b) Carbon atoms, , (c), , (d) Sodium atoms, , Strontium atoms, , (75), , Electrical Properties :, , (79), , Doping of silicon (Si) with boron (B) leads to, (a) n-type semiconductor, (b) p-type semiconductor, , In the formation of alloy brass, copper atoms are, replaced by zinc atoms in the ratio of, (a) 1 : 3, , (74), , 1.8, , (b) 1 : 1, , (c), , 3:3, , (a) Point defect, , (b) Impurity defect, , (c), , (d) Both (a) and (b), , If molten NaCl containing a little amount of SrCl2, is crystallized some the sites of Na+ ions are, occupied by, , (80), , (b) Very small, (c), , (81), , Cl– ions, , (c), , 1, , (d) 2, , The deviations from ideal arrangement around an, atom in a crystalline substance is, (a) Line defect, , (a) n-type and p-type (b) p-type and n-type, , (b) Stoichiometric defect, , (c), , Interstitial defect, , (82), , (83), , (d) Point defect, Select the correct crystal defect observed in ZnS, solid because of Zn2+ ion, (a) Interstitial defect, (b) Schottky defect, (c), , (78), , (b) 5, , If the impurities of arsenic is added to silicon and, impurties of boron is added to silicon shows, semiconductors with respect to, , (c), , (77), , p-type semi conductor is formed when trace, amount of impurity is added to silicon. The, number of valence electrons in the impurity atom, must be, (a) 3, , –, , (d) Both Sr and Cl ions, (76), , Infinite, , (d) Very large, , (b) Sr+ ions, , 2+, , What is the energy gap between valence band and, conduction band in crystal of insulators, (a) Both the bands are overlaped with each other, , (a) Sr2+ ions, , (c), , Metal, , (d) Insulator, , (d) 3 : 1, , The solid solution of CdCl 2 and AgCl is the, example of, , Interstitial defect, , (c), , (84), , Substitution impurity defect, , n-type and a-type (d) t-type and p-type, , Select the corrrect substance in which spacing, between the valence band and conduction band, is relatively more., (a) Silicon, , (b) Diamond, , (c), , (d) None of these, , Copper, , Which of the following shows, the valence bands, and conduction bands are very close to each, other ?, , (d) Interstitial impurity defect, , (a) Metallic crystal, , (b) Semiconductors, , Which one of the following is an incorrect, statement for Frenkel defect ?, , (c), , (d) Non-metals, , Insulators, , (a) It is observed in AgCl solid., , The variation in the property of ability to conduct, electricity is explained by, , (b) Presence of defect alter the density of solid, , (a) Molecular theory, , (b) Valence theory, , (c), , (c), , (d) Band theory, , It is observed when the difference in ionic, radii of two participating ion is large., , (d) It is generally observed in case of relatively, smaller cation., , (85), , Electron theory
Page 15 :
Solid State, , (86), , 15, , The electrons in the higher energy level are, responsible for, , (93), , Doping of silicon with P or Al increases the, conductivity. The difference in the two cases in, , (a) metallic conductivity, , (a) P is non-metal whereas Al is a metal, , (b) electrical conductity, , (b) P is a poor conductor while Al is a conductor, , (c), , (c), , thermal conductivity, , (d) magnetic property, (87), , (c), (88), , (89), , (d) P gives rise to holes while Al gives rise to extra, electrons, , Number of atoms present in 1 g of magnesium, stripe is, (a) 6.022 u 1023, 2.5 u 1022, , (b), , u, , (a) Mixing, , (b) Doping, , (c), , (d) None of these, , Crystallizing, , 1.9, , Magnetic properties :, , (94), , Which one of the following metal oxides is, antiferromagnetic in nature ?, , (d) 0.25 u 1023, , The process of adding an impurity to silicon and, germanium to increase the conductivity is called, , Which one of the following statements is wrong ?, , (a) MnO2 (b) VO2, (95), , (96), , (c), , There is not superconductor at room, temperature, , (d) Ionic solids conduct electricity due to, presence of ions., (90), , (b) CoO, , (c), , (b) Cu, , (c), , (d) GrO2, , (b) n�p�n�p, , n�n�n�p�p, , (d) p�p�n�n, , The ferromagnetic compound is, (a) BaTiO3, , (b) Pb2O3, , (c), , (d) None of these, , K4[Fe(CN)6], , Water is, (a) Diamagnetic, , (b) Ferrimagnetic, , (c), , (d) Ferromagnetic, , Pyromagnetic, , Match the following :, (A) NaCl, , (p) Ferromagnetic, substance, , ReO3 (d) Ti2O3, , (B) Fe3+, , (q) Diamagnetic, , Which substance acts as superconductor at 4 K ?, (a) Hg, , (92), , (98), , TiO2, , (a) n�n�n�n�n, , The oxide that is insulator is, (a) VO, , (91), , (97), , (c), , Which arrangement of electrons describes, ferromagnetism ?, , (c), , (a) The conductivity of metals decreases with, increase in temperature, (b) The conductivity of semiconductors increases, with increase in temperature, , P gives rise to extra electrons while Al gives, rise to holes, , Na, , substance, , (d) Mg, , (C) Gadolinium, , (r), , When n and p-type semiconductors are allowed, to come into contact, , Paramagnetic, substance, , (a) some electrons will flow from n to p, , (a) A-q, B-r,C-p, , (b) A-p, B-q, C-r, , (b) some electrons will flow from p to n, , (c), , (d) A-q, B-p, C-r, , (c), , the impurity element will flow from n to p, , (d) the impurity element will flow from p to n, , (99), , A-p, B-r, C-q, , Oxygen is an example of, (a) ferromagnetic substance, (b) diamagnetic substance, (c), , paramagnetic substance, , (d) none of these
Page 16 :
