Page 1 :
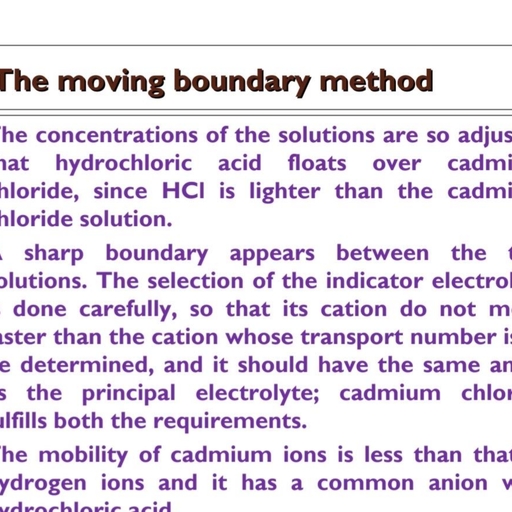
Ear DEGREES OF FREEDOM OF MOTION, Since almost all the mass of the atom is concentrated in, , Consider a molecule made up of N atoms. u l rir? oawaentes i, its nucleus and the nucleus is very small in size (the radius being of the order o} f m as that, of the molccule, the order is 107!’ m), the atoms may be considered as mass points. To represent each, , mass point, we require three co-ordinates. Hence to represent the instantaneous position of N mass, points in space, we require 3N co-ordinates., The number of co-ordinates required to specify the position of all the mass points 7.e. atoms in a., molecule is called the number of degrees of freedom., Thus a molecule made up of N atoms has 3N degrees of freedom., According to kinetic theory of matter, when thermal energy is absorbed by a molecule, it is stored, within the molecule in the form of :, (i) translational motion of the molecule, (ii) internal movement of the atoms within the molecule, i.e., rotational motion and vibrational, , motion. ,, The translational motion of the molecule means the motion of the centre of mass of the molecule i, , as a whole. The centre of mass of the molecule can be represented by three co-ordinates. Hence there, are three translational degrees of freedom. The remaining (3N — 3) co-ordinates represent the internal, , degrees of freedom., However, as already mentioned, the internal degrees of freedom may be subdivided into, , (i) rotational degrees of freedom, and, , (ii) vibrational degrees of freedom., For a rotational motion, there are two degrees of freedom for a linear molecule and three for a nonlinear molecule as shown in Fig. 3.15., , , , ‘, , , , ROTATION ROTATION ROTATION ROTATION ROTATION, _ ABOUT X-AXIS ABOUT Y-AXIS. 7 __ ABOUT X-AXIS. ABOUT Y-AXIS ABOUT Z-AXIS, ° ©, FIGURE 3.15, , , , (a) Rotations of a linear molecule about two mutually perpendicular axes., (b) Rotations of a non-linear molecule about three mutually perpendicular axes., , Remaining degrees of freedom for the linear molecules are equal to (3N — 5) and for the nonlinear molecules, they are equal to (3N — 6). These describe the motion of the nuclei with Tespect to one, another and thus represent the number of vibrational degrees of freedom. Hence, , Vibrational degrees of freedom of a linear molecule containing N atoms = 3N — 5., °! Vibrational degrees of freedom of non-linear molecule containing NV atoms = 3N - 6,, "°°" & few examples on the calculation of vibrational degrees of freedom are given below :, (i) As a diatomic molecule is always linear, its vibrational degrees of freedom, =3N-5=3x2-5=1., , & ’, , , , , , , , , , , , , ete rapes :, PHYSICAL CHEMISTRY VOU
Page 2 :
|, | el, , (ii) For the linear polyatomic molcule CO, N = 3. Hence vibrational degrees of freedom =, 3N -5 =3 = 3-5 =4. Similarly for HC = CH, N = 4. Hence the vibrational degrees of, freedom = 3N-5=3 x 4-S5=7., , (iii) For the non-linear polyatomic molecule H,O, N = 3. Hence vibrational degrees of freedom, = 3N- 6 =3 x 3-6 =3. Similarly, for HCHO, N = 4 the vibrational degrees of freedom, =3N-6=3%*4-6=6., , In terms of co-ordinates, the vibrational degree of freedom represent the interatomic distances and, , angles needed to specify the geometry of the atomic framework within the molecule., , In terms of molecular motions, the vibrational degrees of freedom represent the number of, independent vibrational modes that can occur in the molecule, the vibrational energy being associated, with each mode., , The only one mode of vibration of a diatomic molecule like CO, four modes of vibration of linear, triatomic CO molecule and three modes vibration of non-linear triatomic H,0 molecule are shown Fig., 3.16., , a =>, ©-~(0000000000000 © OM NN0000Q © 0000000 ©, ge, , ie () SYMMETRICAL STRETCHING, , EQUILIBRIUM POSITION —* = —*, ©G000000 ©” 0000000 ©, , (i) ASYMMETRICAL STRETCHING t, , , , , , ©O~D000000000000 -@, , 1, , t, EXTENSION CY JDQQQUVI 0000000 2, , O-ATITHMTNNT IIT XO) A (iii) BENDING IN THE PLANE ZA, , —— OVUTUT-O VHVITI O, , (iv) BENDING OUT OF PLANE, , , , (iii) ASYMMETRICAL STRETCHING, , (i) SYMMETRICAL aaercaneY wi “mer BENDING, , FIGURE 3.16, , Normal modes of vibration of (a) diatomic molecule, CO,, (b) linear triatomic molecule, CO, (c) non-linear triatomic molecule, H,0., , , , , , , , , , To sum up, for a molecule made up of N atoms., Total degrees of freedom = 3N., Further split up of these is as follows :, , Translational Rotational Vibrational, For linear molecule : 3 2 3N-5, For non-linear molecule: 3 3 3N-6, , Now we shall take up the study of the different types of spectra one by one., , MOLECULAR SPECTROSCOPY a
