Page 1 :
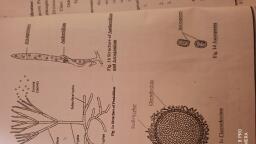
anking., Water Potential and Chemical Potential, Osmosis in the living cells is indirectly energy dependent process., During this process water moves from one side to another side of the, semipermeable membrane only with the energy gradient. The difference, between the free energy of molecules in pure water and the free energy of, water molecules in any other system (i.e. water in the form of solution or, water in the cytoplasmic system of plant cell) is termed as water potential., The amount of free energy available for work, in the molecules of pure, water, is always greater than the available free energy of the water molecules, outside, tes that, sphere, B.Sc. Part II > Semester III> BOTANY Paper y, Plant Water in the, Water, molecules, Water, Soluble, Water, phere., molecules, molecules, molecules, The, (Fig 1.2) Outer, differencers the, permeabl, potential Tonic, the chemd the, Soluble, molecules, C: Highly concentrated, B: Less concentrated, aqueous solution, A: Pure water, in a solution., The energy content of water can easily be described in terms of it's, chemical potential. The chemical potential is defined as the free available, energy per mole (per gram molecular weight) of that substance. In case of, pure water, since all the molecules are available with their free energy, for the work, the amount of free energy per mole (i.e. chemical potential), is always greater than the water molecules in any aqueous solution., (Fig 1.1 (A)) In any solution, since energy in some water molecules (as, solvent) is utilized for binding the solute molecules, the free energy per, mole is always less than that of pure water. (Fig 1.1 (B) and (C) The chemical, potential is the measure of the capacity of a substance to react, or to move,, aqueous solution, Theic or, solution, When we. no, higher s50%, the wat, from hsis., potentisis., (a) Some water molecules, available for work., (a) Very few water mole-, cules available for work., (b) Free energy per mole, (chemical potential) is, very less than pure, (a) All water molecules, available for work., (b) Free energy per mole, (chemical potential) is, maximum., (b) Free energy per mole, (chemical energy) is less, than pure water., (c) Water potential -ve and, less than zero., water., (c) Water potential is zero., (c) Water potential is very, negative and far below, equal, n is, solutic, zero., Fig. 1.1: Concept of water and chemical potential (Diagrammatic)., zero., on tw, This difference in the chemical potential is also because of difference in, the number of water molecules with free energy available for work. In less, concentrated solution, greater number of water molecules with free energy, are available for work than the solution with higher concentration of solute., This difference is finally responsible for movement of water across the, membrane. i.e. when two solutions are separated by a semipermeable, membrane, water from solution having less solute concentration but higher, chemical potential, moves towards the solution, with high solute, concentration but lesser chemical potential and therefore, osmosis, takes place., SOISO, or to work. As free energy per mole, available for work is more, the chemical, potential of pure water is always more than any aqueous solution. The, solute particles decrease the chemical potential of the solvent molecules., Similarly, for a solute (i.e. dissolved chemical substance) in a solvent (in, plants solvent is water), the chemical potential is approximately equal to, the concentration of solute. A diffusing solute tends to move from areas of, high chemical potential (free energy per mole) to areas of low chemical potential., As the solute particles decrease the chemical potential of solvent, when, two solutions of different concentrations (high and low) are separated by a, semipermeable membrane, the solution with less solute concentration have, more chemical potential than the solution with greater amount of solute., in the, parti, Incre, auouso, he, wate, atm, Soil water, (with higher, chemical potential), pres, the, thel,, was, Root hair, Root epidermis, Soil particles, Cytoplasm and cell, sap in root hair, (with lower, chemical potential), sy, Entry of water from, soil to root hair, (from higher chemical, potential to lower, chemical potential), REDMI NOTE 8 PRO, AI QUAD CAMERA, REDMI NOTE 8 PRO Role of water and chemical potential in osmosis., AI QUAD CAMERA
Page 2 :
OTANY Paper V, later, ecules, Plant Water Relationship and Mineral Nutrition, The difference in the chemical potentials is the basis for osmosis., (Fig 1.2) Therefore, it is the rule, that osmosis occurs only, when there is a, difference in the chemical potential of water on two sides of a selectively, permeable membrane. In other words, osmosis occurs only when chemical, potential of water on one side of a selectively permeable membrane exceeds, the chemical potential of water on the other side of the same membrane., The process of osmosis continues till the water potential of both, solutions, on the two sides of a semipermeable membrane, becomes equal., When water diffuses into solution with lesser chemical potential (i.e. with, higher solute concentration), pressure develops in that solution, due to which, the water potential of the solution gradually increases towards zero (i.e., from highly negative to less negative level towards zero). When water, potential in both the solutions becomes equal, it is with some negative but, equal value. The negative value is because, the water potential in both the, solutions will always be less than that of pure water having water potential, zero. Under such conditions, there is no difference in the water potentials, on two sides of the semipermeable membrane and therefore, the process of, osmosis almost stops., The two components of water potentials are pressure potential and, osmotic potential. The pressure potential is the real pressure which develops, in the system, while osmotic potential is caused by the presence of solute, particles (therefore, osmotic potential is also called solute potential)., Increasing concentration of solute particles in the system, decreases the, water potential and therefore, in living plant cells, osmotic potential is, always negative. The pressure potential, by convention, is zero at normal, atmospheric pressure. An increase in the pressure, increases the positive, pressure potential and the tension (i.e. pulling or opposite pressure) increases, the negative pressure potential. In plants, under normal conditions since, the tension exists in the central vascular cylinder due to transpiration pull,, water potential in all living cells is almost, always negative., If we consider the water potentials in three parts soil-plant-air of, system, we find that, the water potential is highest in the soil, it is lowest in, the surrounding atmosphere (air), while in various parts of the plant body,, the values of water potentials are in-between highest (in soil) and lowest, (in air). i.e. intermediate. The difference in the values of water potentials, indicates, that there is a definite water potential gradient, from the soil,, through plant body, to the atmosphere. This water potential gradient is the, driving force for all water transport processes, from soil to plant and from, plant body to the surrounding atmosphere., REDMI NOTE 8 PRO, AI QUAD CAMERA, Soluble, molecules, ntrated, uon, few water mole-, available for work., energy per mole, cal potential) is, ss than pure, potential is very, e and far below, mmatic)., difference in, work. In less, h free energy, on of solute., r across the, ipermeable, but higher, igh solute, , osmosis, d cell, tial), stic, mical
 Learn better on this topic
Learn better on this topic



















































 Learn better on this topic
Learn better on this topic









