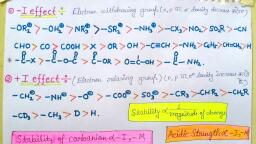Page 1 :
Points to remember in General Organic Chemistry, 1. Inductive effect, The normal C–C bond has no polarity as two atoms of same electronegativity, (EN) value are connected to each other. Hence the bond is nonpolar. Consider, a carbon chain in 1-Chloro butane, here due to more EN of Cl atom C–Cl, bond pair is slightly displaced towards Cl atom hence creating partial negative, ( –) charge over Cl atom and partial positive (+) charge over C1 atom., Now since C1 is slightly positive, it will also cause shifting of C1–C2 bond pair, electrons towards itself causing C2 to acquire small positive charge. Similarly, C3 acquires slightly positive charge creating an induction of charge in carbon, chain. Such an effect is called inductive effect., Diagram showing I effect, , The arrow shows electron withdrawing nature of – Cl group., Thus inductive effect may be defined as a permanent displacement of bond, pair electrons due to a dipole. (Polar bond), Some important points are:, (a) It can also be defined as polarisation of one bond caused by polarisation, of adjacent bond., (b) It is also called transmission effect., (c) It causes permanent polarisation in molecule, hence it is a permanent, effect., (d) The displacement of electrons takes place due to difference in, electronegativity of the two atoms involved in the covalent bond., (e) The electrons never leave their original atomic orbital., (f) Its magnitude decreases with distance and it is almost negligible after 3rd, carbon atom., (g) The inductive effect is always operative through bond, does not involve , bond electron., , Types of inductive effects :, (a) – I Effect : The group which withdraws electron cloud is known as – I, group and its effect is called – I effect. Various groups are listed in their, decreasing – I strength as follows., >–, >–, > –NO2 > –SO2R > –CN > –CHO > –COOH > –F >, –Cl > –Br > –I > –OR > –OH > –CCH > –NH2 > –C6H5 > –CH=CH2 > –H., (b) + I effect : The group which release electron cloud is known as + I group, and effect is + I effect., Page # 119
Page 2 :
>, > –C(CH3)3 > –CH(CH3)2 > –CH2–CH3 > –CH3 > –D > –H, The hydrogen atom is reference for + I and – I series. The inductive effect of, hydrogen is assumed to be zero., Ex. Let us consider effect of COOH & – COO– in carbon chain, (a), , (b), , Due to e¯ donating nature of, carbon chain has become partially, negative but – COOH is – I group therefore carbon chain has become partially, positive., , 2. Resonance, Resonance is the phenomenon in which two or more structures involving in, identical position of atom, can be written for a particular species, all those, possible structures are known as resonating structures or canonical structures., Resonating structures are only hypothetical but they all contribute to a real, structure which is called resonance hybrid. The resonance hybrid is more, stable than any resonating structure., , , , , Resonance hybrid :, The most stable resonating structure contribute maximum to the resonance, hybrid and less stable resonating structure contribute minimum to resonance, hybrid., , Conjugation:, A given atom or group is said to be in conjugation with an unsaturated system, if:(i) It is directly linked to one of the atoms of the multiple bond through a single, bond., (ii) It has ð bond, positive charge, negative charge, odd electron or lone pair, electron., , Page # 120
Page 3 :
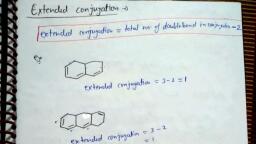
