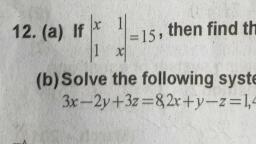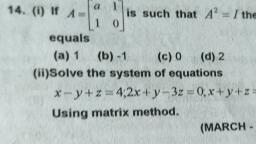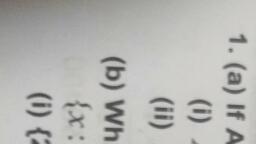Page 1 :
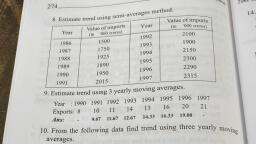
aM POLAT +4 Chemistry, , , , , , 45 [2 Mark Question], , a) Inthe periodic table, elements are classified into, four blocks. Explain any two blocks., b) Account for the following:, , ) First ionization enthalpy of Boron is less than, , that of carbon., ii) First member of a group differs from the rest, , (2) |, , of the members of the same group. (2), , a) The elements are classified into four blocks viz.,, s-block, p-block, d-block and f-block depending, on the type of atomic orbitals that are being filled, with electrons., , 1) The s-block elements : The elements of, Group 1 (alkali metals) and Group 2 (alkaline, earth metals) which have ns‘ and ns”, outermost electronic configuration belong to, this block. They are all reactive metals with, low ionization enthalpies., , The lose the outermost electron(s) readily to, , form 1+ ion (in the case of alkali metals) and |, , 2+ ion (in the case of alkaline earth metals)., , The metallic character and the reactivity |, increase down the group. Because of high |, reactivity they are never found pure in nature., The compounds of s-block element are |, predominantly ionic (except compounds of Li |, , and Be)., , 2) The p-block elements : This block comprises |, , those elements belonging to Group 13 to 18., , The outermost electronic configuration varies |, , from nsnp' to ns? np® in each period., , At the end of each period is a noble gas, element with a closed valence shell ns? np®, configuration., , This configuration is highly stable and hence, noble gases exhibit very low chemical, , reactivity. Preceeding the noble gases are two |, , chemically important groups of non-metals, such as halogens (Group 17) and the, chalcogens (Group 16)., , The non-metallic character increases from left, to right across a period and metallic character, increases down the group., , , , , , 4) The f-bloc, , - Lanthanoids an, , b) i), , ), , giavsitication of Elements ang, | « elements (Trangiy,, . These are the elements of Crp,, Cia e centre of the Periodic tae, to a ed by the filling Of j Inner,, , which e the general outer ect,, , ey hav, orbitals. They )d* 10 ns”. 2, , “1, figuration ("config etals and mostly form cojg,_, , exhibit: variable valence in, P eciueritit and are used as Catalyy,, Petranston elements form ;, , the chemically active metals . OCk a,, less active elements of Groups 1¥ and 14, k elements (Inner-Transit,, ts, Elements) « This include two ns of elem,, at the bottom of the Periodic Table SUCh a, d Actinoids. These ‘i, characterised. by. the outer electron,, configuratin (n-2)f" (n-1)d°' ns?. The bs, electron added to each element is filled j,,, , orbital. |, They are all metals. Within each series, t,, properties of the elements are quite simij,,, The chemistry of actinoids is more Complicate,, due to large number of possible oxidatia, states. Actinoids are radioactive., , c, , They are all’, , The elements after uranium are calle, transuranium elements, (Explanation of an, two blocks is required), , lonization enthalpy increases from left to righ, across a period due to decrease in atomi, size and increase in effective nuclear charge, , Carbon has high first ionization enthalpy tha, boron due to its small atomic size and high, effective nuclear charge., , The first element of each of the groups 4 at’, 2 and groups 13 -17 differs in many respec:, from the other members of their respectiv, group., , This anomalous behaviour is attributed to the, small size,high ionization enthalpy, !ary, charge/radius ratio, high electro negativity, n0”, availability of d-orbitals and greater ability”, form px- px multiple bonds to itself and“, other second period elements (in the casé®, first members Of p-block pernents).