16, , MHT-CET - Part I (Std. 12), , (100) In Guoy’s method, the substance weighs less in, mangetic field is, (a) ferromagnetic, , (b) paramangetic, , (c), , (d) all of these, , diamagnetic, , (101) Which one of the following is a diamangetic, substance ?, (a) NaCl, , (b) Benzene, , (c), , (d) all of these, , Water, , (102) Of the elements Sr, Zr, Mo, Cd and Sb, all of the, which are in the 5 th period, the ones that are, paramagnetic are, (a) Sr, Cd and Sb, , (b) Zr, Mo and Cd, , (c), , (d) Zr, Mo and Sb, , Sr, Zr and Cd, , (103) Which of the following is ferromangetic ?, (a) Ni, (c), , (b) Co, , Fe3O4, , (d) All are correct, , (104) Ferromangetism is maximum in, (a) Fe, , (b) Ni, , (c), , Co, , (d) None, , (105) Which of the following statements is not true ?, (a) Paramangetic substances are weakly, attracted by magnetic field, (b) Ferromagnetic substances cannot be, magnetised permanently, (c), , The domains in antiferromagnetic substances, are oppositely oriented with respected to, each other, , (d) Pairing of electrons cancels their magnetic, moment in the diamagnetic substances
Page 17 :
Solid State, , 17, , HOME WORK, , (9), , The solid NaCl is a bad conductor of electricity since, (a) In solid NaCl there are no ions, , Multiple Choice Questions, , (b) Solid NaCl is covalent, , 1.1, , Classification of solids :, , (c), , (1), , Which one of the following is amorphous ?, , (d) In solid NaCl there are no electrons., , (2), , (3), , (4), , (5), , (a) Polystyrene, , (b) Table salt, , (c), , (d) Diamond, , Silica, , Which one of the following is a good conductor of, electricity ?, , (10), , (11), , (c), , Ice, , (d)Diamond, , Silicon is, , (b) Graphite, , (a) Semiconductor, , (b) Insulator, , (c), , (d) Amorphous carbon, , (c), , (d) None of these, , Silicon, , NaCl is an example of, , (12), , Conductor, , The major binding force in graphite is, , (a) Covalent solid, , (b) Ionic solid, , (a) ionic bond, , (b) covalent bond, , (c), , (d) Metallic solid, , (c), , (d) London force, , Molecular solid, , hydrogen bond, , Quartz is a crystalline variety of, , 1.2, , Unit cell, two and three dimensional lattice :, , (a) Silica, , (b) Sodium silicate, , (13), , (c), , (d) Silicon, , Tetragonal crystal system has the following unit, cell dimensions, , Silicon carbide, , (a) a = b = c and D = E = J = 900, , Solid CO2 is an example of, , Covalent crystal, , (b) a = b z c and D = E = J = 900, (c), , (d) Metallic crystal, (14), , (b) Diamond is soft, (c), , a z b z c and D = E = J = 900, , (d) a = b z c and D = E = 900, J = 1200, , Which of the following is true for diamond., (a) Diamond is a good conductor of electricity, , (8), , (b) Glass, , (a) Diamond, , (c), , (7), , The substance which does not show sharp melting, point is, (a) KCl, , (a) Molecular crystal (b) Ionic crystal, , (6), , In solid NaCl there is no velocity of ions, , How many space lattices are obtainable from the, different crystal systems ?, (a) 7, , Diamond is a bad conductor of heat, , (b) 14, , (c), , 32, , (d) 230, , (d) Diamond is made up of C, H and O, , Example of unit cell with crystallographic, dimensions a z b z c, D = E = 900, y z 900 is, , Crystalline solid is, , (a) Calcite, , (a) Glass, , (b) Rubber, , (c), , (d) Sugar, , Plastic, , (15), , (c), (16), , The lustre of a metal is due to, (a) Its high density, (b) Its high polishing, (c), , Its chemical inertness, , (d) Presence of free electrons, , (17), , (b) Graphite, , Rhombic sulphur (d) Monoclinic sulphur, , Na and Mg crystallize in bcc and fcc type crystal, respecively, then the number of atoms of Na and, Mg present in the unit cell of their respective, crystal is, (a) 4 and 2, , (b) 9 and 14, , (c), , (d) 2 and 4, , 14 and 9, , An FCC unit cell of aluminium contains the, equivalent of how may atoms, (a) 1, , (b) 2, , (c), , 3, , (d) 4
Page 18 :
18, , (18), , MHT-CET - Part I (Std. 12), , If the number of atoms per unit cell in a crystal is, 2, structure of crystal is, , (26), , Total volume of atoms present in a face-centred, cubic unit cell of a metal is (r is atomic radius), , (a) Octahedral, , (c), , Face centred cubic, , (27), , (d) Simple cubic, (19), , (20), , In a face centred cubic cell an atom at the face, contributes to the unit cell, (a) 1/4 part, , (b) 1/8 part, , (c), , (d) 1/2 part, , 1 part, , A compound if formed by elements A and B. This, crystallizes in the cubic structure when atoms A, are the corners of the cube and atoms B are at the, centre of the body. The simplest formula of the, compound is, (a) AB, , (21), , A2B, , (b) 4, , (c), , (28), , (29), , (d) AB4, , 6, , (d) 8, , (b) 45%, , (c), , 90%, , (d) 30%, , The arrangement ABC ABC ABC _ _ _ is referred as, , S, , S, , (c), , (d), , (a) 0.05 NA, , (b) 0.1 NA, , (c), , (d) 2NA, , 0.2 NA, , A cubic closed packed structure of uniform, spheres has edge length of 0.8 nm. What is the, radius of the spherical molecule ?, (a) 2828 pm, , (b) 282.8 pm, , (c), , (d) 2.828 pm, , 28.28 pm, , The density of crystalline NaCl is 2.165 gm cm–3., What would be the volume of the cube containing, one mole of NaCl ?, (c), , 12 cm3 (d) 27 cm3, , 1.4, , Packing in ionic solids and radius ratio rule:, , (30), , How many chloride ions are there around sodium, ion in sodium chloride crystal, (a) 3, , (31), , (b) 8, , (c), , 4, , (d) 6, , Structure of NaCl crystal is, (a) Face centred cubic (b) Monoclinic, , (b) Hexagonal close packing, , (c), , Tetragonal close packing, , (32), , (d) Cubic close packing, 1.3, , Density of unit cell :, , (24), , A metal has BCC structure and the edge length of, its unit cell is 3.04 Ao. The volume of the unit cell, in cm3 will be, (a) 1.6 u 10–21 cm3, (c), , 6.02 u 10–23 cm3, , (b), , (33), , (c), , 2r, , (d), , (34), , (d) Tetragonal, , The ratio of cationic radius to anionic radius in an, ionic crystal is greater than 0.732. Its coordination, number is, (b) 8, , (c), , 1, , (d) 4, , The general formula of an ionic compound, crystallizing in the rock-salt structure is, (a) AB, , (b) 2.81 u 10–23 cm3, , In face centred cubic unit cell, the edge length is, , (a), , Orthorhombic, , (a) 6, , (d) 6.6 u 10–24 cm3, , S, , Number of unit cells in 8 g of X (atomic mass = 40), which crystallizes in BCC pattern is, (NA = Avogadro number), , (a) Octahedral close packing, , (c), , (25), , (b), , (a) 3 cm3 (b) 6 cm3, , Empty space in CCP lattice is, (a) 26%, , (23), , (c), , Potassium crystallizes in a BCC lattice, hence the, coordination number of potassium in potassium, metal is, (a) 0, , (22), , (b) AB2, , S, , (a), , (b) Body centred cubic, , (b) AB2, , (c), , A2 B, , (d) AB3, , The edge length of unit cell of sodium chloride is, 564 pm. If the size of Cl– ion is 181 pm, the size of, Na+ ion would be, (a) 101 pm, , (b) 167 pm, , (c), , (d) 383 pm, , 202 pm