Page 3 :
PURE ROTATIONAL (MICROWAVE) SPECTRA OF DIATOMIC MOLECULES,, , ERE ENERGY LEVELS OF A RIGID ROTOR, at if diatomic molecule is considered to be a, , On the basis of wave mechanics, it can be scen th s, rigid rotator, i.¢., like a rigid dumbbell joined along its line of centres by a bond equal in oan the, distance ry between the two nuclei, then the allowed rotational energies of the molecule aroun = axis, passing through the centre of gravity and perpendicular to the line joining the nuclei are given by, , CENTRE OF, , h?, ms J(J +1) a GRAVITY, 8x27 ¢ @, , , , E, , , where Jis the rotational quantum number that can have values, 0, 1, 2, 3 etc. and J is the moment of inertia of the molecule, , about the axis of rotation, /.e., , he (w2] 72 =ure .Q) FIGURE 3.17 ead, m, +m) Rigid rotator., , , , , , , , , , , , where m, and m, are the atomic masses of the two atoms of the diatomic molecule and, Mm, Mm., D2, mone? -B), , is called the reduced mass., Derivation of the expression for rotational energy. Centre of gravity of a diatomic molecule is, , the point which satisfies the following condition, m, ry =m 1p - (4), The moment of inertia of the diatomic molecule is given by, IT=m rp + my 72 (5) |, Fm rr tm ry ry i, =P Py (m, + my) ...(6), But rntrn=% .(7), .. From eqn. (4),, my My = My (ry - 7), m2 7%, n=, or 1m +m -..(8), Similar! ——1o_, b=, umilarly, 2 my +m, +(9), , Substituting these values in eqn. (5), we get, , 2 2, =m 2 + TS 9a, 2, (m, +m) (m, +m,)? 0, , mm, (m, + m,) m, pa yy Fy, m,, i= 1 r2, , , , (mm, + m,)? m,+m, °, , , , CATCH Rae
Page 4 :
= pr? [wt =m i, mm, +m is reduced os wi(10), , Now, by definition, th, 7 The an;, L= eur momentum of a rotating molecule is given by, where @ is the an as (11, But we know that ‘ani Tocity Gust as linear momentum is mass * velocity)., momentum is quantized whose values are given by, , L=Jy7 pe, : ole (12), , where J= 0, 1, 2, 3, ..., called the rotational quantum numbers., Further the energy of a rotating molecule is given by, E=1@?, , , , , , , , quantized value of the rotational energy will be given by, 1 To)2 2 J Digital, E a1 jy2 - ) _B 12 h2/en21, rg 2. (Or ..(13) 5, Substituting the value of L from eqn. (12), we get ‘ 6h2/an21, _ 2 1, Ey sd Gel) a oF j 2h2/en21, 12 ——_—__2____—, or EE, =—> J (V+ (14, "8221 ee (4) FIGURE 3.18, Putting J= 0, 1, 2,3 etc. in equation (1), pattern of the rotational eT Tat, energy levels obtained will be as shown in Fig. 3.18. as a rigid rotator., Evidently, the spacing between the energy levels increases as J, increases., , ERM ROTATIONAL SELECTION RULES, , According to the selection rules, the transition take place only between those rotational energy, , levels for which, AJ=+1, , the rotational quantum number is unity. ., 0 absorption and AJ =~ 1 corresponds to emission. In other, articular rotational level to just the next higher or just the, take place with equal probability whereas all other, , i.e. the change in, The transition AJ = +1 corresponds ti, , words, a transition can take place from ap, lower rotational level. All these transitions can, , transitions are forbidden., , EXE] ROTATIONAL SPECTRA OF THE DIATOMIC MOLECULES, As already explained, the allowed rotational energies are given by the expression (eqn. 1. sec., , 3.13), _ y+) : w(1), , Er 8n2 I, ©, , , , $$ -TROSCO!
Page 5 :
As E = hy, therefore in terms of frequency, we can write, , v=, , , , J+ (2)!, any 7+) 0),, , Further as c = v= = v/V (because wave number ¥ = 1/},), therefore in terms of wave numbers,, eqn. (2) can be written as, , , , ~ h ', v= salty (3)., ~H0+1) (4), , where B = h/8 72 Ic is called rotational constant,, Putting J = 0, 1, 2, 3 etc. in eqn. (4), the wave numbers of the different rotational levels will be, 0, 2B, 6B, 12B, 20B, 308, ...... and so on. :, , When a transition takes place from a lower rotational level with rotational quantum number Jtoa', higher rotational level with rotational quantum number J’, the energy absorbed will be given by, , AE,=E, ~E, (or Ey- E), , , , , , pray? _ ssa, - ray ars, 8x27 Wt 8n27, 2, = JS 41) -J (S41, teil (J'+1)-J(J +1] an(S), , for which AJ =+ 1, Hence in the present case J’ = J+ 1,, , However, according to the rotational selection rules, only those rotational transitions are allowed \, Substituting this value in eqn. (5), we get, , h2, AE, = Sap lY +0 42)- J 4D), , h2, = yee (6), , To express in terms of wave numbers, we put, , AE, =hv=hS =hev*, , , , -~ Rp, Hence hev =a x2 +), ee v= noe 2841 7,, 8x? Ic ) aoe, where B = is rotational constant (as already explained)., 8x2 Ic, , iebveu oe H, , *Strictly speaking here, we should write Av and AV in place of vand