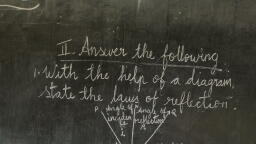Page 1 :
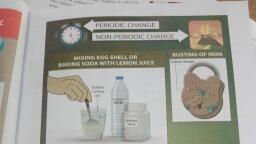
3.4.1 Rusting of iron, , we have already studied that, , In class, , nle of a chemical change, ing is an example of a ch, , rust, Now; shall we read why the process of rusting 1s, , ed a chemical change, , ae, 5 There is an, , iron pillar at the Qutub, complex in Delhi which, is more than 1600 years, age. Even after such a, long period, the iron, pillar kept in open spaces, has not rusted at all. This, shows that Indian scientists made great, advances in metal making technology, even at 16th century which enable, them to make this iron pillar having the, quality of great rust resistance., , , , , , jar at Delhi, , , , The Iron, , * Amazingly there is an, iron that did not rust!, , , , , , , , , , , , , , , , , , Rusting is one change that affects iron, , articles and slowly destroys them. Since iron is, used in making bridges, ships, cars, truck bodies, and many other articles, the monetary loss due, to rusting is huge. The process of forming rust is, , represented as follows:, , iron + oxygen + water-rrust, , 2Fe + 20, from air + 2H,0 —» 2Fe,0;, H,0, , For rusting to take place both oxygen and, water (or even water vapour) is essential. In fact,, if the content of moisture in air is high, the air, is said to be more humid and eventually rusting, , , , , , , How can we prevent rusting?, , es can be prevented from yy,, , , , Iron articl i, contact with oxygen, water/water vapo, ee, simple way is to apply a coat of paint or gr." rermanen, applied regulari,® oft, applied regulary A ity, , nd find, , Another way of preventing a1, , . rusting is to deposit a lay, you, ‘ of a metal like chromiun,, , or zinc on iron. This |,, , These coats should be, , prevent rusting, , ‘ pur, called galvanization and you will Hearn *° e, about this detail in higher classes. gl**, , she bu, , , , 3.4.2 Burning, , we have already studied that burning «, paper is a fast change. Burning a piece of paper M, gives entirely new substances such as carbon-di- p, oxide, water, water vapour, smoke and ash. Heat,, and light are also given out during the burning of