Page 19 :
Solid State, , (35), , 19, , Ions A + and B – have radii 88 and 200 pm, respectively. In the close packed crystal of, compound AB, the coordination number of A+ is, (a) 3, , (36), , (37), , (b) 4, , (40), , (a) increases, , (b) decreases, , (c), , (d) halved, , does not change, , 1.6, , Electrical properites and Magnetic properties:, , (44), , Semi conductors are manufactured by addition of, impurities of, , (b) octahedral, , (a) p-block elements, , (b) actinoids, , (c), , (d) body centred cubic, , (c), , (d) s-block elements, , planar triangular, , In a closed-packed array of a A spheres, the, number of tetrahedral holes are :, (c), , 2A, , (b) A3B4, , (c), , AB2, , (a) tetrahedral, 12, , (b) octahedral, 6, , (c), , (d) octahedral, 8, , Ammonium chloride crystallizes in a body centred cubic lattice with a edge length of 387 pm., If the size of Cl– ion is 181 pm, the size of NH+4 ion, would be, (a) 206 pm, , (b) 116 pm, , (c), , (d) 154 pm, , 174 pm, , (41), , Schottky defect generally appears in, (a), , NaCl, , (b) KCl, , (c), , CsCl, , (a) Density of crystal is increased, (b) Unequal number of cations and anions are, missing from the lattice, An ion leaves its normal site and occupies an, interstitial site, , (d) Equal number of cations and anions are, missing from the lattice., , n type semiconductor is formed when trace, amount of impurity is added to silicon. The, number of electrons in the impurity atom must be, (b) 5, , (c), , 1, , (d) 2, , On doping Ge metal with a little of In, one gets, (a) p - type semi conductor, (b) n - type semi conductor, (c), , insulator, , (d) rectifier, (47), , (48), , If we mix a pentavalent impurity in a crystal lattice, of germanium, what type of semicondcutor, formation will occur?, (a) p - Type, , (b) n - Type, , (c), , (d) none of the two, , both (a) and (b), , Analysis shows that an oxide ore of nickel has, formula Ni0.98 O1.00. The percentage of nickel as Ni3+, ions is nearly, (a) 2, , (d) All of these, , Schottky defect in crystals is oberved when, , (c), , (46), , (49), , Defects:, , lanthanoids, , (a) 3, , (d) AB3, , Ionic radii of Mg2+ and O2– ions are 66 pm and 140, pm respectively. The type of interstitial void and, coordination number of Mg2+ ion respectively are, , tetrahedral, 6, , (45), , (d) 4A, , In a solid of atom A and B, atoms of A are arranged, in CCP array and atoms of B occupy all the, octahedral voids and half of the tetrahedral voids., The formula of the compound is, , 1.5, , (42), , (d) 8, , Due to schottky defect, the volume of crystal, , (a) tetrahedral, , (a) A2B, (39), , 6, , The radius ratio of a compound is 0.193, the, structural arrangement of the compound is, _________., , (a) A / 2 (b) A, (38), , (c), , (43), , (50), , (b) 96, , (c), , 4, , (d) 98, , The unbalance spin of electron exhibit, (a) magnetism, , (b) spectra, , (c), , (d) allotropy, , visible colours, , Crystals where dipoles may align themselves is an, ordered manner so that there is a net zero dipole, moment , exhibit, (a) pyro electricity, , (b) piezo electricity, , (c), , (d) anti ferro magnetic, , ferro electricity
Page 20 :
20, , MHT-CET - Part I (Std. 12), , CLASS WORK - ANSWER KEY, 1, , d, , 2, , c, , 3, , d, , 4, , a, , 5, , c, , 6, , a, , 7, , d, , 8, , d, , 9, , a, , 10 d, , 11 b, , 12 c, , 13 b, , 14 b, , 15 d, , 16 d, , 17 b, , 18 a, , 19 b, , 20 c, , 21 d, , 22 c, , 23 a, , 24 b, , 25 d, , 26 d, , 27 c, , 28 d, , 29 c, , 30 b, , 31 c, , 32 d, , 33 b, , 34 a, , 35 c, , 36 b, , 37 c, , 38 a, , 39 a, , 40 b, , 41 b, , 42 b, , 43 d, , 44 d, , 45 b, , 46 d, , 47 b, , 48 b, , 49 a, , 50 b, , 51 d, , 52 b, , 53 c, , 54 d, , 55 b, , 56 c, , 57 c, , 58 a, , 59 b, , 60 b, , 61 c, , 62 d, , 63 a, , 64 a, , 65 d, , 66 d, , 67 b, , 68 a, , 69 d, , 70 d, , 71 d, , 72 b, , 73 d, , 74 d, , 75 a, , 76 d, , 77 a, , 78 b, , 79 b, , 80 d, , 81 a, , 82 a, , 83 b, , 84 a, , 85 d, , 86 b, , 87 c, , 88 b, , 89 d, , 90 b, , 91 a, , 92 a, , 93 c, , 94 a, , 95 a, , 96 a, , 97 a, , 98 a, , 99 c, , 100 c, , 101d, , 102 d, , 103 d, , 104 a, , 105 b, , 8, , 9, , c, , 10 b, , , , HOME WORK - ANSWER KEY, , 1, , c, , 2, , b, , 3, , b, , 4, , a, , 5, , a, , 6, , c, , 7, , d, , d, , 11 a, , 12 b, , 13 b, , 14 b, , 15 d, , 16 d, , 17 d, , 18 b, , 19 d, , 20 a, , 21 d, , 22 a, , 23 d, , 24 b, , 25 a, , 26 d, , 27 b, , 28 d, , 29 d, , 30 d, , 31 a, , 32 b, , 33 a, , 34 a, , 35 c, , 36 c, , 37 c, , 38 c, , 39 c, , 40 d, , 41 d, , 42 d, , 43 b, , 44 a, , 45 b, , 46 a, , 47 b, , 48 c, , 49 a, , 50 d, ,
Page 21 :