Types of Conjugation :, 1., , Conjugation between C = C and, C=C (, , 2., , Conjugation between +ve charge and, C=C (, , 3., , ), , , , Conjugation between odd electron and, C=C (CH2=CH–, , 5., , ), , , , Conjugation between lone pair and, C=C (, , 4., , ), , , , , , –CH=CH2), , Conjugation between negative charge and, C=C (, , , , ), , 3. Mesomeric effect (or Resonance effect), Mesomeric effect is defined as permanent effect of electron shifting from, multiple bond to atom or from multiple bond to single bond or from lone pair to, single bond. This effect mainly operates in conjugated system of double bond., So that this effect is also known as conjugate effect., Ex., , Types of Mesomeric effects :, (a) Positive Mesomeric effect (+M effect) :, When the group donates electron to the conjugated system it shows + M, effect., Relative order of +M groups (usually followed) :, – O > –NH2 > –NHR > –NR2 > –OH > –OR > –NHCOR > –OCOR > –Ph >, –F > –Cl > –Br > –I > –NO, Ex. (I), , Page # 121
Page 4 :
(b) Negative Mesomeric effect (–M effect) :, When the group withdraws electron from the conjugated system, it shows, – M effect, , Relative order of –M groups (usually followed) :, –NO2 > –CHO >, , —, –, C=O > –C–O–C–R > –C–O–R > –COOH > –CONH2 > –C–O, —, ||, ||, ||, ||, , O, , O, , O, , O, , Ex. (I), , (II), , H 2C = CH – C N:, , +, –, H 2C – CH = C = .N:., , 4. Hyperconjugation, It is delocalisation of sigma electron with p-orbital. Also known as conjugation or no bond resonance. It may takes place in alkene, alkynes,, carbocation, free radical, benzene nucleus., Necessary Condition : Presence of at least one hydrogen at saturated carbon, which is with respect to alkene, alkynes, carbocation, free radical, benzene, nucleus., (i) Hyperconjugation in alkene, , (ii) Hyperconjugation in carbocation, , (iii) Hyperconjugation in radical, , Page # 122
Page 5 :
5., , Aromatic character [The Huckel 4n + 2 rule], The following rules are useful in predicting whether a particular compound is, aromatic or non–aromatic. Aromatic compounds are cyclic and planar. Each, atom in an aromatic ring is sp2 hybridised. The cyclic molecular orbital, (formed by overlap of p-orbitals) must contain (4n + 2) electrons, i.e., 2, 6,, 10, 14 ........ electrons. Where n = an integer 0, 1, 2, 3,.............., Aromatic compounds have characteristic smell, have extra stability and burn, with sooty flame., Comparision between aromatic, anti aromatic and non-aromatic compounds., Aromatic, compounds (A), , Anti Aromatic, compounds (B), , Non-Aromatic, compounds (C), , 1. Structure, , Cyclic, planar all, atoms of ring sp2, hybridised, , Cyclic, planar all, atoms of ring sp2, hybridised, , Cyclic or acyclic, planar,or non planar, 2, 3, sp or sp or sp, , 3. MOT, , Unpaired e¯s in B.M.O., , 4. Overlapping, , Favourable over, lapping of p orbital, , Unfavourable over, lapping of p orbital, , Simple overlaping, like alkenes, , 5. Resonance, energy (R.E.), , Very high, R.E. > 20-25 kcal/mol, , Zero, , 4-8 kcal/mol, like alkenes, , Unstable, not-exist at, room temperature, , Normal stability, like a conjugated, system, , Dimerisation, reaction to attain, stability, , Electrophilic addtion, reaction like alkenes, , Characteristics, , Example, , 6. Stability, , 7. Characteristic, Reactions, , Electrophilic, substitution Reaction, , B.M.O. / Non-bonding, M.O., , Stability of compounds : Aromatic > Non-Aromatic > Anti-Aromatic, , (A) Carbocation :, Definition : A carbon intermediate which contain three bond pair & a positive, charge on it is called carbocation., Hybridisation : Carbocation may be sp2 & sp hybridised, Hybridisation, sp2, sp, , Example, ,, H2 C =, , ,, , ,, , , HC , , Carbocations are electron deficient. They have only six electrons in their, valence shell, and because of this, carbocations act as Lewis acids. Most of, the carbocations are short-lived and highly reactive, they occur as intermediates, Page # 123