Solid State, , 21, , CLASS WORK, , (9), , (a) Ice, Moleculer solids lave wekest intermolecular, force., , Hints & Explanation, (1), , (2), , Solid, , Force of attraction, , Due to intermolecular force of attraction, solids are rigid. Due to orderly long range, arra ngem en t of particles solids have, definite shape and size., , Ice, , H-bonding, , Phosphorous, , Covalent bond, , Naphthalene, , Covalent bond, , Mercury has relatively very low density, , NaF, , Ionic bond, , (d) Definite shape, size and rigidity, , (c), , Mercury has high density (13.6 g mL)., (3), , Ice is an example of H-bonded molecular, solids, , (d) High pressure and low temperature, Liquifaction of gases takes place at high, pressure and low temperature, , (4), , (10) (d) Ice, , (11) (b) Ionic solids, Due to s trong e lectrostatic f orces of, attraction between opposite charged ions, ionic solids have high melting point., , (a) Density, Density of Hg is 13.6 g mL –1., , (5), , (c), , Stability, , (12) (c) Polymorphism, , Stability v melting point, (6), , (a) Changes abrupty from solid to liquid when, heated, On heating solids to it melting point,, sufficient energy is pumped into the solid, to overcome interm olecular forces of, attraction and thus solid m elts and, transforms into liquid state., , (7), , (13) (b) covalent bond, Diamond is an example of covalent solid, (14) (b) covalent bond, Silicon is an example of covalent solid, (15) (d) Arranged in planes, Crystalline solids are arranged in planes, enabling proper cleavage, , (d) B and D are correct, Amorphous solids have short range order, of arrangement of particles and melt over, range of temperature, , (8), , Definition of polymorphism, , (16) (d) AB, In the body centred cubic unit cell of an ionic, compound only one cation and only one, anion is present, hence the formula is AB., , (d) both a and c, Glass is an example of amorphous solid., Amorphous solid s are also called as, supercooled liquids or pseudo solids., , (17) (b) 1, The participation of atom present in the, centre of the cube is 1 per unit cell., (18) (a) AB 3, A ion occupies the corners of the cube., ?, , Number of A ions per unit cell =, B ion occupies centre of each face, , u8=1
Page 22 :
22, , MHT-CET - Part I (Std. 12), , (26) (d) 26%, ?, , Number of B ions per unit cell =, , ?, , AB 3, , u6=3, , Any closed packed structure has volume, occupied 74%., ?, , (19) (b) XYZ 3, , Volume unoccupied = 100 – 74 = 26 %, , (27) (c) 9.033 u 1023, Atoms of X per unit cell = 8 u, , =1, , Number of moles of compound = 0.5 mole, 1 mole = 6.022 u 1023 particles, , Atoms of Y per unit cell = 1, ?, Atoms of Z per unit cell = 6 u, , =3, , Total number of closed particles in packed, structure, = 0.5 u 6.022 u 1023, , Hence the formula is XYZ3., (20) (c), , = 3.011 u 1023 particles, , A2 B 5, , ?, , Effective number of A atoms =, , Number of tetrahedral voids, = 2 u Number of atoms in close packaging, , u� 8 = 1, , = 2 u 3.011 u 1023, Effective number of B atoms =, , = 6.022 u 1023, , u5=, , Number of octahedral voids, Therefore formula is A1B 5/2 = A2B5, , = Number of atoms in close packaging, , (21) (d) 6, , = 3.011 u 1023, , Any unit cell is shared equally by 6 unit cells., (22) (c), , ABABA, , The most efficient packing is seen in ABAB, or ABC ABC type crystal lattice with, coordination number 12., (24) (b) 6, HCP contains 6 atoms per unit cell, , S, In FCC lattice effectively 4 atoms remain, present in a unit cell., Total volume of atoms present in a fcc unit, cell = 4 u, , = 6.022 u 1023 + 3.011 u 1023, = 9.033 u 1023, , (23) (a) Twelve, , ?, , Total number of voids, = Tetrahedral void + octahedral void, , A and B type layer of spheres are arranged, alternately leading to ABAB type of packing., , (25) (d), , ?, , Sr 3 =, , Sr 3, , (28) (d) Triangular voids, Triangular voids are formed in 2D close, packing., (29) (c) Twelve, In both HCP and CCP methods of stacking,, a sphere is in contact with 6 other sphere, in its own layer. It also touches directly 3, spheres in the layer above and three, spheres in the layer below., Thus a sphere has 12 close neighbours., It is said to have a coordination no of 12.
Page 23 :
Solid State, , 23, , (30) (b) HCP structure, Be, Mg and Mo show ABAB... arrangement, i.e HCP., (36) (b) 4.25 g/cm3, u, , U =, , u, =, (31) (c), , =, , = 68.04 %, ?, , Packing efficiency = 0.68, , 4.25 g/cm 3, , 58.5 g NaCl = 1 mole = 6.02 u 1023 Na+Cl–, units, , Volume occupied in BCC crystal lattice, , One unit cell contains 4 Na+Cl – units., , = 68.04%, , Hence number of unit cell present, , Volume unoccupied = 100 – 68.04 = 32%, =, , (33) (b) 2 Z, Number of tetrahedral voids in the unit cell, = 2 u number of atoms = 2 Z., (34) (a), , u, , (37) (c) 1.5 u 1023, , (32) (d) 32%, , ?, , u, , u, , 0.68, Volume occupied in BCC crystal lattice, , �, , = 1.5 u 1023., , (38) (a) 2.57 u 10 21 unit cell, u, (i), , S, , u, , Mass of unit cell, , =, , =, P.F =, , u, u, , = 38.85 u 10–23 g, (ii) 38.85 u 10–23 g = 1 unit cell, , S, , ?, , =, , 1g, , = x, , x = 2.57 u 10 21 unit cell, (39) (a) 1.857 u 10–8 cm, , S, For a simple unit a = 2r =, , =, , S, , =, , S, , Radius of Na (if BCC lattice), =, , =, , u, , (35) (c), = 1.8574A° = 1.8574 u 10–8 cm, SC a = 2r, BCC, , a = 4r, , FCC, , a = 4r, , (40) (b) 127 pm, In FCC unit cell 4r =, atom, a = edge length), , (r = radius of Cu
Page 24 :
24, , MHT-CET - Part I (Std. 12), , So, r =, , a, , u, , r=, , Z =, , = 127 pm., , ?, , (41) (b) 154 pm, , =, , In body centred cubic unit cell, ions touch each, other along the body diagonal of the cube., , u, , Z =, , u, , u, , 4, , Hence it is face centred cubic cell., (45) (b) 2 u 1024, , 2rc + 2ra =, , a, , 2(rc + 181 pm) =, , §, rc = ¨, ¨, ©, , The number of atoms in one unit cell of, BCC crystal is 2., , (387 pm), , ·, ¸ (387 pm) – 181 pm = 154.15 pm, ¸, ¹, , (42) (b) 335 pm, , number of unit cell present =, , u, , = 2 u 1024, (46) (d) 609.6 pm, , For BCC the dis tance between two, oppositely charged ions, =, , u 387 = 335 pm, , =, , (43) (d) 4, , For FCC arrangement, distance of nearest, neighbour (d) is, d=, , =, , = 609.6 pm, , (47) (b) 0.414 r, u, u, , Density =, , ?, , ?, , Limiting value of r+/r– of octahedral type, of hole is 0.414 – 0.732., (48) (b) 4, , ZN =, , Number of molecules,, , MW =, , Molecular weight, , In NaCl (rock salt ) :, , a3 =, , Volume,, , Number of Na+ ions, , NA=, , Avogadro’s number, u, , Z =, , u, , u, , = 12 (at edge centres) u, u, , + 1 (at body centre), , u 1, = 4, , u, , =, =, , 4.06, , u, , u, , Number of Cl – ions, = 8 (at corners) u, , + 6 (at face centre) u, , (44) (a) face centred, U =, , u, = 4, Thus 4 formula units per unit cell.
Page 25 :
Solid State, , 25, , (49) (a) 1, , (58) (a) 1.123, , Number of octahedral site = Number of, spheres, ?, , �, , �, , = 0.74, , 1 octahedral sites per sphere, , (50) (b) 2, , �, , Number of tetrahedral sites = 2 u Number, of spheres, ?, , = 0.55 and, , 2 tetrahedral sites per sphere, , �, , �, , + 1 = 0.55 + 1 and, , �, , �, , �, , = 1.55 and, , (51) (d) 0.732 - 1.000, Limiting value of r +/r– of CsCl is > 0.732, , + 1 = 0.74 + 1, , = 1.74, , Now edge length ratio of KCl and NaCl is, , (52) (b) Face centred cubic, =, , ZnS has 4Zn+2 and S2– each ions, , = 1.122, , Thus, it’s structure is of FCC type, (53) (c), , (59) (b) NaWO 3, , 4, , In a unit cell, W atoms at the corner, , Number of spheres in CCP structure = 4, Number of octahedral voids = Number of, spheres = 4, , =, , (54) (d) X4Y 3, , u8=1, , O atoms at the centre of edges =, , u 12 = 3, , Number of atoms in CCP lattice = 4, ?, , y =, , Number of atoms at the centre of the cube = 1, , 4, , W : O : Na = 1 : 3 : 1,, , Number of tetrahedral voids = 8, , Hence formula = NaWO3, ?, , x, , =, , u8, , =, , (60) (b) 4, Co-ordination number of Zn+2 ion is 4., , ?, , y4 , , y1 x 4 y 3, , (61) (c) remains same, , (55) (b) 50%, , In Frenkel defect, the density of ionic solid, remains same., , In FCC, number of tetrahedral voids = 8, In ZnS structure, Zn ++ ions = 4, ?, (56) (c), , Ag ions occupies 50% (i.e. 4) of tetrahedral, voids, 6, Ionic crystal with coordination number 6, has r +/r– ratio of 0.414 –732, , (57) (c), , CsI, Cs+ and I – have large sizes., , (62) (d) Both b and c, By definition, (63), , (a), , Positive ions and negative ions are of different, size, Schottky defect is caused if some of the, lattice points are unoccupied. The points, which are unoccupied are called vacancies, or holes. The number of missing positive, and negative ions is same keeping the, crystal neutral. Cations and anions are of, similar size.
Page 26 :
26, , MHT-CET - Part I (Std. 12), , (64) (a) both schottky and frenkel defect, , (76) (d) Point defect, , AgBr shows both type of defect, i.e. Schottky and Frenkel defect, , By definition, (77) (a) Interstitial defect, , (65) (d) Schottky defect, Definition, , Refer interstitial defect, (78) (b) Presence of defect alter the density of solid, , (66) (d) transition metals only, Metal de ficiency def ect is shown by, transition metals because they possess, variable valency., (67) (b) equal number of cations and anions are, missing from the lattice, , In Frenkel defect, density remains constant, (79) (b) p-type semiconductor, Dopin g of Si with B lea ds to p-type, semiconductor, (80) (d) Very large, When insulators (non metal atoms) interact, to form a solid, their atomic orbitals mix to, form two bunch of orbitals, separated by a, large band gap. Electrons cannot therefore, be promoted to an empty level, where they, could move freely., , Schotkky defect is observed when equal, number of cations and anions are missing, from their lattice sites., (68) (a) low, Frenkel defect is observed in crystals in, which the radius ratio is low., , (81) (a) 3, , (69) (d) All the above, , When atoms with valence electron one less, than Si is added the p-type semiconductor, is formed., , (70) (d) Interstitial defect, Definition of interstitial defect, , (82) (a) n-type and p-type, , (71) (d) None of these, , Ar + Si�, � n-type semiconductor, , NaCl doped with MgCl2 is simply impurity, defect., (72) (b) Carbon atoms, , B + Si � p-type semiconductor, (83) (b) Diamond, Diamond is bad conductor of electricity., Thus has large spacing between valence, and conduction band., , Stainless steel is formed when ion atoms, are replaced by C, (73) (d) 3 : 1, , (84) (a) Metallic crystal, , In the formation of brass, copper atoms are, replaced by zinc atoms in the ratio 3 : 1., (74) (d) Both (a) and (b), , Metallic crystals has least gap between, valence and conduction band., (85) (d) Band theory, , Point defects are due to the presence of, foreign atoms or impurities, (75) (a) Sr2+ ions, , By definition, (86) (b) electrical conductity, Electron in higher e nergy lev el are, responsible for electrical conductivity., , Some of the sites of Na + ions are occupied, by Sr+2 ions leading to defect in crystal, , (87) (c) 2.5 u 1022, 1 mole = 24 g = 6.022 u 10 23 atoms, ?, , 1 g = 2.5 u 1022 atoms
Page 27 :
Solid State, , 27, , (88) (b) Doping, Definition, (89) (d) Ionic solids conduct electricity due to, presence of ions., By definition, (90) (b) CoO, Oxide that is insulator is CoO., (91) (a) Hg, Hg acts as a superconductor at 4 K., (92) (a) some electrons will flow from n to p, When n and p-type semiconductors come, in contact, some electrons flow from n to, p., (93) (c), , P gives rise to extra electrons while Al gives, rise to holes, Doping of Si with P gives extra electrons, while doping with Al gives rise to holes., , (94) (a) MnO2, MnO 2 is anti-ferromagnetic in nature., (95) (a) n�n�n�n�n, Ferromagnetism is due to large number of, unpaired electrons., (96) (a) BaTiO3, BaTiO 3 is ferromagnetic, (97) (a) Diamagnetic, Water is dimagnetic, (98) (a) A-q, B-r,C-p, NaCl o Diamagnetic, Fe +3 o Paramagnetic, Gradolinium o Ferromangetic, (99) (c), , paramagnetic substance, Oxygen is paramagnetic, , (100) (c), , diamagnetic, In Gouy’s methods, diamagnetic substance, weigh less in magnetic field, , (101) (d) all of these, All of these are diamagnetic, (102) (d) Zr, Mo and Sb, The first three include Cd which has fullyfilled orbitals and hence is diamagnetism., (103) (d) All are correct, Ni, Co and Fe 3O4 all are ferromagnetic, (104) (a) Fe, Ferromagnetism is maximum in Fe out of, Fe, Co and Ni., (105) (b) Ferrom agnetic substances can not b e, magnetised permanently, Ferrom agnetic s ubstance s can b e, mangetised permanently. Other given, statements are true.