Page 6 :
in some organic reactions. Carbocations react with Lewis bases or ions that, can donate the electron pair, that they need to achieve a stable octet of, electrons (i.e., the electronic configuration of a noble gas):, , Because carbocations are electron seeking reagents, chemists call them, electrophiles. All Lewis acids, including protons, are electrophiles. By, accepting an electron pair, a proton achieves the valence shell configuration, of helium; carbocations achieve the valence shell configuration of neon., Stability : Carbocations are stabilised by, (i) + I effect, (ii) + M effect, (iii) Hyperconjugation, (iv) delocalisation of charge, General stability order :, >, , >, , >, , >, , >, , >, , >, , (B) Carbanion :, Definition : A carbon intermediate which contain three bond pair and a negative, charge on it, is called carbanion., Hybridisation : Hybridisation of carbanion may be sp3, sp2 & sp., Hybridisation, Example, sp3, , , CH3–, , sp2, , H2C=, , sp, , HC , , ,, , , CH2=CH–, , ,, , ,, , ,, , Page # 124
Page 7 :
Stability of carbanion : Carbanions are stabilised by electron withdrawing, effect as, (i) – I effect, (ii) – m effect, (iii) Delocalisation of charge, , (C) Free Radicals :, Homolysis of covalent bond results into free radical intermediates possess, the unpaired electrons., , It is generated in presence of Sun light, Peroxides or High temperature, , Free Radical : An uncharged intermediate which has three bond pair and an, unpaired electron on carbon., Note : (i) It is Neutral species with odd e¯, (ii) It is paramagnetic in nature due to odd e¯, (iii) No rearrangement is observed generally., (iv) Carbon atom having odd electron is in sp2 hybridised state, (v) Any reaction if it is carried out in the presence of sunlight, peroxide or high, temperature it generally proceeds via free radical intermediate., , , Stability of free radical : It is stabilised by resonance, hyperconjugation, and + I groups., , , Ex. (H3C)3C >, , , , , , H3 C – C H – CH3 H3C – C H2 C H3, , (Stability order), , (D) Carbenes (Divalent Carbon intermediates) :, Definition : There is a group of intermediates in which carbon forms only two, bonds. These neutral divalent carbon species are called carbenes. Most, carbenes are highly unstable that are capable of only fleeting existence., Soon after carbenes are formed, they usually react with another molecules., , Methods of preparation of carbene :, CHCl3 +, , :CCl2, , ;, , CH2I2 + Zn, , :CH2, , (E) Nitrenes :, The nitrogen analog of carbenes are nitrenes. They are very much reactive, since in them octet of N is incomplete. In nitrenes only one valencies of N, are satisfied., , Page # 125
Page 8 :
(F) Benzyne :, The benzene ring has one extra C – C bond in benzyne, , Clearly, we can see that the newly formed bond cannot enter in resonance, with other orbitals of ring. since it is in perpendicular plane., It is also important to note that hybridisation of each carbon involved in, 'Benzynic bond' is sp2 since the overlap between these sp2 hybrid orbitals is, not so much effective., , Points to remember in Alkane, Wurtz reaction (Reagent : Na, ether) 1º & 2º alkyl halides give this, reaction., ether, , R – R ;, R – X + 2Na , , R – X + R’ – X + 2Na, , ether, , , R – R’ + R – R + R’ – R’, ether, + 2Na , , , , Points to remember in Alkene & Alkyne, Characteristic reaction of Alkene & Alkyne is Electrophilic addition reaction., Mechanism, Step 1 : Attack of the electrophile on bond forms a carbocation., +, , + E, , |, –C–C, |, E, , +, , C=C, , + on the more substituted carbon, , Step 2 : Attack by a nucleophile gives the product of addition., +, , |, –C–C, |, E, , + Nu:, , |, |, CC, |, |, E Nu, , Page # 126