Page 28 :
28, , MHT-CET - Part I (Std. 12), , HOME WORK, , (8), , (d) Presence of free electrons, The atoms of metal have an outer layer of, electrons. Thus when light shines on lustrous, metal, the loosely bound electrons reflect, incoming light, giving the metal a shiny, appearance., , Hints & Explanation, (1), , (c), , Silica, The substances that appear like solids do not, have well developed perfectly ordered, crystalline structure are called amorphous, solids., , (2), , (b) Ionic solid, Ionic solids are formed due to electrostatic, force of attraction between cation and anion., , (4), , (6), , (10) (b) Glass, Glass being amorphous solid melts over a, range of temperature., (11) (a) Semiconductor, Silicon is an example of semi conductor., (12) (b) covalent bond, Graphite is a covalent solid and hence,, covalent bonding is present, (13) (b) a = b z c and D = E = J = 90o, , (a) Molecular crystal, Solid CO2 is a perfect example of a molecular, crystal. The vander waal’s forces holding, the CO 2 molecules together are weak, enough that dry ice sublimes and passes, directly from solid to gas phase at –78oC, (c), , Tetragonal unit cells has two sides equal, and third side with different length, a = b z, c and D = E = J = 90o, (14) (b) 14, The lattice points can be arranged in, maximum of fourteen ways., , Diamond is a bad conductor of heat, i. All carbon atoms in graphite are sp 2, hybridised which are convalently bonded, to three other sp2 hybridised carbon atoms, and form interlinked six membered rings, of carbon atoms., ii. The remaining half filled unhybridised 2pz, orbital is used for p bonding so that layers, of carbon atoms i.e. graphite is formed., , (15) (d) Monoclinic sulphur, :, , a=b=c;, , D = E�=, E� J z 90o, , Graphite :, , a=bzc;, , D = E = 90 o J ��120o, , Calcite, , Rhombic sulphur, , (d) Sugar, Yes, sugar is a covalent type of crystalline solid, , :, , Monoclinic sulphur :, , a z b z c ; D = E = J = 90o, a z b z c ; D = E = 90o �J z 90o, , (16) (d) 2 and 4, BCC o 8 atoms at the corners and 1 atom at, centre., , Also due to no delocalized electron diamond, is a bad conductor of electricity., (7), , In solid NaCl there is no velocity of ions, In ionic solids the ions occupy fixed, position where the velocity of ions is zero,, thus these solids are bad conductor of, electricity., , (a) Silica, Quartz is a crystalline variety of silica, because it is obtained from silicon dioxide., , (5), , (c), , (b) Graphite, Graphite has delocalized molecular orbitals, and the delocalized electrons have the, freedom to move in the delocalized molecular, orbitals. Therefore, graphite is a good, conductor of electricity., , (3), , (9), , ?, , §, ·, n= ¨ u ¸+1=2, ©, ¹, FCC o 8 atoms at the 8 corners and 1 atom, at each of the 6 faces. Thus total number of, atoms in FCC is 4
Page 29 :
Solid State, , 29, , (17) (d) 4, (25) (a), , In FCC unit cell, Atoms at corner = 8 u, , In FCC, , =1, , a =, Atoms at face centre = 6 u, , =3, , Total number of atoms = 4, , S, , (26) (d), , (18) (b) Body centred cubic, Total volume, , In a body centred cubic crystal system, the, number of particle per unit cell is 2., , = number of atoms u volume of sphere per, unit cell, , part, , (19) (d), , S, , = 4u, , S, , =, part inside the unit cell, , (27) (b) 0.1 NA, u, , (20) (a) AB, , Mass of unit cell, , =, , Acorner B body centre, B1 u 1 = A1B1 = AB, , u, , =, , (21) (d) 8, , g, , u, , Co-ordination number of metal crystallized, in BCC lattice is 8., , g, , =, , 1 unit cell, , 8g, , =, , x, , x, , =, , (22) (a) 26%, In a CCP lattice the volume occupied is 74%, (23) (d) Cubic close packing, The ABC ABC type arrangement is called, cubic close packed CCP structure, , =, (28) (d) 282.8 pm, , (24) (b) 2.81 u 10–23 cm3, V =, , a3, , V =, , (3.04 A °)3, , r =, , ?, , r =, , =, , (3.04 u 10–8 cm)3, , |, , 27 u 10 –24 cm3, , =, , 0.282 nm, , =, , 2.7 u 10 –23 cm3, , =, , 282 u 10–12 m, , =, , 282 pm, , u, , 0.1 NA
Page 30 :
30, , MHT-CET - Part I (Std. 12), , (29) (d) 27 cm3, , (37) (c), , In a closed packed crystal, number of, tetrahedral voids are twice the number of, sphere., , Density =, 1 mole of NaCl = 58.5 g, ?, , 2A, , (38) (c), , A (CCP), , = 27 cm3, , Volume =, , AB2, B(all octahedral and half, tetrahedral voids), , (30) (d) 6, In NaCl crystal coordination number of Na, and Cl are 6, 6 respectively, (31) (a) Face centred cubic, NaCl is a FCC lattice where Cl forms FCC, lattice and Na is present in octahedral voids, , ?, (39) (c), , 4, , 4+4, , 4, , 8, , 1, , 2, , Formula = AB2, tetrahedral, 6, �, , (32) (b) 8, �, , If, , 0.471comes in range of 0.414 – 0.732. Hence,, co-ordination number of cation is 6 and the, void is octahedral., , value is between 0.073 to 1, then the, , coordination numeber of cation is 8., (33) (a) AB, , (40) (d) 154 pm, , The rock-salt structure involves face-centred, cubic unit cell. It has four cations and four, anions per unit cell. The formula of ionic, compound is A4B4 i.e. AB., , Q =, , 387, , r– =, , 181 pm, , r+ =, , ?, , (34) (a) 101 pm, rNa+ + rCl–, , r Na +, , ?, , r+, , =, , ?, , r+, , =, , 334.75 – 181, , ?, , r+, , =, , 153.75 pm, , u, , – 181, , =, , = 101 pm, 6, , (41) (d) All of these, NaCl, KCl and CsCl all shows schottky defect., , =, , (36) (c), , =, , =, , = 282 – 181, , (35) (c), , For BCC, r+ + r–, , = 0.44, , which is in the range of 0.414 and 0.732., Hence the co-ordination number of A+ is 6., planar triangular, Radius ratio of compoudn is 0.193, which is, in the range of 0.155 – 0.225. Hence the, structural arrangement is planar triangular., , (42) (d) Equal number of cations and anions are, missing from the lattice., In schottky defect equal number of cation, and anion are missing, (43) (b) decreases, Due to schottky defect, volume of crystal, decreases.
Page 31 :
Solid State, , 31, , (44) (a) p-block elements, Semiconductors are formed by addition of, group 13 to group 14 elements or impurities, of group 15 to group 14 elements., (45) (b) 5, n-type semiconductor where major charge, carries are electrons which are negative, charge. Silicon belongs to group 14, the, number of electrons in valence shell is 4. Thus, to have negative charge, the number of, electrons in impurity must be more than 4., (46) (a) p- type semi conductor, Germanium belongs to Group 14 which is, doped with In of group 13, which gives, p type semiconductor., (47) (b) n - Type, By definition, (48) (c), , 4, Let number of nickel ions = 98, , ?, , Number of oxide ions, , = 100, , Total negative charge on O2– ions, = 2 u 100, = 200, Let number of Ni3+ ions = x, ?, , Number of Ni2+ ions, , = 98 – x, , x u 3 + (98 – x) u 2 = 200, 3x – 2x, , = 200 – 196, , x = 4, Percentage of nickel as Ni3+ =, , u 100, , = 4.08, (49) (a) magnetism, By definition, (50) (d) anti ferro magnetic, Crystals where dipoles may align themselves, is an ordered manner so that there is a net, zero dipole moment exhibit anti ferro, magnetism.
Page 32 :
32, , MHT-CET - Part I (Std. 12), , Points to remember, , , Isotropic substances : The substances which show same physical properties (electrical conductivity, thermal, conductance refractive index etc.) in all directions are called isotropic substances., , , , Anisotropic substances : The substances which show different physical properties (electrical conductivity,, then conductivity, refractive index, etc.) in different directions are called anisotropic substances., , , , Crystal lattice or Space lattice : It is the regular arrangement of the constituent particles which when, repeated over again in different directions produces the complete crystal lattice., , , , Unit cell : It is the smallest three dimensional repeating portion of a space lattice which when repeated, over again in different directions produces the complete crystal lattice., , , , Close packing : The arrangement in which maximum available space is occupied leaving minimum available space., , , , Interstitial voids or sites : The holes or voids in the crystal lattice are called interstitial voids or sites., , , , Coordination number : It is the number of nearest (or closest) neighbours of any constituent particles in, the crystal lattice., , , , Radius ratio : The ratio of the radius of the cation to the radius of the anion (i.e. r+ /r– ) is called radius ratio., , , , Disorder or defect : Any deviation from completely ordered arrangement of constituent particles in a, crystal is disorder or defect., , , , In simple cubic structure, 52% of the space is occupied while in body centred cubic structure 68% of the, spheres occupied., , , , In close packing of spheres, there are vacant spaces, holes or voids called interstitial sites. These are tetrahedral, sites and octahedral sites. For each atom, there is one octahedral site and two tetrahedral sites., , , , The coordination number in HCP and CCP arrangement is 12., , , , Ideal crystals exist ony at 0 K temperature. At temperatures above 0 K, defects in crystals occur., , , , Non-stoichiometric defects are of metal excess type (anion vacancies or cations in interstitial sites) and, deficient type (cation vacancies or anions in interstitial sites)., , , , Impurity defects are caused by the presence of impurity in the crystal., Example : SrCl2 in NaCl, CdCl2 in AgCl.
Page 33 :
Solid State, , 33, , , , Paramagnetic substances are those which have permanent magnetic dipoles and are attracted by the, magnet. Diamagnetic substances are repelled by the mangetic field. Ferromagnetic substances are strongly, attract the magnetic field and show permanent magnetism even when mangetic field is removed., , , , Solids may be classified as conductors (104 to 107 ohm–1 m–1), semi-conductors (10–6 to 10–4 ohm–1m–1) and in, solids (10–20 to 10–10 ohm–1m–1)., , , , Some times calculated amounts of impurities are added by doping in semi-conductors which change their, properties. Doping can be of P or As in Ge (electrons rich) or B or In (electrons deficient) to Ge or Si., , , , Seven crystal systems and the Bravais lattice are, , Crystal system, , Cubic, , Orthorhombic Tetragonal Monoclinic Triclinic, , Hexagonal Rhombohedral, , Bravais lattice, , Primitive, , Primitive, , Primitive, , Primitive, , Primitive, , Primitive, , Primitive, , Face centred Face centred, Body centred Body centred Body, centred, End centred, Total, , , , 3, , 4, , Coordination Number, , 1, , 1, , 1, , Shape, , Example, , 3, , Planar triangular, , B2O3, , 0.225 – 0.414, , 4, , Tetrahedral, , ZnS, , 0.414 – 0.732, , 6, , Octahedral, , NaCl, , 0.732 – 1, , 8, , Body centred cubic, , CsCl, , Face centred cubic, , End centred cubic, , Number of atoms per unit cell is, Body centred cubic, , 1, , 2, , 4, , 2, , Relationship between nearest neighbour distance (d) and radius of atom (r) and edge of unit cell (a) for, unit cell of a cubic crystals are :, Simple cubic, , , , 1, , 0.155 – 0.225, , Simple cubic, , , , 2, , The limiting radius ratio (r+/r–) and the coordination numbers in different crystals are :, r+/r–, , , , End centred, , Body centred, , Face centred, , d=a, , d=, , , , d=, , r=, , r=, , , , r=, , Density of unit cell,, , d=, , u, u, , g cm–3
Page 34 :
34, , MHT-CET - Part I (Std. 12), , EVALUATION PAPER SOLID STATE, Time : 30 Min., , (1), , (2), , CO2 belongs to, (a) covalent crystal, , Marks : 25, , (b), , molecular crystal, , (c), , ionic crystal, , The relation a z b z c and D = E = J z 90o belongs to the crystal system., (a) tetrahedral, (b) triclinic, (c), rhombohedral, , (d), , metallic crystal, , (d), , hexagonal, , (3), , The ratio of close packed atoms to octahedral holes in hexagonal close packing is, (a) 2 : 2, (b) 1 : 2, (c), 2:1, (d) 1 : 3, , (4), , The unit cell present in the crystal lattice of Aluminium is ________ ., (a) trigonal, (b) cube, (c), tetragonal, , (d), , hexagonal, , Beryllium crystallise in _______ structure., (a) FCC, (b) CCP, , (d), , HCP, , (5), , (c), , BCC, , (6), , The intermetallic compound LiAg crystallizes in cubic lattice in which both lithium and silver, have co-ordination number eight. The crystal class is ________, (a) simple cubic, (b), body centred cubic, (c) hexagonal close packed, (d), face centred cubic, , (7), , In a solid ‘AB’, A atoms occupy the corners of the cubic unit cell. If all the face centred atom along, one of the axis are removed, then the resultant stoichiometry of the solid is, (a) A2B, (b) AB2, (c), A3B4, (d) A4B3, , (8), , The general formula of an ionic compound crystallizing in the body centred cubic structure is, _______, (a) AB3, (b) A2B, (c), AB2, (d) AB, , (9), , The anion A forms hexagonal closest packing and cations B occupy only, , octahedral voids in, , it, then the general formula of the compound is, (a) BA, (b) AB2, , (d), , (c), , B 2A3, , B3A2, , �, , (10) An ionic compound is expected to have tetrahedral structure if, (a), (c), , is in the range of 0.414 to 0.732, is in the range of 0.225 to 0.414, , (b), (d), , is in the range of 0.155 to 0.225, is more than 0.732
Page 35 :
Solid State, , 35, , (11) The inter ionic distance for cesium chloride crystal is, (b), , (a), , (c), , (d), , (12) Ammonium chloride crystallizes in a body-centred cubic lattice with edge length of unit cell, equal to 387 pm. If the size of Cl– ion is 181 pm, the size of NH+4 ion would be, (a) 116 pm, (b) 154 pm, (c), 174 pm, (d) 206 pm, (13) Which of the following fcc structure contains cations in alternate tetrahedral voids ?, (a) NaCl, (b) ZnS, (c), Na2O, (d) CaF2, (14) If the positions of Na+ and Cl– are interchanged in NaCl , having fcc arrangement of Cl– ions then, in the unit cell of NaCl, (a) Na+ ions will decrease by 1 while Cl– ions will increase by 1, (b) Na+ ions will increase by 1 while Cl– ions will decrease by 1, (c) Number of Na+ and Cl– ions will remain the same, (d) The crystal structure of NaCl will change, (15) In CaF2 crystal, F– ions are located in, (a) tetrahedral voids, (c) octahedral voids, , (b), (d), , half of tetrahedral voids, half of octahedral voids, , (16) If NaCl is doped with 10–4 mol % of SrCl2 , the concentration of cation vacancies will be, (NA = 6.02 u 1023 mol–1), (a) 6.02 u 1014 mol–1 (b) 6.02 u 1015 mol–1, (c), 6.02 u 1016 mol–1 (d) 6.02 u 1017 mol–1, (17) The crystal with metal deficiency defect is, (a) NaCl, (b) FeO, , (c), , KCl, , (d), , ZnO, , (18) Diamagnetism is exhibited by, (a) Cobalt, (b) Water, , (c), , Oxygen, , (d), , Iron, , (19) Which of the following is ferromagnetic substance ?, (a) Water, (b) NaCl, (c), , Benzene, , (d), , CrO2, , (20) Zinc oxide loses oxygen on heating according to the reaction, ZnO, , Zn2+ +, , O2 + 2e–., , It becomes yellow on heating because, (a) Zn2+ ions and electrons move to interstitial sites and F-centres are created, (b) Oxygen and electrons move out of the crystal and ions become yellow, (c) Zn2+ again combine with oxygen to give yellow oxide, (d) Zn2+ are replaced by oxygen
Page 36 :
36, , MHT-CET - Part I (Std. 12), , (21) If three elements X, Y and Z crystallize in a ccp lattice with X atoms at the corners, Y atoms at, the cube centre and Z atoms at the edge, the formula of the compound will be, (a) XXZ, (b) XYZ2, (c), XYZ3, (d) X2Y2Z, (22) Number of unit cells in 8g of X (atomic mass = 40) which crystallizes in bcc pattern in, (Na = Avogadro number), (a) 0.05NA, (b) 0.1 NA, (c), 0.2NA, (d) 2NA, (23) For a solid with the following structure, the co-ordination number of the points A and B respectively are, , (a), , 6, 8, , (b), , 8, 8, , (c), , 6, 6, , (d), , 4, 6, , (24) With reference to Guoy’s method which of the following statement is not correct ?, (a) The diamagnetic substance weighs less in the magnetic field, (b) The paramagnetic substance weighs more in the magnetic field, (c) The diamagnetic substance weighs maximum in the magnetic field, (d) The ferromagnetic substance weighs maximum in the magnetic field, (25) Which metal among the following has highest packing efficiency ?, (a) Iron, (b) Tungesten, (c), Aluminium, , (d), , Polonium, , EVALUATION PAPER - SOLID STATE ANSWER KEY, , 1, , b, , 2, , c, , 3, , a, , 4, , b, , 5, , d, , 11 b, , 12 b, , 13 b, , 14 b, , 15 a, , 21 c, , 22 b, , 23 c, , 24 c, , 25 a, , 6, , b, , 16 d, , , , 7, , bd 8, , 17 b, , d, , 18 b, , 9, , c, , 10 c, , 19 d, , 20 a